| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 12, Number 5, October 2019, pages 263-266
Acute Liver Injury in a Patient Treated With Rosuvastatin: A Rare Adverse Effect
Jamil Shaha, c, Vivek Lingiaha, Nikolaos Pyrsopoulosa, Mark Galanb
aDivision of Gastroenterology and Hepatology, Department of Medicine, Rutgers New Jersey Medical School, Medical Science Building, 185 South Orange Avenue, Newark, NJ 07103, USA
bDepartment of Pathology and Laboratory Medicine, Rutgers New Jersey Medical School, Medical Science Building, 185 South Orange Avenue, Newark, NJ 07103, USA
cCorresponding Author: Jamil Shah, 899 Woodmere Drive, Valley Stream, NY 11581, USA
Manuscript submitted August 8, 2019, accepted August 20, 2019
Short title: Acute Liver Injury After Rosuvastatin Therapy
doi: https://doi.org/10.14740/gr1212
| Abstract | ▴Top |
Drug-induced liver injury (DILI) is among the challenging liver conditions encountered by clinicians today. It has a low incidence in the general population with an approximated annual incidence of 10 - 15 cases per 10,000 - 100,000 persons who have taken prescription medications. Nevertheless, DILI remains the most frequent cause of acute liver injury in the United States. Rosuvastatin is a commonly prescribed medication that, similar to other statins, is associated with serum aminotransferase elevations that are mild, asymptomatic and usually self-limited. Here, we report a case of a man who developed acute liver injury after taking rosuvastatin for hypercholesterolemia treatment. Moreover, DILI with autoimmune features represents a key subgroup of hepatotoxicity attributable to medication exposure. Similar to idiopathic autoimmune hepatitis, circulating autoantibodies and a hypergammaglobulinemia are often present in the serum of such individuals. However, such findings are not invariable. In the case reported here, these laboratory features were absent, but a liver biopsy demonstrated interface hepatitis with a prominent plasma cell infiltrate, histologic components consistent with an immune-mediated drug reaction. After withdrawal of the offending medication did not result in complete resolution, corticosteroid therapy was administered with a subsequent clinical response, confirming the diagnosis.
Keywords: Rosuvastatin; Acute liver injury; Drug-induced liver injury; Drug-induced hepatotoxicity
| Introduction | ▴Top |
Rosuvastatin, like other statins, is commonly used to lower cholesterol levels. Furthermore, when prescribed in higher doses, it may be used as moderate- or high-intensity statin therapy for patients with atherosclerotic cardiovascular disease to reduce the risk of coronary events and stroke. As recently as 2014, rosuvastatin was the most prescribed brand name drug in the United States with 22.3 million prescriptions filled [1].
Approximately 1-3% of individuals taking rosuvastatin will develop serum aminotransferase elevations that are mild, asymptomatic and usually self-limited [2]. Furthermore, aminotransferase levels greater than three times the upper limit of normal occur no more frequently among those treated with rosuvastatin (0.2%) as those receiving placebo (0.3%). Rarely, patients taking rosuvastatin can present with frank, clinically apparent acute liver injury, occurring in less than 1 in 10,000. The threshold to investigate for drug-induced liver injury (DILI) should be low, and early identification and discontinuation of the offending agent without delay is essential. Also, in cases of autoimmune hepatitis-like injury, corticosteroids have been administered successfully.
| Case Report | ▴Top |
A 47-year-old healthy Peruvian man was transferred from a community hospital to a tertiary care liver transplant center for advanced liver care. Six weeks earlier, his primary care physician had started him on rosuvastatin 5 mg daily for hypercholesterolemia. At the time, his liver function tests (LFTs) showed an aspartate aminotransferase (AST) of 17 (10 - 40 U/L) and an alanine aminotransferase (ALT) of 19 (10 - 40 U/L). Now, his LFTs showed aminotransferase levels in the 1,000’s, and he was advised to visit his local emergency department.
The patient was admitted and the rosuvastatin was discontinued. However, his LFTs continued to rise and showed AST of 1,142 U/L (10 - 40 U/L), ALT of 2,260 U/L (10 - 40 U/L), total bilirubin (TB) of 3.53 mg/dL (0.2 - 1.2 mg/dL), alkaline phosphatase (ALP) of 277 U/L (40 - 115 U/L) and international normalized ratio (INR) of 1.1 (0.8 - 1.2). He underwent an interventional radiology-guided liver biopsy. He was then transferred to the tertiary care center for acute liver injury suspected to be secondary to rosuvastatin-induced liver injury.
