| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 2, April 2023, pages 59-67
Lumen-Apposing Metal Stent With and Without Concurrent Double-Pigtail Plastic Stent for Pancreatic Fluid Collections: A Comparative Systematic Review and Meta-Analysis
Azizullah Berana, i, Mouhand F.H. Mohamedb, Thaer Abdelfattahc, Yara Sarkisd, Jonathan Montrosed, Wasef Sayehe, Rami Musallamf, Fouad Jaberg, Khaled Elferth, Eleazar Montalvan-Sanchezd, Mohammad Al-Haddada
aDivision of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN 46204, USA
bDepartment of Internal Medicine, Warren Alpert Medical School Brown University, Providence, RI, USA
cDivision of Gastroenterology and Hepatology, Allegheny Health Network, Pittsburgh, PA, USA
dDepartment of Internal Medicine, Indiana University, Indianapolis, IN, USA
eDepartment of Internal Medicine, University of Toledo, Toledo, OH, USA
fDepartment of Medicine, Case Western Reserve University, Cleveland, OH, USA
gDepartment of Internal Medicine, University of Missouri, Kansas City, MO, USA
hDepartment of Medicine, St. Barnabas Hospital Health System, Bronx, NY, USA
iCorresponding Author: Azizullah Beran, Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN 46204, USA
Manuscript submitted January 26, 2023, accepted March 20, 2023, published online April 28, 2023
Short title: LAMS With DPPS vs. LAMS Alone for PFCs
doi: https://doi.org/10.14740/gr1601
| Abstract | ▴Top |
Background: Lumen-apposing metal stents (LAMSs) are often used to drain pancreatic fluid collections (PFCs). However, adverse events, such as stent obstruction, infection, or bleeding, have been reported. Concurrent double-pigtail plastic stent (DPPS) deployment has been suggested to prevent these adverse events. This meta-analysis aimed to compare the clinical outcomes of LAMS with DPPS vs. LAMS alone in the drainage of PFCs.
Methods: An extensive search was conducted in the literature to include all the eligible studies that compared LAMS with DPPS vs. LAMS alone for drainage of PFCs. Pooled risk ratios (RRs) with the 95% confidence intervals (CIs) were obtained within a random-effect model. The outcomes were technical and clinical success, and overall adverse events, including stent migration and occlusion, bleeding, infection, and perforation.
Results: Five studies involving 281 patients with PFCs (137 received LAMS plus DPPS vs. 144 received LAMS alone) were included. LAMS plus DPPS group was associated with comparable technical success (RR: 1.01, 95% CI: 0.97 - 1.04, P = 0.70) and clinical success (RR: 1.01, 95% CI: 0.88 - 1.17). Lower trends of overall adverse events (RR: 0.64, 95% CI: 0.32 - 1.29), stent occlusion (RR: 0.63, 95% CI: 0.27 - 1.49), infection (RR: 0.50, 95% CI: 0.15 - 1.64), and perforation (RR: 0.42, 95% CI: 0.06 - 2.78) were observed in LAMS with DPPS group compared to LAMS alone but without a statistical significance. Stent migration (RR: 1.29, 95% CI: 0.50 - 3.34) and bleeding (RR: 0.65, 95% CI: 0.25 - 1.72) were similar between the two groups.
Conclusions: Deployment of DPPS across LAMS for drainage of PFCs has no significant impact on efficacy or safety outcomes. Randomized, controlled trials are necessary to confirm our study results, especially in walled-off pancreatic necrosis.
Keywords: Lumen-apposing metal stent; Double-pigtail plastic stent; Pancreatic fluid collection; Walled-off pancreatic necrosis
| Introduction | ▴Top |
Acute pancreatitis (AP) remains one of the most prevalent gastrointestinal-related hospital discharge diagnoses, and it has a high morbidity and death rate [1]. More than 200,000 people in the USA are hospitalized annually due to AP [2]. Mortality can reach up to 21% in AP, especially in severe cases [3]. Pancreatic fluid collections (PFCs) can form in about 43% of AP patients [4]. Although most PFCs resolve spontaneously, almost 15% can persist beyond 4 weeks [4]. PFCs can persist as homogeneous fluid collections called pancreatic pseudocysts (PPs) [5]. Heterogenous collections called walled-off pancreatic necrosis (WOPN) can occur as a complication of acute necrotizing pancreatitis [5]. PFCs can become infected or symptomatic by compressing the adjacent organs and structures [6]. Infection of PFCs or symptomatic compression are an indication to drainage [6, 7].
