| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 3, June 2023, pages 171-183
Trends in Hospitalizations of Esophageal Varices From 2011 to 2018: A United States Nationwide Study
Abdelwahap Elghezewia, Mohamad Hammada, Mohammed El-Dallalb, Mujtaba Mohamedb, c, Ahmed Sherifb, Wesam Frandahb
aDepartment of Internal Medicine. Marshall University Hospital, Huntington, WV 25701, USA
bDepartment of Gastroenterology and Hepatology, Marshall University Hospital, Huntington, WV 25701, USA
cCorresponding Author: Mujtaba Mohamed, Department of Gastroenterology and Hepatology, Marshall University Hospital, Huntington, WV 25701, USA
Manuscript submitted April 11, 2023, accepted May 17, 2023, published online June 11, 2023
Short title: Trends in Hospitalizations of EV
doi: https://doi.org/10.14740/gr1627
| Abstract | ▴Top |
Background: Prevalence of gastroesophageal varices is around 50% of patients with cirrhosis. In compensated cirrhosis they are present in 30-40%. Progression from small to large varices occurs at rate of 10-12% annually. That percentage increases significantly in decompensated liver cirrhosis with gastroesophageal varices found in 85% of patients. Variceal hemorrhage occurs at a rate around 10-15% per year. The outcome of variceal hemorrhage depends on the severity of liver disease, size of varices, and presence of stigmata of recent bleeding (red whale sign). Six-week mortality of variceal hemorrhage ranges between 15% and 25%. Without treatment, variceal hemorrhage tends to recur in 60% of patients within 1 - 2 years. The aim of the study was to assess demographics of esophageal varices with and without bleeding, geographic distribution, comorbidities, outcomes, main payers, and cost of hospitalizations.
Methods: The National Inpatient Sample (NIS) database from year 2011 to 2018 was used. Patients who had a primary diagnosis of esophageal varices with or without bleeding were identified using the International Classification of Diseases, Ninth Revision (ICD-9) codes (456.0 for esophageal varices with bleeding, and 456.1 for esophageal varices without bleeding), and International Classification of Diseases, 10th Revision (ICD-10) codes (I85.01 for esophageal varices with bleeding, and I85.00 for esophageal varices without bleeding) in the first two discharge diagnoses. The propensity score to calculate the inverse probability treatment weighting (IPTW) to adjust between the differences of the compared groups was implemented. Two groups were compared in terms of their hospitalization outcomes, including LOS, hospital charges, hospital mortality, and disposition.
Results: A total of 322,761 patients were admitted with esophageal varices between 2011 and 2018, with 236,802 (73.6%) had bleeding esophageal varices and 85,959 (26.4%) had nonbleeding esophageal varices. The majority of the patients from both groups were white (66%), covered with Medicare (38% in the esophageal varices with bleeding vs. 41% in the nonbleeding group). There was a steady increase of patients admitted with nonbleeding esophageal varices. Most common comorbidities were liver diseases, alcohol abuse, uncomplicated hypertension and depression in both groups. There were no significant changes in OLS over the years in both groups, but there was a significant increase in hospital charges, especially in the patients with bleeding esophageal varices starting in 2015, and no change in mortality throughout the years. Regarding hospital disposition, there was a notable decline in rehab discharge in the bleeding esophageal varices group.
Conclusions: Esophageal varices with and without bleeding have been steadily increasing since the beginning of this century. This may result in a substantial impact on increasing health care costs and utilization due to acute variceal hemorrhage. Odds of death, transfer to urban hospital, and transfer to visiting nursing assistance remained unchanged.
Keywords: Nonbleeding esophageal varices; Bleeding esophageal varices; Liver cirrhosis
| Introduction | ▴Top |
Cirrhosis is an end result from different mechanisms of liver insults that lead to necrosis, inflammation and fibrogenesis; histologically it is characterized by diffuse nodular regeneration surrounded by dense fibrotic septa with subsequent parenchymal extinction and collapse of liver structures, resulting in a pronounced distortion of hepatic vascular architecture [1, 2]. The prevalence of cirrhosis in the United States was approximately 0.27%, corresponding to 633,323 adults [3]. The prevalence was higher in non-Hispanic blacks and Mexican Americans, those living below the poverty level, and those with less than a 12th grade education [3]. Main leading causes of liver cirrhosis in developed countries are hepatitis C virus (HCV), alcohol misuse, and, increasingly, nonalcoholic liver disease, while hepatitis B virus (HBV)infection is the leading cause in sub-Saharan Africa and most parts of Asia [4]. Liver cirrhosis is the 12th leading cause of death in USA. According to a study, cirrhosis accounted for 37,890 deaths in 2013 [5]. One-year mortality in cirrhosis varies widely, from 1% to 57%, depending on the occurrence of clinical decompensating events such as variceal bleeding, ascites, and hepatic encephalopathy [6]. Median survival in the compensated stage exceeds 12 years, whereas it is only 1.8 years in patients who develop decompensation [7]. One study estimated the annual cost of care of patients with cirrhosis to be about $2 billion [8]. One of main decompensations of liver cirrhosis is developing gastroesophageal varices. Gastroesophageal varices present in around 50% of patients with cirrhosis [9, 10]. In compensated cirrhosis they are present in 30-40% of patients with varices develop at rate of 7-8% annually [11], as well, progression from small to large at rate of 10-12% yearly [12]. That increases significantly in decompensated liver cirrhosis with gastroesophageal varices found in 85% of patients [12]. Variceal hemorrhage occurs at a rate of around 10-15% per year. Outcomes of variceal hemorrhage depend on the severity of liver disease, size of varices, and presence of red wale mark [12, 13]. Six-week mortality