| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 10, Number 1, February 2017, pages 21-27
Predictors of Fetal and Maternal Outcome in the Crucible of Hepatic Dysfunction During Pregnancy
Indrajit Suresha, b, Vijaykumar TRa, Nandeesh HPa
aDepartment of Gastroenterology & Hepatology, JSS University Hospital & Medical College, Mysore, Karnataka, India
bCorresponding Author: Indrajit Suresh, Department of Gastroenterology & Hepatology, JSS University Hospital & Medical College, Mysore, Karnataka 570004, India
Manuscript accepted for publication February 01, 2017
Short title: Feto-Maternal Outcomes in Hepatic Dysfunction
doi: https://doi.org/10.14740/gr787w
| Abstract | ▴Top |
Background: Hepatic dysfunction during pregnancy places both the mother and the fetus at risk. Investigations which are efficient, cost effective and easily available for prognostication are required to tackle this global problem. We studied the etiologies and evaluated investigations for predictive efficiency.
Methods: One hundred ninety-seven pregnant women with hepatic dysfunction during pregnancy were identified. All patients were followed up till 8 weeks after termination of pregnancy or death. Clinico-demographic, biochemical and hematological data were collected and analyzed.
Results: One hundred ninety-seven of 6,122 females had abnormal liver function tests. Pre-eclampsia (57%), eclampsia (19%), HELLP syndrome (8%), viral infection (6%), hyperemesis gravidarum (5%), intrahepatic cholestasis of pregnancy (4%), chronic liver disease (1%) and sepsis were encountered. There were 41 fetal deaths, 42% preterm deliveries, and NICU admission rate was 27%. Five maternal deaths occurred. Maternal anemia, thrombocytopenia, hyperbilirubinemia and coagulopathy were statistically significant in adverse fetal outcomes. Serum bilirubin performed better than INR as a predictor of both maternal and fetal outcomes.
Conclusions: Hepatic dysfunction during pregnancy is associated with adverse events for both the mother and the fetus and hypertensive disorders remain the major cause. Maternal bilirubin levels and INR have a role in predicting adverse feto-maternal outcome.
Keywords: Pregnancy; Liver disease; Bilirubin; INR; Fetal outcome
| Introduction | ▴Top |
Pregnancy is a dynamic process which involves anatomical, physiological and metabolic changes in the future mother as her body adapts to facilitate the growth and development of the fetus. Some changes in hepatic function occur normally in pregnancy, but need to be differentiated from pathologies which carry significant risk to the mother and her baby.
Serum total bilirubin (STB) levels are generally lower in pregnant women during all three trimesters, while low conjugated bilirubin concentration is observed during the second and third trimesters. This phenomenon is often attributed to hemodilution and hypoalbuminemia [1].
Serum gamma-glutamyl transferase (GGT) activity levels decrease while serum 5'-nucleotidase activity marginally increases during the second and third trimesters.
Serum alkaline phosphatase (ALP) activity may increase two- to fourfold in late pregnancy, due to the production of the placental isoenzyme and the increase in bone isoenzyme, thus rendering it unsuitable for diagnosing cholestasis during pregnancy.
Being a hypercoagulable state, pregnancy alters the plasma levels of clotting factors. Fibrinogen and thrombin levels increase. Protein S level decreases. Protein C and antithrombin III levels remain constant [1-3].
Hepatic dysfunction may occur in 3-10% of pregnancies and jaundice is observed in 0.1% [4]. The common causes that are associated with pregnancy are: 1) pre-eclampsia (PEC)/eclampsia (EC); 2) HELLP syndrome (hemolysis, elevated liver enzymes and low platelets); 3) hyperemesis gravidarum (HG); 4) intrahepatic cholestasis of pregnancy (ICP); and 5) acute fatty liver of pregnancy (AFLP).
In addition to the above, various pregnancy unrelated liver diseases also contribute to the spectrum such as: 1) pre-existing liver disease: autoimmune liver disease, Wilson’s disease, cirrhosis, and viral hepatitis B/C; 2) co-incidental disease: viral hepatitis, drug-induced liver injury, and sepsis; and 3) exacerbations of unrelated illness: hepatic adenomas and Budd-Chiari syndrome.