Upon further questioning, the patient reported a yellow tinge to the skin, mild pruritus and fatigue. He denied experiencing additional symptoms recently. He denied regular alcohol use and reported that he drank an occasional can of beer on weekends. He denied taking any medications (other than rosuvastatin), complementary and alternative medicines, or herbal/dietary/bodybuilding/weight-loss supplements. He denied any drug allergies. He denied a history of intravenous (IV) drug use, smoking, acquiring tattoos or piercings, receiving transfusions of blood or blood products, sexual promiscuity, sexually transmitted infections or rashes, occupational exposure to toxins (he worked as a truck driver), or prior liver diseases or viral hepatitis. He denied any family history of liver disease.
The patient was afebrile and hemodynamically stable. The physical exam was remarkable for icteric sclera, generalized jaundice, mild right upper quadrant tenderness and a palpable liver edge. His LFTs upon arrival to the tertiary care center showed AST of 1,400, ALT of 2,253 and TB of 9.9. The serum electrolytes and the CBC were within normal limits. The Roussel Uclaf Causality Assessment Method (RUCAM) scale, also referred to as the Council for International Organizations of Medical Sciences (CIOMS) scale, a well-established and validated tool commonly utilized to quantitatively assess causality in cases of suspected DILI, was used. The RUCAM score was calculated to be 9, which was suggestive of a highly probable adverse drug reaction. An abdominal ultrasound revealed non-specific findings often seen in acute hepatitis (Fig. 1).