of variceal hemorrhage ranges between 15% and 25% [14]. Without treatment, variceal hemorrhage tends to recur in 60% of patients within 1 - 2 years [15]. Poor prognostic factors include high hepatic portal vein gradient more than 20 mm Hg, and bacterial infection at the time of variceal hemorrhage [16]. According to Solanki et al, hospitalizations due to esophageal varices were increasing in the first decade of this century. Mostly white males, with mean of hospital stay around 2 - 7 days and mean hospitalization cost of $12,322 [17]. One of major concerns regarding acute variceal bleeding is prolonged LOS, complications during hospitalization including sepsis, rebleeding, and acute renal failure [18, 19]. Another major concern is the risk of readmission to hospital. Weissman et al reported in his retrospective review that one in four patients with variceal hemorrhage was readmitted within 30 days, and over one in three was readmitted within 90 days of discharge [19]. Most common reason for readmission in 30 and 90 days was recurrence of acute variceal upper gastrointestinal (GI) hemorrhage [19]. In this study we are looking into demographics of esophageal varices with and without bleeding, geographic distribution, comorbidities, outcomes (LOS, and discharge destination) as a factor that may affect risk of readmission and increased health care utilization, odds of death, main payers, and cost of hospitalizations.
| Materials and Methods | ▴Top |
Data source
We used the National Inpatient Sample (NIS) database from year 2011 to 2018. The NIS is an all-payer, annually collected abstraction of approximately 7 million hospital discharges from 46 states in addition to the District of Columbia, representing more than 97% of the US population. It is available through the Healthcare Cost and Utilization Project (HCUP) for research purposes. Since 2012, there have been changes in the sampling strategy of the NIS data to improve its precision and decrease sample error. These strategies include sampling 20% from all participating hospitals instead of sampling hospitals, using statewide data from the HCUP to feed the NIS database, and removing all identifiers of hospitals or states, as well as data not uniformly available in all states [20]. Starting in the last quarter of 2015, the hospital administrative data in the whole USA started using the International Classification of Diseases, 10th Revision (ICD-10) instead of International Classification of Diseases, Ninth Revision (ICD-9), accordingly, the coding for diagnoses and procedures in all the HCUP data had changed to ICD-10 [20]. NIS data do not include model of end stage liver disease (MELD) laboratory data, or medications given during admission. NIS admission data do not include whether this is a new admission or readmission.
Study design and study population
Patients who had a primary diagnosis of esophageal varices with or without bleeding were identified using the ICD-9 codes (456.0 for esophageal varices with bleeding, and 456.1 for esophageal varices without bleeding), and ICD-10 codes (I85.01 for esophageal varices with bleeding, I85.00 for esophageal varices without bleeding) in the first two discharge diagnoses (Supplementary Material 1, www.gastrores.org). These codes have been used in multiple previous studies [17, 19, 20]. We excluded all subjects less than 18 years of age. The weighted comorbidity score [21] was calculated for all individuals in the study population, based on the ICD-9 and ICD-10 codes definition of the included comorbidities, and was used to adjust between esophageal varices with bleeding and esophageal varices without bleeding. Demography (age, race, sex), median household income for the patient’s ZIP code based on the current year, the expected primary payer for the patient’s hospital stay, patient location (based on a six-category urban-rural classification scheme for the United States counties developed by the National Center for Health Statistics), whether the patient was admitted to the hospital electively, and the type of facility that the patients were transferred from (if any) were considered as potential confounders.
Outcomes
Our primary outcomes of interest were: 1) hospital length of stay (LOS); 2) total charges of hospital admission; and 3) in-hospital mortality in bleeding and nonbleeding esophageal varices groups. We also examined other secondary outcomes including: 1) odds of discharge to rehabilitation; 2) odds of discharge home with visiting nurse; 3) and odds of transfer to other hospital in bleeding and nonbleeding esophageal varices groups.
Statistical analysis
R version 3.5.1 (R foundation for statistical computing, Vienna, Austria) was used for the analysis. Descriptive statistics were used to describe the baseline hospital and patient characteristics. We used the Chi-square test to compare categorical variables and two-group analysis of variance to compare continuous variables, while the Kruskal and Fisher tests were used for variables not fitting a normal distribution. We then estimated propensity scores for each patient based on their demographic, primary insurance, median household income for their living areas, Elixhauser comorbidity score, sepsis, acute kidney injury, and hospital characteristics (Supplementary Material 2, www.gastrores.org). We then used the propensity score to calculate the Inverse Probability Treatment Weighting (IPTW) to adjust between the differences of the compared groups. The overlap between of propensity scores of the two groups was evaluated visually using density and box plots (Supplementary Material 2, www.gastrores.org), and the absolute standardized mean deference of ≤ 0.1 was used as an indicator to achieve covariate balance after implementing the IPTW. We compared the two groups in terms of their hospitalization outcomes, including LOS, hospital charges, hospital mortality, and disposition. All analyses were performed using survey-adjusted methods accounting for NIS-specific hospital weighting and the IPTW. As a confirmatory measure, we re-estimated propensity scores using generalized boosting modeling (GBM) and compared final results to the previous ones.