Endoscopic transluminal drainage has emerged as the preferred modality of therapy for managing symptomatic PFCs [8]. PFCs can be drained endoscopically using double-pigtail plastic stent (DPPS) or lumen-apposing metal stent (LAMS) [9]. LAMSs have largely supplanted DPPS due to their advantages in terms of simplicity of deployment, shorter procedure duration, higher clinical success rates, and lower migration rates [10-12]. However, recent studies reported higher rates of bleeding, buried LAMS syndrome, stent occlusion, and infection with the use of LAMS alone [13-16]. Therefore, some studies suggested that simultaneous placement of DPPS may help decrease the risk of LAMS-related adverse events [16-18]. LAMS with concurrent DPPS placement was compared to LAMS alone for drainage of PFCs in recent studies [19-21]. However, the results were inconsistent, and the sample sizes were small [17-21]. This meta-analysis aimed to assess and compare the clinical outcomes of LAMS with DPPS and LAMS alone for drainage of PFCs.
| Materials and Methods | ▴Top |
The Institutional Review Board (IRB) approval was not applicable. This study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [22, 23].
Search strategy
We extensively searched PubMed, EMBASE, and Web of Science databases for all relevant articles published from inception until December 10, 2022 (“pancreatic fluid collection” or “pancreatic necrosis”, and “lumen-apposing metal stent” or “double pigtail plastic stent”, were utilized as MeSH words. The complete search terms used for each database search are detailed here (Supplementary Material 1, www.gastrores.org).
Eligibility criteria
Studies comparing LAMS with concurrent DPPS vs. LAMS alone for drainage of PFCs and reporting one of the following clinical outcomes: technical success, clinical success, and overall adverse events, including stent migration, stent occlusion, infection, bleeding, or perforation, were eligible for inclusion. Conference abstracts were excluded in our analysis. The studies for the final review were separately evaluated and selected by two authors (YS and JM). A third author resolved any disagreement (WS).
Data extraction
The data on study, patient, and disease characteristics and outcome measure were extracted by two independent authors (AB and YS). The extracted disease characteristics included PFC features (type, etiology, size, and location), drainage site, definition of clinical success, number of necrosectomy procedures performed, and LAMS size used. Outcome measures retrieved were efficacy outcomes (technical success and clinical success) and safety outcomes (i.e., overall adverse events, stent migration and occlusion, infection, bleeding, and perforation).
Outcome measures
Technical success, clinical success (PFC resolution or improvement), and overall adverse effects were our primary outcome measures. Secondary outcome measures included stent migration and occlusion rates, as well as bleeding, infection, and perforation rates.
Data analysis
A random-effects model was used to assess the outcome data, which were summarized as a pooled risk ratio (RR) with a corresponding 95% confidence intervals (Cis). P values < 0.05 were considered statistically significant. The I2 statistic, as specified in the Cochrane handbook for systematic reviews, was used to assess heterogeneity and I2 value of 50% or more was deemed substantial heterogeneity. If continuous data were reported in median and interquartile range, they were converted to mean and standard deviation [24]. All statistical analyses were conducted via Review Manager 5.4 and Open Meta Analyst softwares. We further intended to perform leave-one-out sensitivity analysis for outcomes reported by ≥ 5 studies.
Quality assessment
The Newcastle Ottawa scale (NOS) was used to assess the quality of the included studies [25]. Studies having scores of 6 or higher were deemed to be of high-quality. Two authors (AB and YS) evaluated each study independently for bias. Disagreements were settled by a third reviewer (TA). Publication bias assessment was not feasible given that the number of eligible studies were < 10.
| Results | ▴Top |
Study selection
Our search yielded a total of 1,119 studies. Eventually, 10 studies were suitable for systematic review. Consequently, we omitted five studies due to improper comparison [13, 26-29]. Four [13, 27-29] studies compared LAMS versus DPPS. In one [26] study, all patients received LAMS plus DPPS with no comparison with LAMS alone. Eventually, five [17-21] studies were included in our analysis. Figure 1 summarizes the selection process in this study (PRISMA flow chart).