The characteristics of the pregnancy-specific liver diseases are described in Table 1, and it is evident that hepatic dysfunction has a significant impact on maternal as well as fetal outcome in pregnancy [5].
 Click to view | Table 1. Pregnancy-Specific Causes for Hepatic Dysfunction |
A few Indian studies have brought forth data regarding the prevalence of pregnancy-specific liver diseases, and these are similar to international results [6-10]. The global nature of this problem necessitates continued research so as to identify individuals at risk and to provide better monitoring, care and delivery facilities. We found only one study which focused on the utility of a few blood investigations as a tool for prognostication in females with pregnancy-specific liver disease [11].
The present study thus seeks to address this important issue by analyzing a larger number of variables of interest/investigations and deriving logical conclusions regarding prognostic indicators for both fetal and maternal outcome in hepatic dysfunction during pregnancy.
| Materials and Methods | ▴Top |
This was a prospective cohort study conducted in the Department of Gastroenterology & Hepatology in a tertiary care hospital in Mysore district, Karnataka, India.
Patient selection
During the period from September 1, 2014 to September 1, 2016, pregnant females who reported to the Department of Gastroenterology and Hepatology as well as Department of Obstetrics at the Jagadguru Sri Shivarathreeshwara University Hospital (Mysore, India) underwent routine blood investigations and liver function tests (LFTs). Patient history, progress notes and investigation details were stored in a computerized database. Patients with aspartate aminotransferase (AST) > 40 IU/L, alanine aminotransferase (ALT) > 40 IU/L or STB > 1 mg/dL were included in the cohort of “hepatic dysfunction in pregnancy”. Patient categorization into different illness subsets was done according to diagnostic criteria formulated by bodies such as the American College of Obstetricians and Gynaecologists (ACOG) and in consultation with senior obstetricians. Standards of care provided to the patients were condition-specific and as per established guidelines.
All patients were followed up until 8 weeks after termination of pregnancy/delivery, irrespective of the outcome. This study was approved by the institutional ethics committee, and informed, valid consent for inclusion in the study was obtained from the patients. The study was also registered in the WHO approved Thai clinical trials registry (ID- TCTR20161215001).
Data analysis
Tabulated data sheets were created which included patient demographic characteristics, relevant laboratory investigations and notes on feto-maternal outcome as well as complications if they had been encountered. Fetal and maternal deaths were considered as the outcomes of interest. Statistical analyses were done using XLSTAT 2016 statistical software and online calculators at www.vassarstats.net. All reported P values are two-tailed and P < 0.05 was considered statistically significant.
| Results | ▴Top |
Patient characteristics
During the study period from September 1, 2014 to September 1, 2016, 6,122 pregnant females were screened for hepatic dysfunction as evidenced by abnormalities in LFTs. A cohort of 197 (3.2%) patients was identified.
Table 2 describes the clinico-demographic characteristics and etiology of hepatic dysfunction in the study cohort. The median age was 25 years (range 22 - 28), 122 (62%) were primigravida, 179 (91%) were booked, six (3%) had twin pregnancies, while 53 (27%) had bad obstetric history. Co-morbidities such as hypothyroidism (32, 16%), diabetes (14, 7%) and obesity (15, 8%) were observed. Seventy-two (37%) women were anemic. Sixty percent of the cohort was aged 25 years or less. In 76% of the patients, the mode of delivery was cesarean section.
 Click to view | Table 2. Age Wise Demographics and Etiology of Hepatic Dysfunction |
Causes of hepatic dysfunction
Hepatic dysfunction in PEC was most commonly encountered (113, 57%), followed by EC (37, 19%), HELLP syndrome (15, 8%), viral infection (V) (12, 6%), HG (9, 4.5%), ICP (eight, 4%), chronic liver disease (CLD) (two, 1%), and sepsis (S) (one, 0.5%), respectively. Young, primis aged below 25 years made up the majority of the PEC, EC as well as HELLP groups. The patients included in the CLD group comprised one individual suffering from autoimmune hepatitis (AIH) and the other from extra-hepatic portal venous obstruction with liver cirrhosis.