In the trend analysis, we utilized discharge or trend weight that was provided by the NIS data, used year 2011 as a reference, and adjusted for inflation for the hospital charges to the year 2018.
Institutional review board statement
This study was done using the NIS database, which does not require approval from the Institutional Review Board (IRB), thus no IRB approval was needed for this study.
Ethical compliance with human study
All study procedures were carried out in accordance with the Declaration of Helsinki regarding research involving human subjects.
| Results | ▴Top |
Baseline characteristics
Basic demographics revealed that our patient population was mostly white men (66%) and average age of 57 years in both groups. The rest of the characteristics were summarized in Tables 1, 2.
 Click to view | Table 1. Baseline Characteristics |
 Click to view | Table 2. Comorbidities of Patients With Esophageal Varices With and Without Bleeding |
Our study showed that 322,761 patients were admitted with esophageal varices between 2011 and 2018, with 236,802 (73.6%) had bleeding esophageal varices and 85,959 (26.4%) had nonbleeding esophageal varices. The percentage of patients with nonbleeding esophageal varices had increased over the years (Table 3, Fig. 1). The majority of the patients from both groups were white (66%), covered with Medicare (38% in the esophageal varices with bleeding vs. 41% in the nonbleeding group), other baseline characteristics are summarized in Table 1. Patient with esophageal varices with bleeding had higher Elixhauser comorbidity score (mean 20 (standard deviation (SD) 19.78)) compared with patient with nonbleeding esophageal varices (mean 16.13 (SD 18.2)), and the most common comorbidities were liver diseases, alcohol abuse, uncomplicated hypertension and depression in both groups (Table 2).
 Click to view | Table 3. Baseline Characteristics for Hospitalized Patients With Esophageal Varices From 2011 to 2018 |
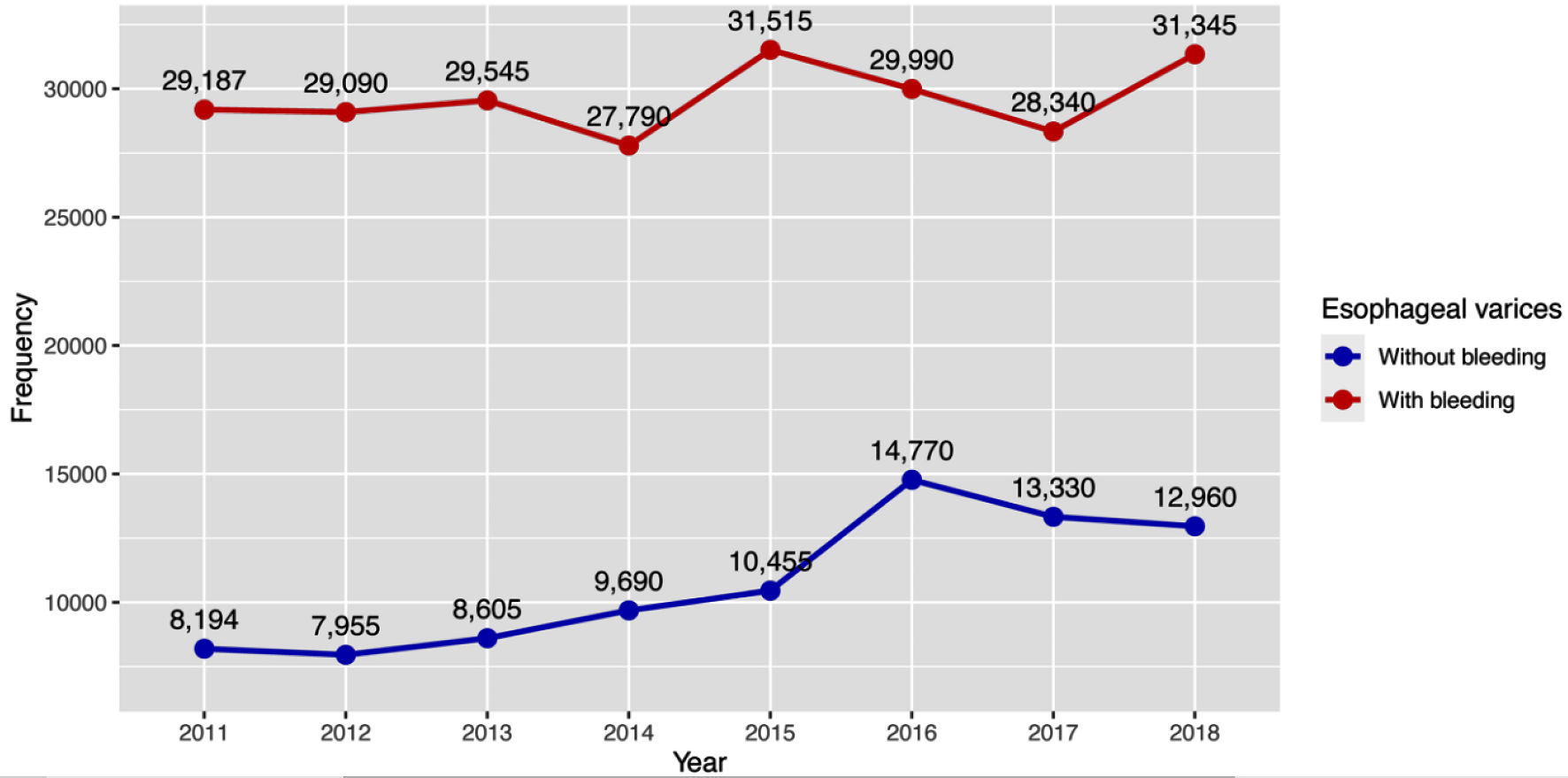 Click for large image | Figure 1. Trend of hospital admissions of esophageal varices with and without bleeding. |
Primary outcomes
In the outcome analyses, the bleeding esophageal varices group had a higher LOS with mean difference (MD) of 1.33 days (95% confidence interval (CI): 1.25 - 1.41, P < 0.001), total hospital charges (MD $20,445.8, 95% CI: 19,354.5 - 21,537.1, P < 0.001), odds ratio (OR) of death (11.8, 95% CI: 9.4 - 14.73, P < 0.001) (Fig. 2). In the trend analysis, there was no significant changes in LOS over the years in both groups, but there was a significant increase in hospital charges, especially in the patients with bleeding esophageal