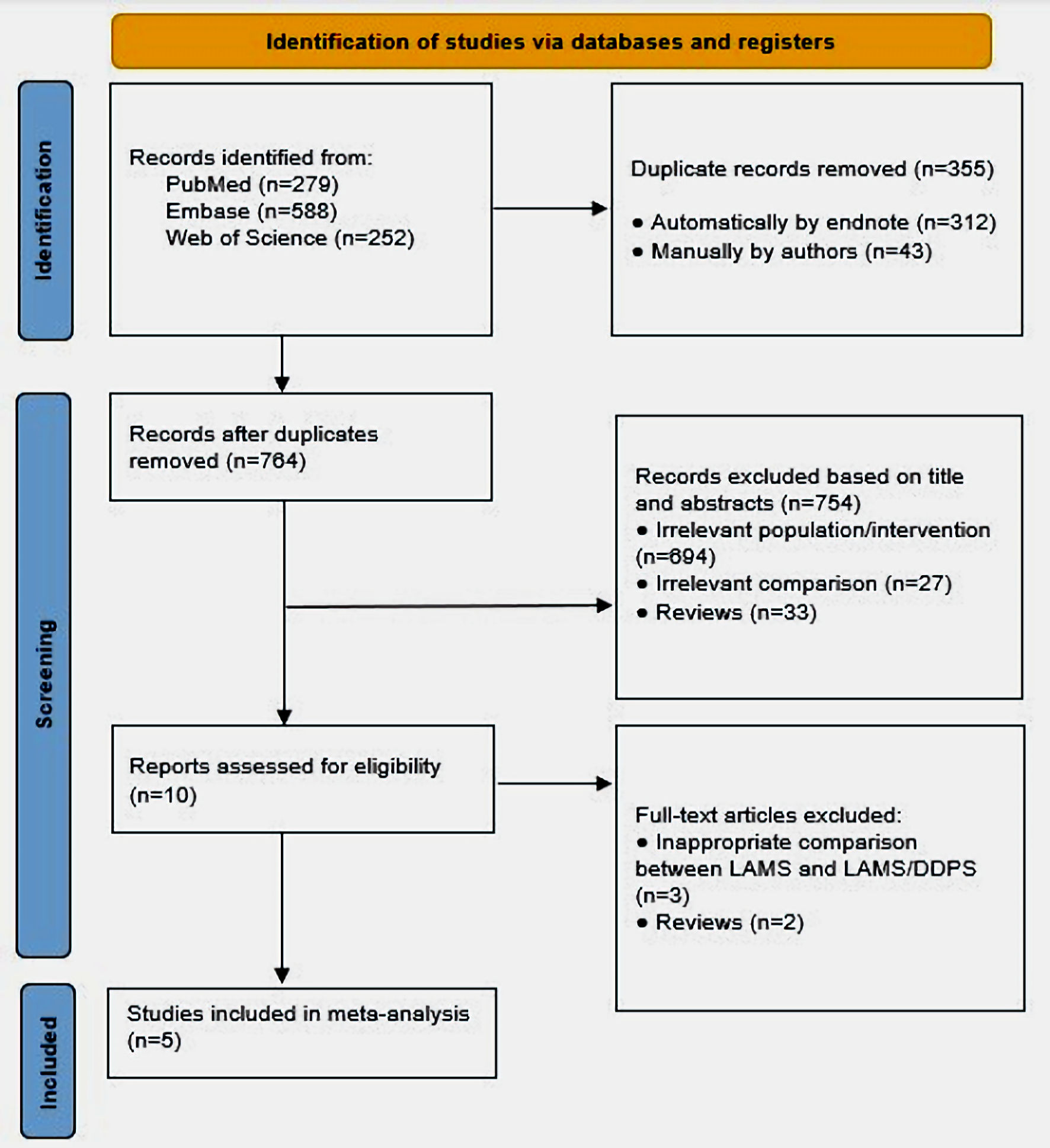 Click for large image | Figure 1. PRISMA flow diagram of included studies. PRISMA: preferred reporting items for systematic reviews and meta-analyses; DPPS: double-pigtail plastic stent; LAMS: lumen-apposing metal stent. |
Study and patients’ characteristics
Table 1 [17-21] shows the study and patient characteristics of the studies involved in the meta-analysis. All of the studies [17-21] that evaluated individuals with PFC, were retrospective, and were published between December 2017 and December 2022. One [19] study was a multicenter study, while four [17, 18, 20, 21] studies were single-center studies. Four [18-21] studies were carried out in the USA and one [17] in Spain. A total of 281 participants were included in the study (137 received LAMS with concurrent DPPS and 144 received LAMS alone), with males accounting for 64.4% of the total patients.
 Click to view | Table 1. Study Characteristics of the Included Studies |
The patients, PFCs, and procedural characteristics of the included studies in the meta-analysis are outlined in Table 2. In terms of age, gender, proton pump inhibitor (PPI) use, type, etiology, and location of PFCs, the LAMS plus DPPS group and the LAMS alone group were similar. The mean PFC size was larger in the LAMS alone group compared to the LAMS plus DPPS group (12.2 cm vs. 10.7 cm, respectively, P = 0.02). PP and WOPN represented 51.2% and 44.5% of PFCs, respectively. The most common etiologies of PFCs were gallstones (33%) and alcohol (31%). The drainage site was mostly transgastric (85%).
 Click to view | Table 2. Baseline Patients and Procedural Characteristics Included in the Meta-Analysis |
Outcomes of interest
Technical and clinical success
Technical and clinical success were reported by all five [17-21] studies. There was no significant difference in technical success between the LAMS with concurrent DPPS and LAMS alone groups (100% vs. 98.6%, respectively) (RR: 1.01, 95% CI: 0.97 - 1.04, P = 0.70, I2 = 0%) (Fig. 2a). Similarly, there was no significant difference in clinical success between the two groups (82.5% vs. 86.1%, respectively) (RR: 1.01, 95% CI: 0.88 - 1.17, P = 0.85, I2 = 60%) (Fig. 2b). The results remained consistent on sensitivity analysis for both technical and clinical success as shown here (Supplementary Material 2, www.gastrores.org).