Symptoms and complications
Table 3 describes the symptoms, complications and co-morbidities observed in the study cohort. Overall, 78% of the cohort subjects reported symptoms. Pruritus (26%) and gastrointestinal (GI) symptoms (22%) were most often encountered. The prevalence of jaundice was highest among those suffering from viral infections. Pre-existing co-morbidities were observed with higher frequency in ICP patients. None of the patients with HG developed jaundice or pruritus during the entire course of their pregnancies. The incidence of headache and GI symptoms was relatively higher in the HELLP group when compared to PEC and EC groups. Pruritus was reported maximally amongst patients with ICP.
 Click to view | Table 3. Population Demographics and Symptomatology |
Complications such as placental abruption (2%) and renal dysfunction (3%) occurred. These were predominantly observed in the PEC, EC, HELLP and sepsis groups, and not in others.
Laboratory investigations
The highest median levels of STB were seen in viral infection and sepsis, while the highest AST and ALT levels were seen in viral infection, sepsis and HELLP, respectively (Table 4). Anemia (72, 37%), thrombocytopenia (61, 31%) and coagulopathy (52, 26%) were observed. Median hemoglobin levels were lowest in sepsis, CLD and HELLP. LFTs of majority (92%) of the population had improved within 2 weeks of delivery; however, 12 patients with viral infection, one with AIH and three with ICP had a relatively prolonged course of recovery, and required a mean period of 26 ± 4 days for resolution.
 Click to view | Table 4. Laboratory Investigations in Various Etiologies and Outcomes |
The viruses encountered in decreasing order of frequency were hepatitis B virus (7, 58%), dengue (2, 17%), hepatitis E (2, 17%), and hepatitis A (1, 8%), respectively. Two fetal deaths occurred in the hepatitis B virus group, while no mothers had adverse outcomes.
Fetal outcomes
Table 5 highlights the fetal and maternal outcomes in the cohort. Majority of the pregnancies were singleton (191, 94%) and 105 (52%) were males. Eighty-six (42%) were pre-term deliveries and 127 (63%) had low weight when adjusted for gestational age. There were 41 (20%) fetal deaths and 54 (27%) NICU admissions. A higher number of fetal adverse events were encountered in patients with sepsis (50%), CLD (50%), HELLP (47%) and EC (38%). In the CLD group, fetal demise occurred in the female suffering from AIH. There were six miscarriages, 14 stillbirths and 21 infant deaths. Overall, the HG and ICP groups had relatively good fetal outcomes. All twin pregnancies had favorable outcomes. The median age of the mothers who had adverse fetal outcome was 24 years (range 21 - 26), and 56% were aged 25 years or lesser.
 Click to view | Table 5. Feto-Maternal Outcomes in Patients With Hepatic Dysfunction |
Maternal outcomes
One hundred fifty (76%) patients underwent cesarean sections. Ten patients (5%) required ICU admission. Five (2.5%) maternal deaths collectively occurred in the EC (two), HELLP (two) and sepsis (one) groups. The median age was 24 years (range 21 - 28) for the patients who expired. Majority of the mothers recovered well after their deliveries.
Risk factors in feto-maternal outcome
Median values of laboratory parameters in the fetal and maternal adverse event groups are provided in Table 4. Low values of hemoglobin, platelet counts and high levels of total bilirubin, AST and ALT were observed in maternal adversities, and these were significantly different from that of the fetal adversities. Median age of the mothers in both groups was 24 years.