varices starting in 2015 (Fig. 3a, b, Table 4). That may be attributed to increased coding for nonbleeding variceal bleeding. There were changes in hospital mortality throughout the years, but without a specific trend (Fig. 3c, Table 4).
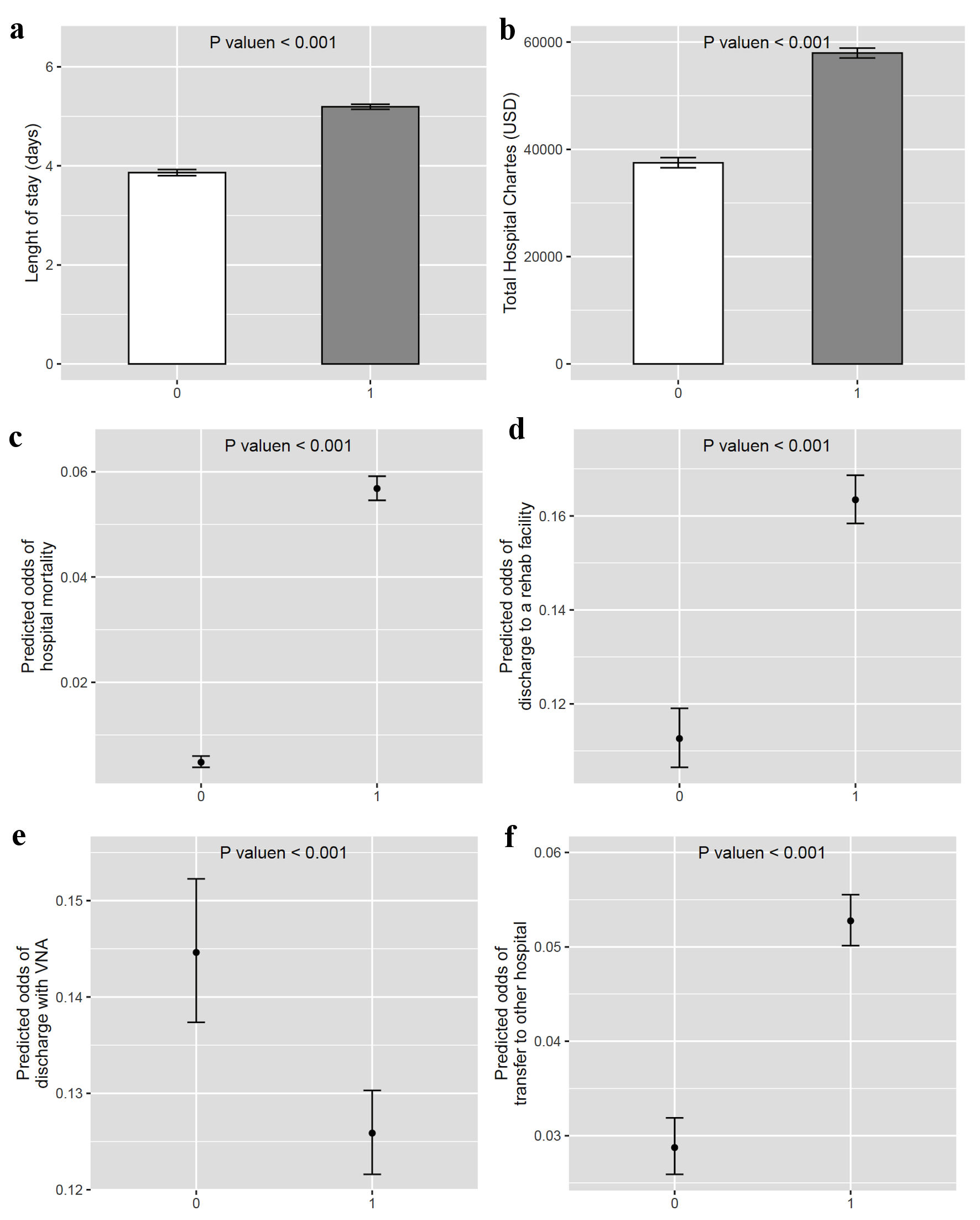 Click for large image | Figure 2. Hospital outcomes of patients with nonbleeding esophageal varices versus bleeding esophageal varices. 0 represents nonbleeding esophageal varices and 1 represents bleeding esophageal varices. VNA: visiting nursing assistance. |
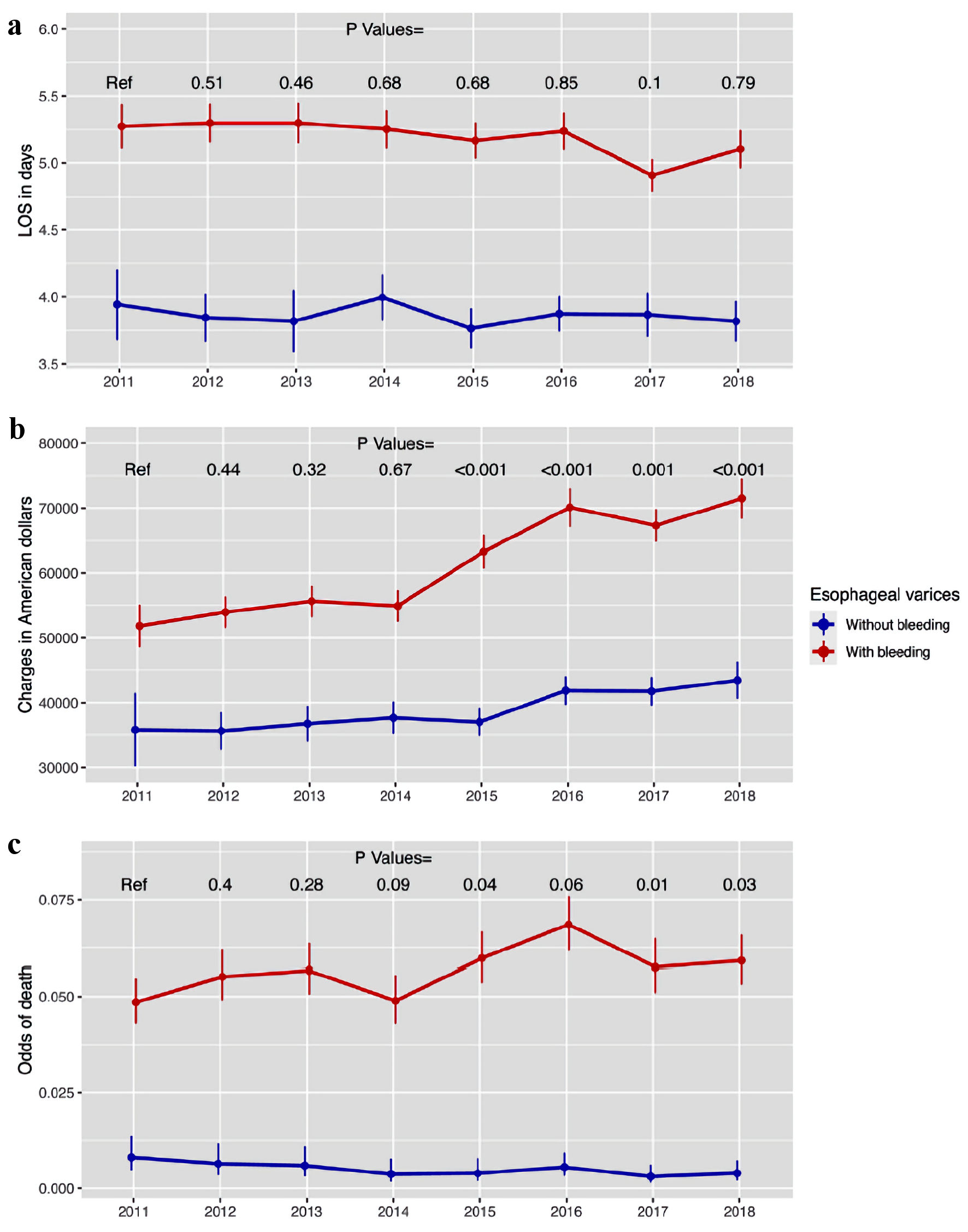 Click for large image | Figure 3. The trend in the hospitalization outcomes for patients admitted with esophageal varices (part 1). (a) Hospital LOS. (b) Total hospital charges. (c) Hospital mortality. LOS: length of stay. |
 Click to view | Table 4. Outcomes |
Secondary outcomes
In the secondary outcomes, the group with bleeding esophageal varices had higher odds of rehabilitation facility discharge (OR: 1.45, 95% CI: 1.36 - 1.55, P < 0.001), and hospital transfer (OR: 1.84, 95% CI: 1.64 - 2.06, P < 0.001), compared to the nonbleeding esophageal varices group (Fig. 2d, f). However, they had lower odds of discharge with a visiting nurse (OR: 0.87, 95% CI: 0.82 - 0.92, P < 0.001) compared to the nonbleeding esophageal varices group (Fig. 2e).