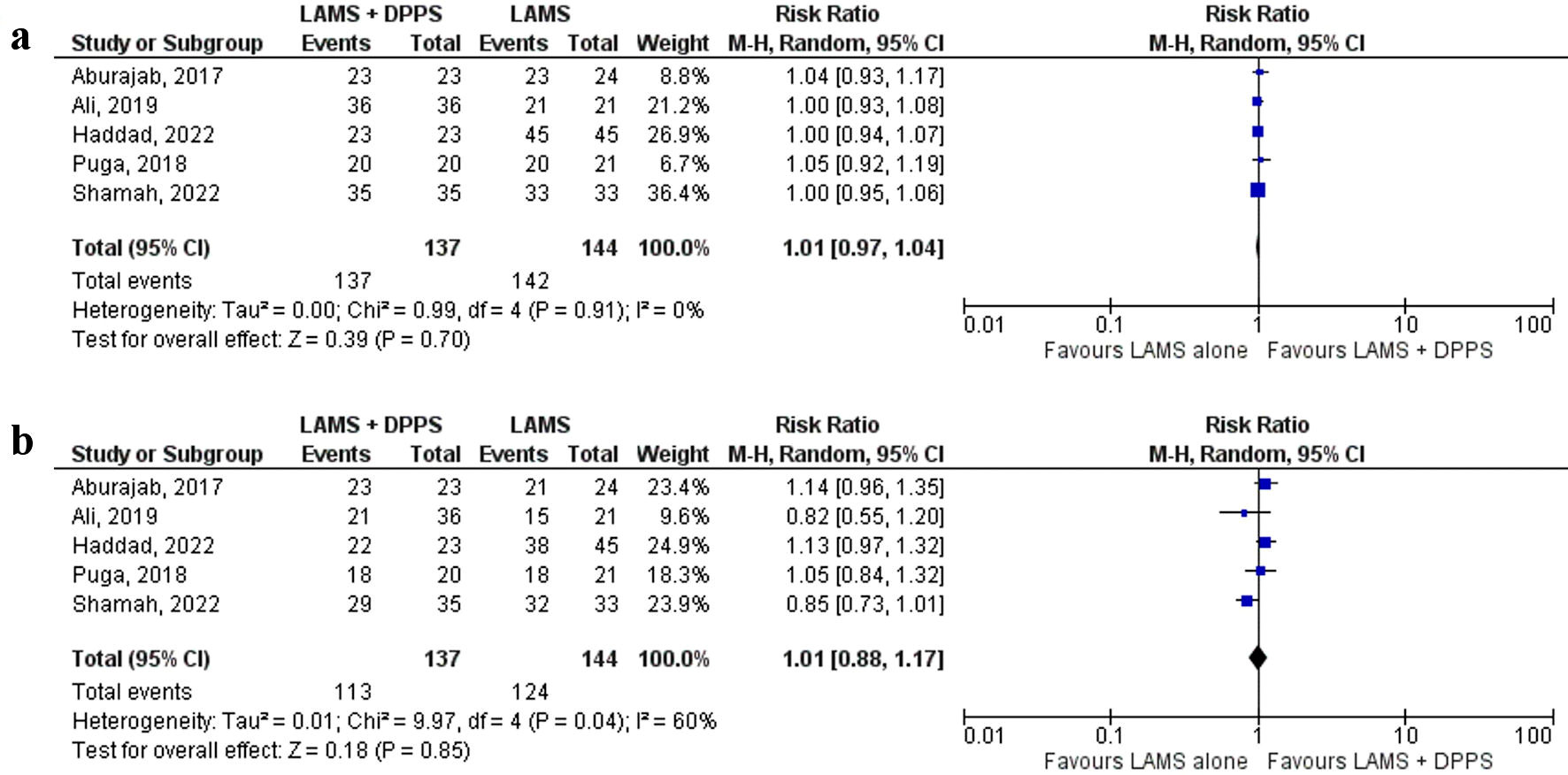 Click for large image | Figure 2. Forest plots comparing between LAMS plus DPPS and LAMS alone for drainage of pancreatic fluid collections regarding: (a) technical success and (b) clinical success. DPPS: double-pigtail plastic stent; LAMS: lumen-apposing metal stent; CI: confidence interval. |
Overall adverse events
All five [17-21] studies reported overall adverse events. Although lower trend of overall adverse events was observed in the LAMS with concurrent DPPS group compared to the LAMS alone group (21.2% vs. 30.6%, respectively), the difference was not statistically significant (RR: 0.64, 95% CI: 0.32 - 1.29, P = 0.22, I2 = 50%) (Fig. 3a).