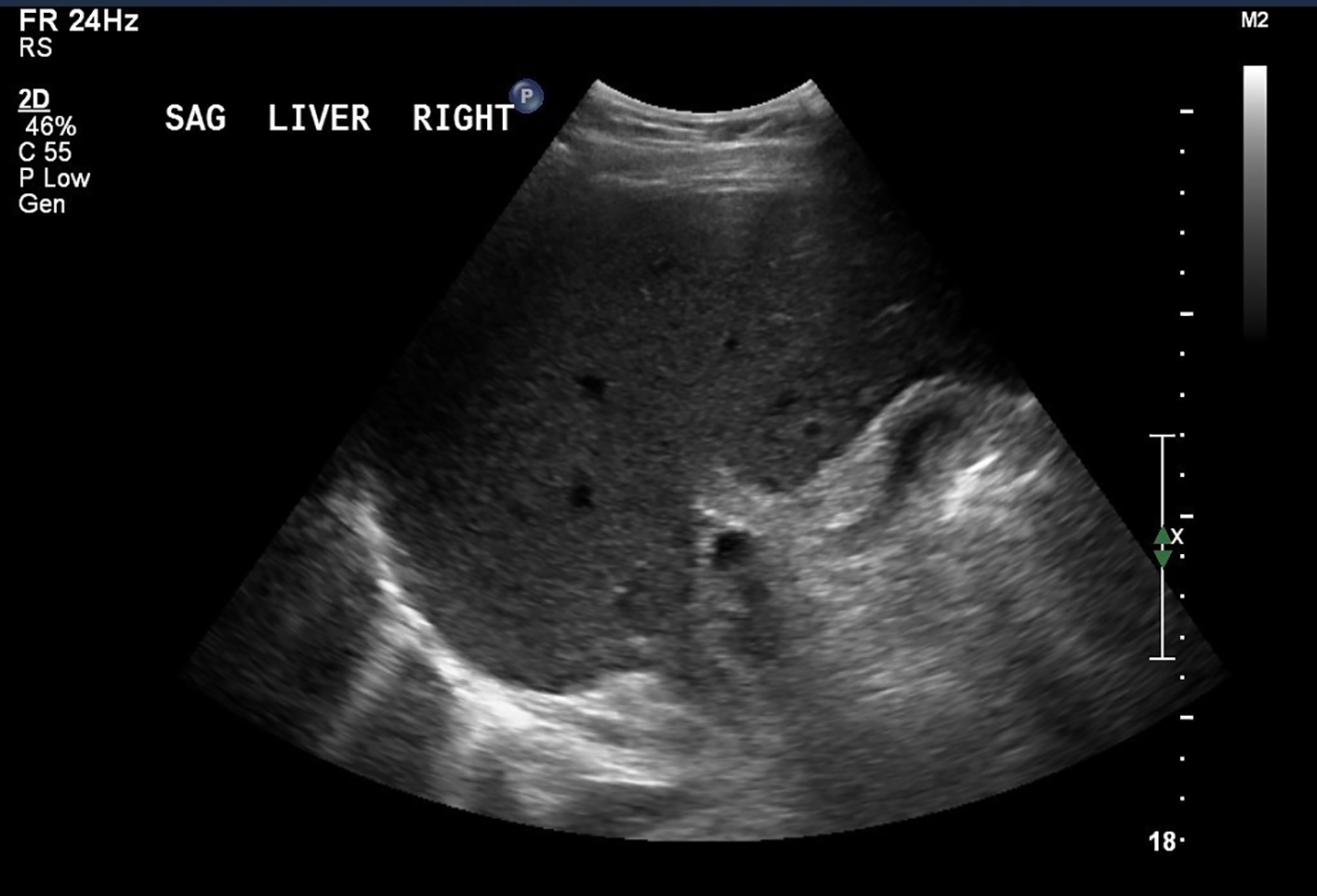 Click for large image | Figure 1. Abdominal ultrasound. The liver is mildly heterogeneous with coarsened echotexture, non-specific findings that can be seen in hepatitis. |
The patient was started on IV N-acetylcysteine (NAC). Workup for chronic liver diseases was sent to evaluate for other etiologies of acute liver failure. An autoimmune panel, including anti-smooth muscle antibodies (ASMA), antinuclear antibodies (ANA) and anti-liver-kidney microsomal antibodies (anti-LKM); an immunoglobulin G (IgG) level; and workup for hepatotropic viruses were negative, and all test results were ultimately unrevealing. The liver biopsy slides were acquired from the transferring hospital and, interestingly, showed multiple histologic components of autoimmune hepatitis (Fig. 2). In particular, the liver biopsy showed acute severe hepatitis with a portal and lobular mixed inflammatory infiltrate comprised of neutrophils, lymphocytes, plasma cells and scattered eosinophils. There was interface activity and bile duct damage. The histopathology was consistent with a likely immune-mediated drug reaction. Furthermore, immunostains for herpes simplex virus (HSV), cytomegalovirus (CMV) and in situ hybridization of Epstein-Barr virus (EBV), as well as the copper, iron and trichrome stains were performed by the liver pathologist and were unrevealing.