The trend analysis revealed a noticeable decline in rehabilitation discharges within the bleeding esophageal varices group (Fig. 4a, Table 4). The trends for discharges with a visiting nurse and hospital transfers are summarized in Figure 4b, c, as well as Table 4.
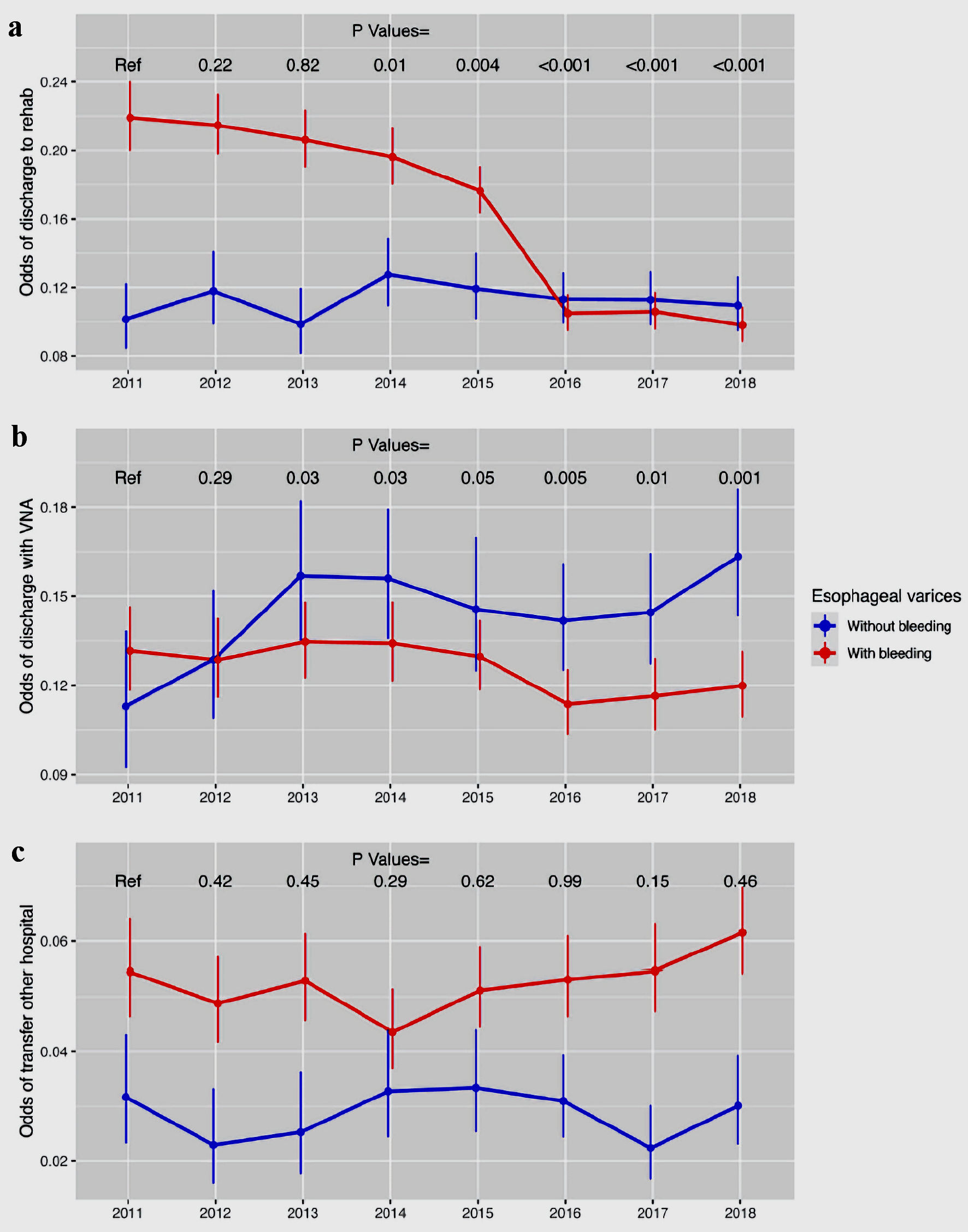 Click for large image | Figure 4. The trend in the hospitalization outcomes for patients admitted with esophageal varices (part 2). (a) Discharge to rehab. (b) Discharge with visiting nurse. (c) Outside hospital transfer. VNA: visiting nursing assistance. |
| Discussion | ▴Top |
Important data related to hospitalizations of patients with esophageal varices with and without bleeding during an 8-year period in the USA are reported in our study. Overall number of hospitalization of patients with esophageal varices has increased significantly from 2011 to 2018, from 37,381 in 2011 to 44,305 in 2018, with number of patients admitted with esophageal varices with hemorrhage remaining relatively steady (Table 3). Similar findings noted in the first decade of this century were reported by Solanki et al [17]. This is consistent with reports of increased alcohol consumption and obesity as two main etiological factors for alcohol liver disease and nonalcohol steatohepatitis-related cirrhosis [21, 22]. Steadiness of number of admissions related to bleeding esophageal varices may reflect effectiveness of preventive measures such as beta blockers monotherapy for portal hypertensive patients, who never experienced variceal bleeding, or serial varices banding and beta blockers for patient who had a variceal bleeding [23, 24].