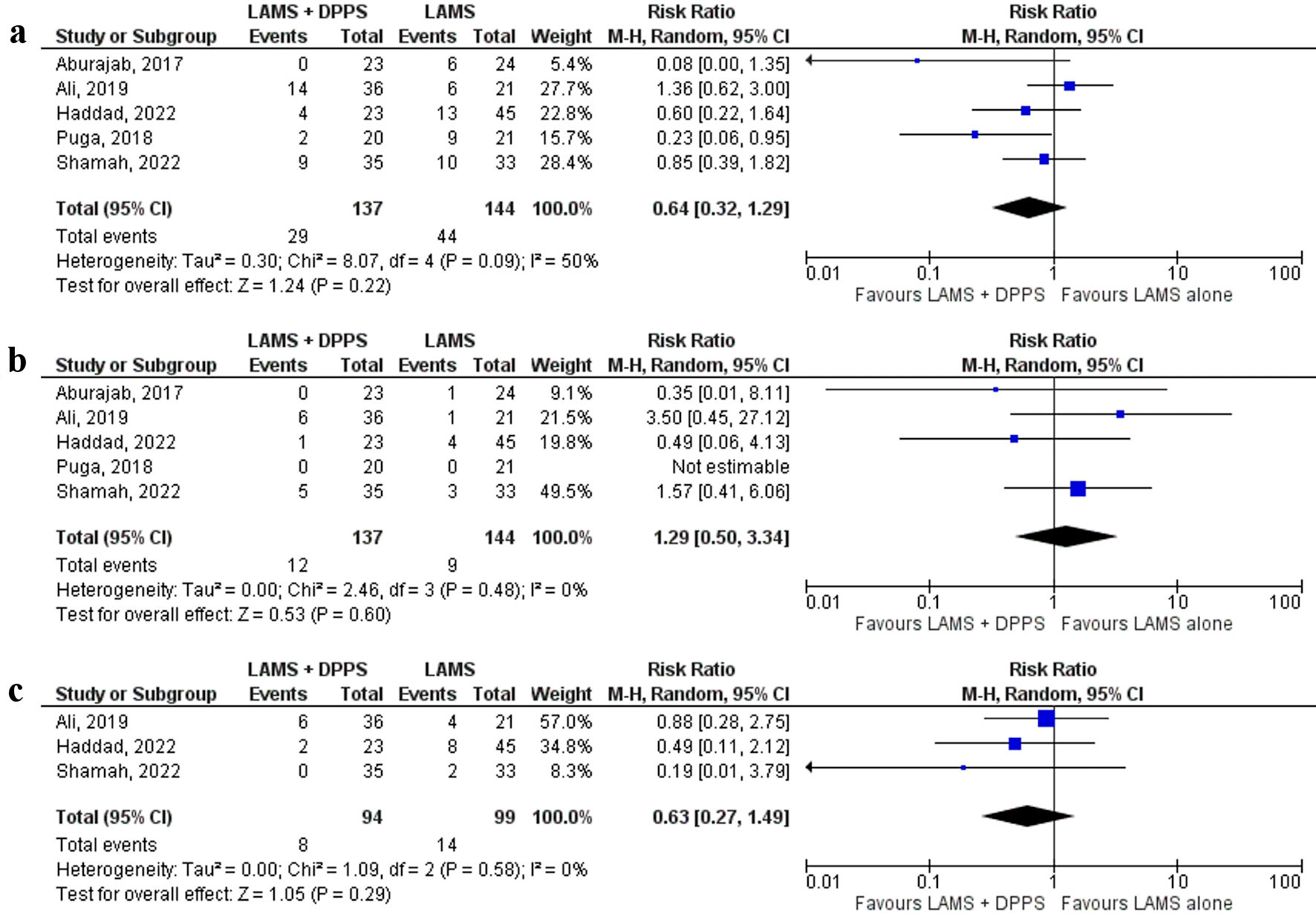 Click for large image | Figure 3. Forest plots comparing between LAMS plus DPPS and LAMS alone for drainage of pancreatic fluid collections regarding: (a) overall adverse events, (b) stent migration, and (c) stent occlusion. DPPS: double-pigtail plastic stent; LAMS: lumen-apposing metal stent; CI: confidence interval. |
Stent migration and occlusion
All five [17-21] studies reported stent migration and the rate was similar between the two groups (LAMS plus DPPS vs. LAMS alone: 8.8% and 6.3%, respectively) (RR: 1.29, 95% CI: 0.50 - 3.34, P = 0.60, I2 = 0%) (Fig. 3b). Stent occlusion was reported by three [19-21] studies. Although lower trend of stent occlusion was observed in the LAMS with concurrent DPPS group (8.5%) compared to the LAMS alone group (14.1%) (RR: 0.63, 95% CI: 0.27 - 1.49, P = 0.29, I2 = 0%) (Fig. 3c), the difference did not reach statistical significance.