Statistical analysis of the association between the co-morbidities and hematological parameters to feto-maternal outcome yielded interesting results (Table 6). We observed the presence of maternal factors such as anemia (odds ratio (OR): 2.16; 95% CI: 1.07 - 4.34; P = 0.03), thrombocytopenia (OR: 3.47; 95% CI: 1.7 - 7.08; P = 0.0006), and hyperbilirubinemia (OR: 5.18; 95% CI: 1.7 - 15.2; P = 0.002) and coagulopathy (OR: 1.36; 95% CI: 1.36 - 5.7; P = 0.005) to be significantly associated with adverse fetal outcome, while only thrombocytopenia (OR: 9.4; 95% CI: 1.03 - 86.6; P = 0.03) and hyperbilirubinemia with a cut-off of 2.7 mg/dL (P < 0.0001) were deemed statistically significant in maternal adversities. Co-morbidities such as diabetes or hypothyroidism did not have statistical significance in either outcome. Upon applying Fisher’s exact test (two-tailed) to the outcome groups at various maternal age cut-offs, no statistical significance could be detected.
 Click to view | Table 6. Risk Factors in Maternal and Fetal Outcomes |
Receiver operating characteristic (ROC) curve analyses of maternal levels of hemoglobin, platelet counts, total bilirubin and INR and their impacts on fetal as well as maternal outcome were done. The area under the ROC (AUROC) for maternal INR (0.65) (95% CI: 0.55 - 0.75; P = 0.004), and maternal total bilirubin level (0.81) (95% CI: 0.72 - 0.89; P < 0.0001) were interpreted as clinically significant, and maternal bilirubin levels performed better as a prognostication tool for fetal outcome (Fig. 1a).
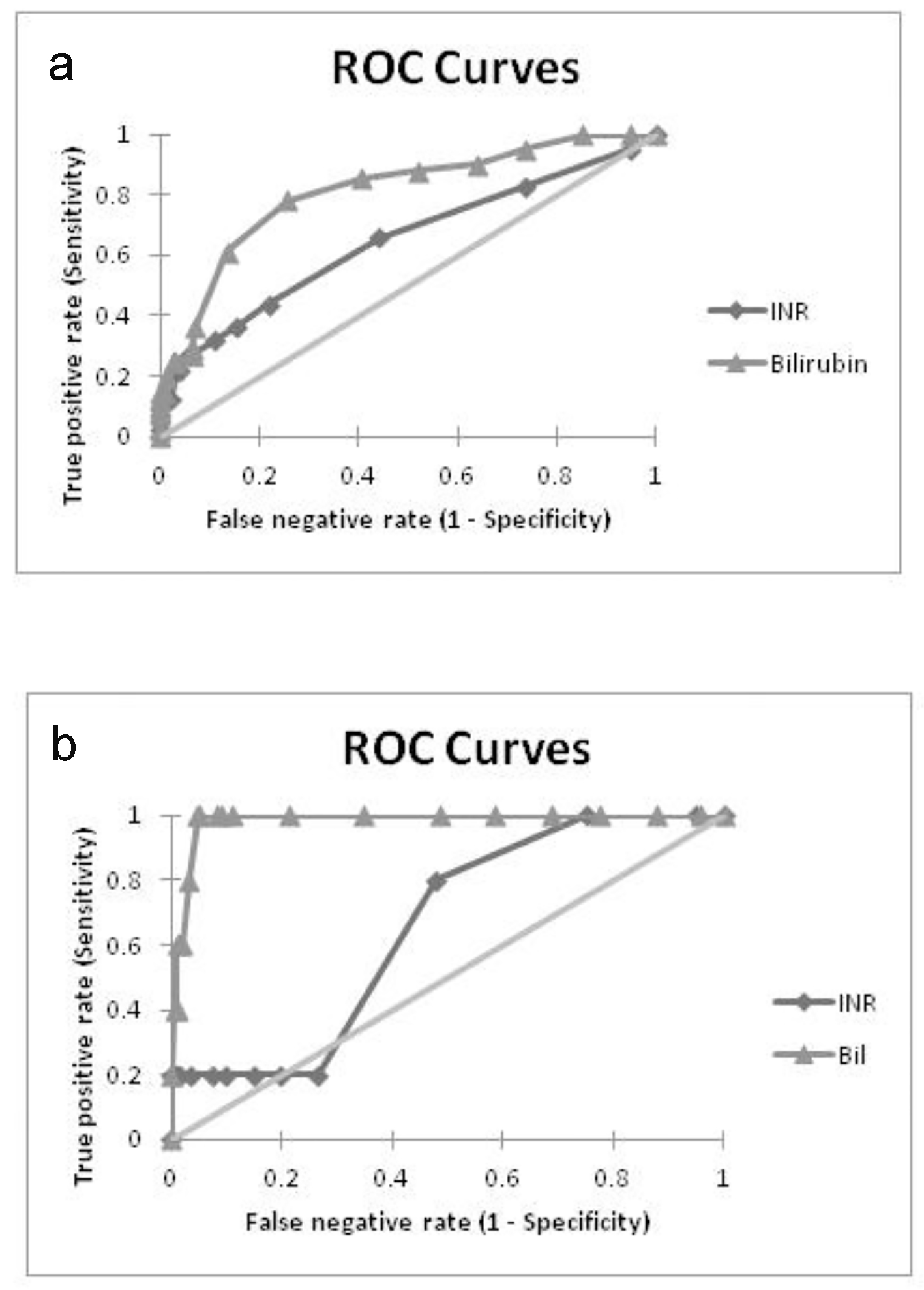 Click for large image | Figure 1. ROC curves showing predictive performance of bilirubin and INR in (a) fetal adversities and (b) maternal adversities. |
In relation to maternal outcome, the AUROC for INR (0.65) (95% CI: 0.39 - 0.92, P = 0.25) and bilirubin (0.98) (95% CI: 0.91 - 1.0, P < 0.0001) indicated the superiority of bilirubin levels as an outcome predictor (Fig. 1b). The threshold level for STB was computed to be 2.7 mg/dL with a sensitivity of 100% and a specificity of 95%.
| Discussion | ▴Top |
The overall incidence of hepatic dysfunction observed in pregnancies was 3.2% which corroborates rates observed in other studies [4]. PEC with hepatic dysfunction was most commonly encountered (1.8%), followed by EC (0.6%), HELLP (0.24%), viral infection (0.19%), HG (0.14%), ICP (0.13%), CLD (0.03%) and sepsis (0.01%), respectively. The observation of PEC with hepatic dysfunction as a major subset of the illness spectrum is also similar to prior data, although in contrast, ICP occurrence was not as frequent [7-10].