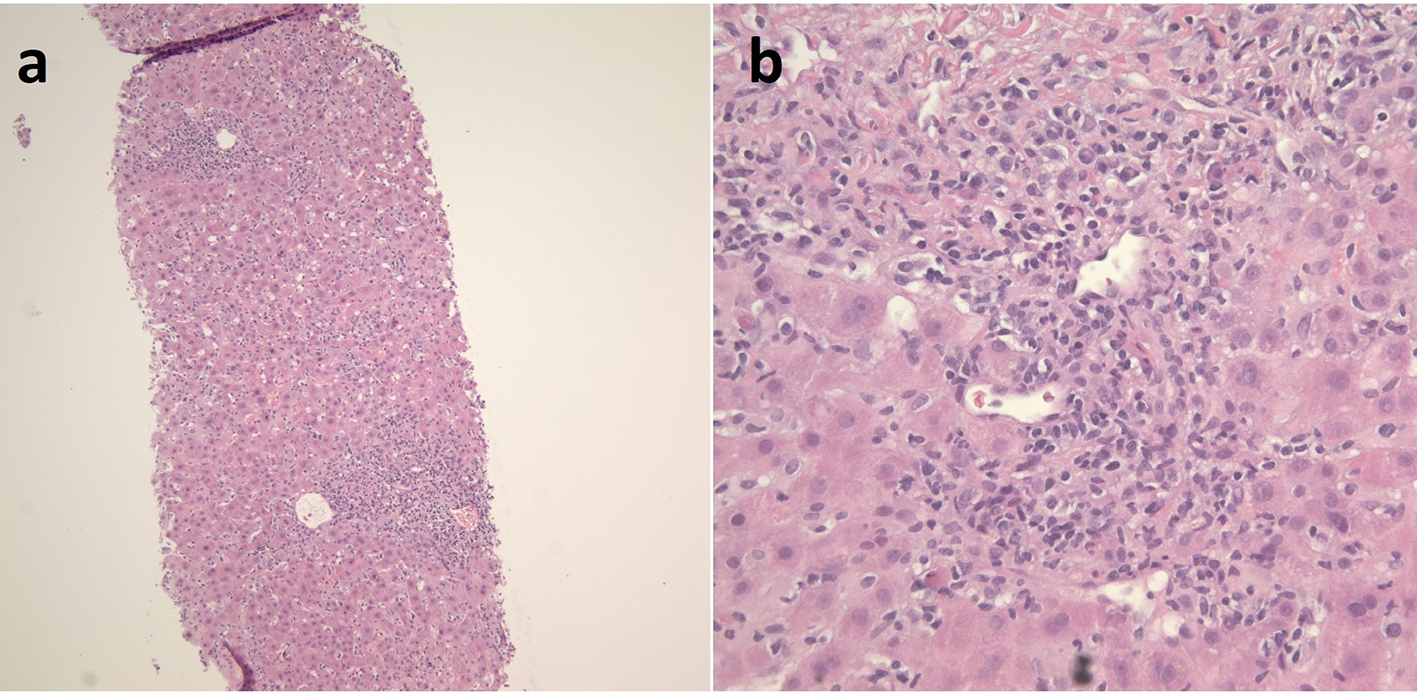 Click for large image | Figure 2. Histopathologic images of the patient’s DILI from liver biopsy showing acute severe hepatitis. There is a portal and lobular mixed inflammatory infiltrate comprised of neutrophils, lymphocytes, plasma cells and scattered eosinophils. There is interface activity present and bile duct damage. The histopathology is consistent with an immune-mediated drug reaction vs. less likely an infectious etiology. (a) H&E stain (× 200). (b) H&E stain (× 400). DILI: drug-induced liver injury. |
Despite IV NAC therapy for 5 days as well as the initiation of Ursodiol, the patient’s liver enzymes did not improve. A triple-phase abdominal computed tomography (CT) scan was performed in anticipation of possible evaluation for liver transplantation, and it revealed good hepatic vascular anatomy (Fig. 3).
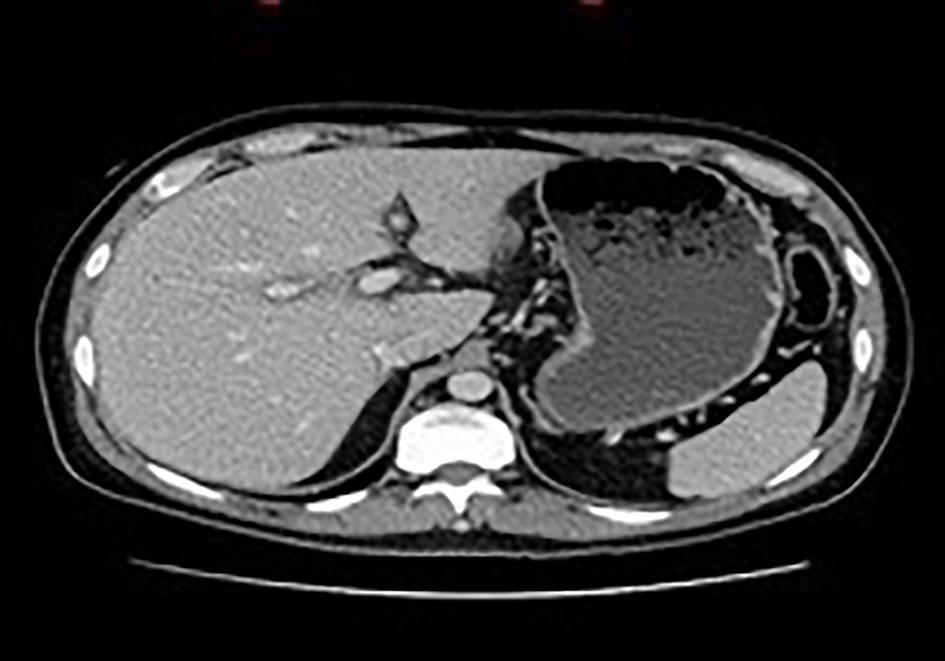 Click for large image | Figure 3. Abdominal CT scan. There is hypoattenuation around the portal area consistent with mild periportal edema. CT: computed tomography. |
During the hospital course, the patient remained hemodynamically and neurologically stable with no evidence of hepatic encephalopathy. His LFTs began to show some improvement with AST of 942, ALT of 1,312 and ALP of 167, but the TB increased to 17.2. He was discharged with a plan to closely monitor the LFTs on an outpatient basis.