Bleeding, infection, and perforation
All five [17-21] studies reported bleeding. Bleeding was similar in LAMS plus DPPS and LAMS alone (5.1% and 8.3%, respectively) (RR: 0.65, 95% CI: 0.25 - 1.72, P = 0.39, I2 = 0%) (Fig. 4a).
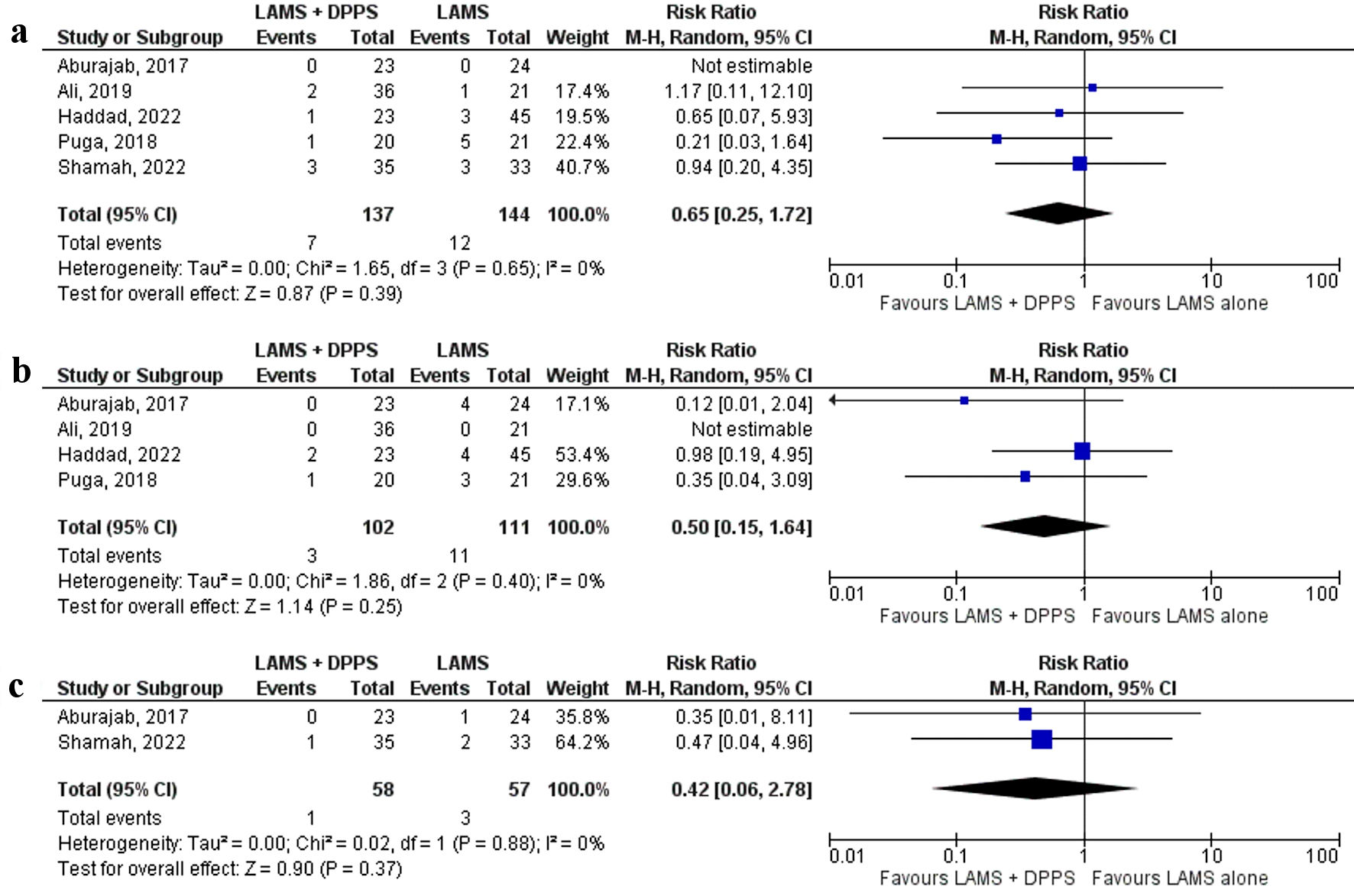 Click for large image | Figure 4. Forest plots comparing between LAMS plus DPPS and LAMS alone for drainage of pancreatic fluid collections regarding: (a) bleeding, (b) infection, and (c) perforation. DPPS: double-pigtail plastic stent; LAMS: lumen-apposing metal stent; CI: confidence interval. |
Infection was reported by four [17, 18, 20, 21] studies and a lower trend of infection rate was observed with the LAMS with concurrent DPPS group (2.9%) compared to the LAMS alone group (9.9%) but without a statistical significance (RR: 0.50, 95% CI: 0.15 - 1.64, P = 0.25, I2 = 0%) (Fig. 4b).