Majority reported pruritus and GI symptoms such as nausea and vomiting, although a significant proportion of individuals belonged to the categories of ICP and HG, respectively. Jaundice was observed mostly in cases of viral hepatitis.
The fetal mortality rate was 20% in patients with hepatic dysfunction, and there were also more instances of premature deliveries, low birth weight babies and NICU admissions. Women with EC and HELLP generally had a higher incidence of fetal and maternal adversities than other subgroups while HG and ICP were comparatively safer.
The overall incidence of anemia in patients with hepatic dysfunction was 37%, and 58% of them were young mothers. Although median levels of maternal hemoglobin were normal overall, a significantly lower level was seen in females who had adverse events. Various studies have described the relationship between maternal anemia and adverse fetal outcome [12, 13]. In our study, although low maternal hemoglobin levels had significant statistical correlation with both fetal and maternal adversities, its use as a predictor of outcome could not be validated.
Thrombocytopenia can occur in 8-10% of all pregnancies, with lower levels reported during the last trimester. Gestational thrombocytopenia (75%), hypertensive disorders (15-20%) and immune processes (3-4%) contribute more to the spectrum [14]. In the cohort, lowest median levels of platelets were encountered in the HELLP and CLD groups. Thrombocytopenia was associated with poor outcome in both mother and the fetus, albeit with an unsatisfactory predictive capability.
INR and STB consistently demonstrated good statistical and clinical significance in feto-maternal outcomes. STB was superior to INR as a diagnostic test in correctly identifying high-risk patients and thereby aiding in prognostication. Its utility in predicting maternal outcome was excellent and also demonstrated good sensitivity and specificity at a cut-off value of 2.7 mg/dL. We identified studies which had looked into the association of elevated bilirubin levels with pregnancy outcomes. Two studies had associated elevated bilirubin levels to maternal adversities [11, 15]. Only one study had provided ROC-based data regarding the utility of serum bilirubin and INR levels on maternal health, and demonstrated their prognostic capabilities [11]. No ROC-based data regarding the influence of maternal blood investigations on the fetal outcome were provided in any previous studies.