After adjusting to inflation, hospital charges ranged between $51,839.98 - $71,473.25 and $35,827.21 - $43,452.13 for patients with and without bleeding esophageal varices, respectively. This represents a sharp increase in comparison to $15,202 for esophageal varices with hemorrhage and $12,322 for esophageal varices without hemorrhage which was reported by Solanki et al [17]. This could be attributed to increased coding for patients without hemorrhage.
Incidence of esophageal varices without bleeding remained steady throughout the study period and remained between 8,194 and 12,960. It is important to mention that they occur in patients with clinically significant portal hypertension (CSPH) with hepatic vein pressure gradient (HVPG) of 10 - 12 mm Hg. The main goal in this clinal setting is to prevent the first bleeding episode by reducing HVPG to less than 12 mm Hg or more than 20% from baseline [24]. Prophylaxis is indicated in patients with medium/large varices (more than 5 mm in size), patients with small varices and stigmata of recent bleeding (red wale sign); and decompensated patients with small varices [12, 25]. Options are pharmacological therapy including non-selective beta blockers such as propranolol and nadolol [13], non-elective beta blockers and selective alpha blocker carvedilol [26]. The second option is serial esophageal varices ligation (EVL) [12]. It is unclear from our data whether patients were on beta blockers or not, as NIS data do not reflect patient’s home medications at the time of admission.
Incidence of esophageal varices with bleeding fluctuated throughout study period but remained mostly between 27,790 and 31,515 patients. Increase in HVPG can result from untreated underlying pathology that contributes into worsening of cirrhosis (untreated HCV, HBV, persistent alcohol use) [27, 28], or development of portal vein thrombosis [29]. According to our study, Elixhauser comorbidity score showed high alcohol abuse, chronic liver disease as most common comorbidities in both groups. HVPG > 20 mm Hg (mostly prevalent among Child-Pugh C patients) is strongly associated with risk of bleeding and rebleeding [28].
We found in our analysis that rates of sepsis and acute kidney injury were steadily increasing (1.2-3.4% and 13.7-16.9%). Both are associated with increased risk of readmission in 30 and 90 days according to Weissman et al [19].
Regarding outcomes, LOS was higher in esophageal varices with bleeding with average of 5.3 days versus 4.1 days in nonbleeding esophageal varices, which may correlate with acuity of patients with esophageal varices bleeding. It is thought that there has been no change in LOS trend over the years, which may reflect the effectiveness of protocol being used for esophageal varices bleeding. Treatment protocol is usually conducted within 5 days, which includes stabilizing patient, restrictive blood transfusion with hemoglobin (Hb) goal of 7 - 9 g/dL [30], short-term prophylactic antibiotics for maximum 7 days [31], and vasoactive peptides (somatostatin, octreotide and terlipressin) [32]. Transjugular intrahepatic portosystemic shunt (TIPS) is reserved for endoscopic treatment failure. Increased LOS is a risk of readmission as was reported by Bilal et al [33].
Odds of transfer to visiting nursing assistant, transfer to other hospital and odds of death remained the same throughout the study period (Fig. 4 a-c). Transfer to tertiary teaching hospital can be explained by tendency to refer those patients for higher level of care or liver transplant evaluation. As well, there was a noticeable decrease of discharge to rehab in variceal bleeding group. We were not to capture the effects of these trends on readmission risk as our data do not support that trend.
One limitation of this study is the accuracy of the NIS database in capturing ICD-9 and ICD-10 codes. For instance, some acute variceal hemorrhage-related admissions could have been reported as upper GI bleeding, lower GI bleeding, or acute severe anemia, which would exclude those patients from our analysis. There was also no information regarding the outcomes in terms of rebleeding after discharge. We were not able to capture whether those are primary (first time acute variceal hemorrhage) bleeding or a readmission. As well, it was not clear whether patients received prophylaxis measures for variceal varices (with or without bleeding) after discharge.
Conclusions
Esophageal varices with and without bleeding have been steadily increasing since the beginning of this century. This may result in a substantial impact on increasing health care costs and utilization due to acute variceal hemorrhage. Odds of death, transfer to urban hospital, and transfer to visiting nursing assistance remained unchanged.
| Supplementary Material | ▴Top |
Suppl 1. ICD-10 codes for diagnoses and procedures that were used in the study.