Only two [18, 19] studies reported perforation. There was a trend toward lower perforation with the LAMS with DPPS group compared to LAMS alone, but it was not statistically significant (1.7% and 5.2%, respectively) (RR: 0.42, 95% CI: 0.06 - 2.78, P = 0.37, I2 = 0%) (Fig. 4c).
Our findings remained consistent on the sensitivity analysis for overall adverse events, stent migration, and bleeding (Supplementary Material 3, www.gastrores.org).
Quality assessment
The quality of included studies was assessed and summarized here (Supplementary Material 4, www.gastrores.org). All included studies [15-19] were of high-quality and scored ≥ 6 in the NOS (Supplementary Material 4, www.gastrores.org). Since there were less than 10 studies, we could not analyze publication bias.
| Discussion | ▴Top |
This systematic review and meta-analysis of five studies with 281 patients compares LAMS with DPPS to LAMS alone in PFC. We found no significant differences in the safety or efficacy outcomes of PFC drainage with LAMS plus DPPS vs. LAMS alone.
Although LAMS has become the preferred therapeutic option for the management of symptomatic PFCs, recent studies reported increasing rates of adverse events such as bleeding, and stent occlusion associated with LAMS use [14-16]. Simultaneous placement of DPPS across LAMS has been proposed to decrease the risk of LAMS-related adverse events such as stent occlusion and bleeding. However, our study showed that deployment of DPPS across LAMS for drainage of PFCs did not significantly affect safety outcomes.
The study of Aburajab et al [18] was the first to show that the DPPS plus LAMS reduced the risk of infection in PFCs (0% in LAMS plus DPPS vs. 17% in LAMS alone). The primary limitation of that study was that the only evaluated PFCs were PPs and WOPN were not included [18]. More recently, multiple studies that included both types of PFCs (PP and WOPN), have been published and reported that concurrent placement of DPPS with LAMS did not reduce the risk of adverse events [19-21]. Furthermore, Shamah et al [19] published the first multicenter study to date and showed similar outcomes between the two therapeutic strategies in terms of adverse events regardless of PFC type (PP vs. WOPN).
Our study results matched the largest single-center study conducted by Haddad et al [20], which showed numerical but not statistically significant fewer rates of overall adverse events and stent occlusion in the LAMS plus DPPS strategy compared to the LAMS only strategy. In addition, in our analysis, stent migration rate was similar between the two strategies, which is in line with the multicenter study by Shamah et al [19]. Based on our study findings, placement of DPPS across LAMS might not provide any additional clinical benefit to patients with PFCs despite requiring more time, cost, and resources.
This study has several limitations. To begin, our study included only observational studies with relatively small sample sizes. These observational studies are susceptible to selection biases. In light of this, it would be prudent to conduct randomized, controlled trials to validate our findings. Second, significant heterogeneity was noted in measuring clinical success and overall adverse events, which could be due to variations in baseline patient characteristics, follow-up duration, and differences in PFC size, indwelling duration of stents, and endoscopic techniques in the included studies. Third, most studies were conducted in the USA, which limits our results’ generalizability to other populations. Fourth, DPPS was placed at the endoscopist’s discretion, and there were no predefined criteria for selecting therapeutic strategies (LAMS with DPPS or LAMS alone) in patients in the included studies. Lastly, we could not account for the type of PFC (PP vs. WOPN) and differences in the number of necrosectomies needed with each strategy due to limited reported information. Further studies are warranted to evaluate the efficacy of deployment of DPPS across LAMS in patients with WOPN.
In conclusion, our meta-analysis shows that deployment of DPPS across LAMS for drainage of PFCs has no significant impact on clinical success and complications, including stent migration and occlusion, bleeding, infection, or perforation. Randomized, controlled trials are necessary to confirm our study results, especially in patients with WOPN.
| Supplementary Material | ▴Top |
Suppl 1. Search strategy used in each database searched.
Suppl 2. Leave-one-out sensitivity analysis for technical success and clinical success.
Suppl 3. Leave-one-out sensitivity analysis for overall adverse events, stent migration, and bleeding.
Suppl 4. Quality assessment of the included studies in the meta-analysis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors do not have any conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
AB, TA, MM designed the study and drafted the manuscript. MA critically revised the manuscript. YS, JM, WS, FJ, RM, KE, EMS, and MM interpreted and collected the data, reviewed the literature, and revised the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AP: acute pancreatitis; DPPS: double-pigtail plastic stent; LAMS: lumen-apposing metal stent; NOS: Newcastle Ottawa scale; PFC: pancreatic fluid collection; PP: pancreatic pseudocyst; WOPN: walled-off pancreatic necrosis
| References | ▴Top |
- Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, Jensen ET, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731-1741.e1733.
doi pubmed pmc - Ingraham NE, King S, Proper J, Siegel L, Zolfaghari EJ, Murray TA, Vakayil V, et al. Morbidity and mortality trends of pancreatitis: an observational study. Surg Infect (Larchmt). 2021;22(10):1021-1030.