Overall maternal death rate was 2.5%, and this figure was largely achieved due to early identification of the illness and appropriate management strategy including monitoring, prompt delivery and intensive care. Maternal ICU admission rates were similar to other studies [7-10]. A large number of women had been hospitalized within a week of onset of symptoms, and 73% delivered within the first 36 h after admission. In 165 (84%) of the patients, the LFT improved within the first 96 h after delivery, irrespective of the fetal outcome, and this figure rose to 92% over a 2-week period, and near total by 4 weeks. Viral infections in the mother were generally associated with a longer recovery period.
Conclusion
Patients with hepatic dysfunction form a special subset in pregnancies and it is frequently encountered in hypertensive disorders of pregnancy. The symptomatology is usually non-specific. It could occur as a novel incident or as a flare of a pre-existing liver disease. Overall, there is an increased incidence of fetal adverse events in pregnant females with hepatic dysfunction. The impact of hepatic disorders on the mother is also significant. Maternal anemia, thrombocytopenia, coagulopathy and hyperbilirubinemia have significant association with fetal outcome. Identification of at-risk patients is important, and careful monitoring coupled with expeditious delivery may reduce harm to both mother and her baby. Maternal levels of STB and INR could serve as reliable tools in risk-stratification as well as prognostication in patients with hepatic dysfunction during pregnancy.
Conflicts of Interest
None.
| References | ▴Top |
- Bacq Y. The Liver in Normal Pregnancy. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6005/.
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003;29(2):125-130.
doi pubmed - Ryan JM, Heneghan MA. Pregnancy and the liver. Clinical Liver Disease. 2014;4:51-54.
doi - Angel Garcia AL. Effect of pregnancy on pre-existing liver disease physiological changes during pregnancy. Ann Hepatol. 2006;5(3):184-186.
pubmed - Walker I, Chappell LC, Williamson C. Abnormal liver function tests in pregnancy. BMJ. 2013;347:f6055.
doi pubmed - Satia MN, Jandhyala MA. Study of fetomaternal outcomes in cases of jaundice at a tertiary care centre. Int J Reprod Contracept Obstet Gynecol. 2016;5:2352-2357.
doi - D'Souza AS, Gupta G, Katumalla FS, Goyal S. Maternal and fetal outcome in liver diseases of pregnancy: a tertiary hospital experience. International Journal of Scientific and Research Publications. 2015;5(9):1-4.
- Reddy MG, Prabhakar GC, Sree V. Maternal and fetal outcome in jaundice complicating pregnancy. J NTR Univ Health Sci. 2014;3:231-323.
doi - Allen AM, Kim WR, Larson JJ, Rosedahl JK, Yawn BP, McKeon K, Hay JE. The Epidemiology of Liver Diseases Unique to Pregnancy in a US Community: A Population-Based Study. Clin Gastroenterol Hepatol. 2016;14(2):287-294 e281-282.
- Ch'ng CL, Morgan M, Hainsworth I, Kingham JG. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. 2002;51(6):876-880.
doi pubmed - Murali AR, Devarbhavi H, Venkatachala PR, Singh R, Sheth KA. Factors that predict 1-month mortality in patients with pregnancy-specific liver disease. Clin Gastroenterol Hepatol. 2014;12(1):109-113.
doi pubmed - Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J Matern Fetal Neonatal Med. 2014;27(9):887-891.
doi pubmed - Nair Manisha, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Global Health. 2016;1(1):e000026.
doi - Myers B. Diagnosis and management of maternal thrombocytopenia in pregnancy. Br J Haematol. 2012;158(1):3-15.
doi pubmed - Nagaria T, Agarwal S. Fetomaternal outcome in jaundice during pregnancy. J Obstet Gynecol India. 2005;55(5):424-427.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