Afterward, repeat blood work showed an increasing TB. The patient was re-admitted and given a trial course of IV methylprednisolone after which the TB proceeded to decrease. Ultimately, his liver enzymes continued to improve, and he was discharged home in improved and stable condition.
| Discussion | ▴Top |
DILI is among the most commonly cited reasons for medication removal from the market as well as for termination of drug development during and after preclinical studies [3-5]. Both prescription and over-the-counter drugs can cause this liver condition. DILI is frequently overlooked, particularly when patients are taking many medications. Also, the clinical presentation can imitate any type of liver disease, including acute and chronic hepatitis. Moreover, several risk factors, such as intake of acetaminophen and/or alcohol, can potentiate the harmful effects of a medication. Thus, in every patient who presents with acute liver injury, the possibility of DILI should be considered, independent of any known pre-existing liver disease.
DILI can be categorized as intrinsic, or dose-dependent (e.g. acetaminophen toxicity), or idiosyncratic, or dose-independent, with the latter being frequently associated with rosuvastatin therapy [6, 7]. The etiology of hepatic injury from rosuvastatin is unclear. It is minimally (about 10%) metabolized in the liver via CYP 2C9 [8, 9]. The mild, transient serum aminotransferase elevations may be secondary to formation of an inconsequential toxic intermediate of drug metabolism. The rarer clinically apparent acute liver injury attributed to rosuvastatin is frequently accompanied by autoimmune features and, so, may be due to immune mechanisms [8, 9].
In conclusion, the threshold to investigate for DILI should be low, and early identification and discontinuation of the offending agent without delay is essential. In cases of autoimmune hepatitis-like injury, corticosteroids have been administered successfully, particularly when recovery did not occur immediately [8]. In this case, because there was a pathologic diagnosis of probable drug-induced autoimmune hepatitis, steroids could have been used earlier after the infectious etiologies had been clinically excluded. If given, the dose and duration of therapy should be held to a minimum, and close follow-up after cessation is crucial. Recovery is typically complete within 1 - 2 months. Switching treatment to another statin appears to be safe, but it should be performed with close monitoring for recurrence [7].
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed patient consent was obtained for publication of the details of this case.
Author Contributions
JS conceived of the idea for the manuscript. JS designed and drafted the manuscript. VL, NP and MG evaluated and critically revised the manuscript for important intellectual content. MG provided the images.
| References | ▴Top |
- IMS Health. 1 October 2013 to 30 September 2014. IMS Health, 2014.
- Shepherd J, Hunninghake DB, Stein EA, Kastelein JJ, Harris S, Pears J, Hutchinson HG. Safety of rosuvastatin. Am J Cardiol. 2004;94(7):882-888.
doi pubmed - Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947-954.
doi pubmed - Xu JJ, Diaz D, O'Brien PJ. Applications of cytotoxicity assays and pre-lethal mechanistic assays for assessment of human hepatotoxicity potential. Chem Biol Interact. 2004;150(1):115-128.
doi pubmed - Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
doi pubmed - Gunawan BK, Kaplowitz N. Mechanisms of drug-induced liver disease. Clin Liver Dis. 2007;11(3):459-475, v.
doi pubmed - Bjornsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56(2):374-380.
doi pubmed - Wolters LM, Van Buuren HR. Rosuvastatin-associated hepatitis with autoimmune features. Eur J Gastroenterol Hepatol. 2005;17(5):589-590.
doi - Sanchez M, Castiella A, Zapata E, Zubiaurre L, Perez-Yeboles J, Mendibil L, Iribarren A. Autoimmune hepatitis (Immune-Mediated Liver Injury) induced by rosuvastatin. Gastroenterol Hepatol. 2018;41(5):311-313.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