doi pubmed pmc - Popa CC, Badiu DC, Rusu OC, Grigorean VT, Neagu SI, Strugaru CR. Mortality prognostic factors in acute pancreatitis. J Med Life. 2016;9(4):413-418.
pubmed pmc - Cui ML, Kim KH, Kim HG, Han J, Kim H, Cho KB, Jung MK, et al. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci. 2014;59(5):1055-1062.
doi - Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
doi - Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, Sharaiha RZ, et al. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol. 2016;22(7):2256-2270.
doi pubmed pmc - Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, Horvath KD, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176-1194.
doi - van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491-1502.
doi - Dorrell R, Pawa S, Pawa R. Endoscopic management of pancreatic fluid collections. J Clin Med. 2021;10(2):284.
doi pubmed pmc - Bazerbachi F, Sawas T, Vargas EJ, Prokop LJ, Chari ST, Gleeson FC, Levy MJ, et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87(1):30-42.e15.
doi - Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(1):67-75.e61.
doi - Lyu Y, Li T, Wang B, Cheng Y, Chen L, Zhao S. Comparison between lumen-apposing metal stents and plastic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collection: a meta-analysis and systematic review. Pancreas. 2021;50(4):571-578.
doi - Bang JY, Hasan M, Navaneethan U, Hawes R, Varadarajulu S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2017;66(12):2054-2056.
doi pubmed pmc - DeSimone ML, Asombang AW, Berzin TM. Lumen apposing metal stents for pancreatic fluid collections: Recognition and management of complications. World J Gastrointest Endosc. 2017;9(9):456-463.
doi pubmed pmc - Stahl K, Busch M, Fuge J, Schneider A, Manns MP, Seeliger B, Schmidt JJ, et al. Therapeutic plasma exchange in acute on chronic liver failure. J Clin Apher. 2020;35(4):316-327.
doi - Lang GD, Fritz C, Bhat T, Das KK, Murad FM, Early DS, Edmundowicz SA, et al. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc. 2018;87(1):150-157.
doi - Puga M, Consiglieri CF, Busquets J, Pallares N, Secanella L, Pelaez N, Fabregat J, et al. Safety of lumen-apposing stent with or without coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a retrospective study. Endoscopy. 2018;50(10):1022-1026.
doi - Aburajab M, Smith Z, Khan A, Dua K. Safety and efficacy of lumen-apposing metal stents with and without simultaneous double-pigtail plastic stents for draining pancreatic pseudocyst. Gastrointest Endosc. 2018;87(5):1248-1255.
doi - Shamah SP, Sahakian AB, Chapman CG, Buxbaum JL, Muniraj T, Aslanian HA, Villa E, et al. Double pigtail stent placement as an adjunct to lumen-apposing metal stentsfor drainage of pancreatic fluid collections may not affect outcomes: A multicenter experience. Endosc Ultrasound. 2022;11(1):53-58.
doi pubmed pmc - Haddad JD, Tielleman T, Fuller A, Tavakkoli A, Vanderveldt D, Goldschmiedt M, Kubiliun N, et al. Safety and efficacy of lumen-apposing metal stents with and without coaxial plastic stents for pancreatic fluid collections. Techniques and Innovations in Gastrointestinal Endoscopy. 2022
- Ali SE, Benrajab K, Mardini H, Su L, Gabr M, Frandah WM. Anchoring lumen-apposing metal stent with coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: any benefit? Ann Gastroenterol. 2019;32(6):620-625.
doi pubmed pmc - Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012.
doi - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed pmc - Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785-1805.
doi - Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605.
doi - Ahmad W, Fehmi SA, Savides TJ, Anand G, Chang MA, Kwong WT. Protocol of early lumen apposing metal stent removal for pseudocysts and walled off necrosis avoids bleeding complications. Scand J Gastroenterol. 2020;55(2):242-247.
doi - Brimhall B, Han S, Tatman PD, Clark TJ, Wani S, Brauer B, Edmundowicz S, et al. Increased incidence of pseudoaneurysm bleeding with lumen-apposing metal stents compared to double-pigtail plastic stents in patients with peripancreatic fluid collections. Clin Gastroenterol Hepatol. 2018;16(9):1521-1528.
doi pubmed pmc - Ge PS, Young JY, Jirapinyo P, Dong W, Ryou M, Thompson CC. Comparative study evaluating lumen apposing metal stents versus double pigtail plastic stents for treatment of walled-off necrosis. Pancreas. 2020;49(2):236-241.
doi pubmed pmc - Valente R, Zarantonello L, Del Chiaro M, Vujasinovic M, Baldaque Silva F, Scandavini CM, Rangelova E, et al. Lumen apposing metal stents vs double pigtail plastic stents for the drainage of pancreatic walled-off necrosis. Minerva Gastroenterol (Torino). 2022.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