Suppl 2. (A) Box plot to compare the overlap of propensity score between the two intervention groups. (B) Density plot of the propensity scores of the groups. (C) Love plot to summarize covariate balance before and after applying ATT. Red dots represent covariates before applying ATT and blue triangles represent covariates after applying ATT.
Acknowledgments
We would like to acknowledge library staff at Marshall University School of Medicine for helping us with obtaining similar studies to cite and compare to our study.
Financial Disclosure
Authors have no financial interest to disclose.
Conflict of Interest
All authors have no relevant conflict of interest.
Informed Consent
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data using NIS database which contains no identifying patient information and does not require informed consent to use the data.
Author Contributions
Abdelwahap Elghezewi, Mohamad Hammad, Mujtaba Mohamed and Mohammed El-Dallal: manuscript writing, methodology, editing, and project administration. Ahmed Sherif, Wesam Frandah: reviewing and editing. Mohammed El-Dallal: statistical analysis, and data extraction. Wesam Frandah: supervision, reviewing and editing. All authors have read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838-851.
doi pubmed pmc - Dooley J, Lok A, Burroughs AK, Heathcote E, eds. Sherlock’s diseases of the liver and biliary system, 12th edn. Oxford: Wiley-Blackwell; 2011.
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The epidemiology of cirrhosis in the united states: a population-based study. J Clin Gastroenterol. 2015;49(8):690-696.
doi pubmed - Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593-608.
doi pubmed - Yoon YH, Chen CM. Liver cirrhosis mortality in the United States: national, state, and regional trends, 2000-2013. Bethesda: National Institute on Alcohol Abuse and Alcoholism (NIAAA). 2016.
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217-231.
doi pubmed - D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tine F, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39(10):1180-1193.
doi pubmed - Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y). 2011;7(10):661-671.
pubmed pmc - Pagliaro L, D’Amico G, Pasta L, Politi F, Vizzini G, Traina M, et al. Portal hypertension in cirrhosis: natural history. In: Bosch J, Groszmann RJ, eds. Portal Hypertension. Pathophysiology and Treatment. Oxford, UK: Blackwell Scientific; 1994:72-92.
- Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65(1):82-88.
doi pubmed - Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254-2261.
doi pubmed - North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983-989.
doi pubmed - D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19(4):475-505.
doi pubmed - Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146(2):412-419.e413.
doi pubmed - Bosch J, Garcia-Pagan JC. Prevention of variceal rebleeding. Lancet. 2003;361(9361):952-954.
doi pubmed - Tandon P, Abraldes JG, Keough A, Bastiampillai R, Jayakumar S, Carbonneau M, Wong E, et al. Risk of Bacterial Infection in Patients With Cirrhosis and Acute Variceal Hemorrhage, Based on Child-Pugh Class, and Effects of Antibiotics. Clin Gastroenterol Hepatol. 2015;13(6):1189-1196.e1182.
doi pubmed - Solanki S, Haq KF, Chakinala RC, Khan Z, Aronow WS, Ali Khan M, Siddiqui MT, et al. Inpatient burden of esophageal varices in the United States: analysis of trends in demographics, cost of care, and outcomes. Ann Transl Med. 2019;7(18):480.
doi pubmed pmc - Pinto C, Parra P, Magna J, Gajardo A, Berger Z, Montenegro C, Munoz P. [Variceal and non-variceal upper gastrointestinal bleeding. Analysis of 249 hospitalized patients]. Rev Med Chil. 2020;148(3):288-294.
doi pubmed - Weissman S, Sharma S, Aziz M, Ehrlich D, Perumpail M, Sciarra M, Tabibian JH. Impact of Readmission for Variceal Upper Gastrointestinal Bleeding: A Nationwide Analysis. Dig Dis Sci. 2022;67(6):2087-2093.
doi pubmed - HCUP-US NIS overview. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp [Accessed 5 January 2021].
- Axley PD, Richardson CT, Singal AK. Epidemiology of alcohol consumption and societal burden of alcoholism and alcoholic liver disease. Clin Liver Dis. 2019;23(1):39-50.
doi pubmed - Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020;360:1-8.
pubmed - Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
doi pubmed - D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131(5):1611-1624.
doi pubmed - Puente A, Hernandez-Gea V, Graupera I, Roque M, Colomo A, Poca M, Aracil C, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int. 2014;34(6):823-833.
doi pubmed - Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50(3):825-833.
doi pubmed - de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743-752.
doi pubmed - Moitinho E, Escorsell A, Bandi JC, Salmeron JM, Garcia-Pagan JC, Rodes J, Bosch J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117(3):626-631.
doi pubmed - Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73(1):366-413.
doi pubmed - Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, Graupera I, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11-21.
doi pubmed - Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29(6):1655-1661.
doi pubmed - Wells M, Chande N, Adams P, Beaton M, Levstik M, Boyce E, Mrkobrada M. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35(11):1267-1278.
doi pubmed - Bilal M, Abougergi MS, Tayyem O, Parupudi S, Rockey DC. Thirty-day readmission after esophageal variceal hemorrhage and its impact on outcomes in the United States. J Clin Gastroenterol. 2020;54(5):477-483.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


