| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 11, Number 1, February 2018, pages 75-78
A Rare Cause of Abdominal Pain and Mass in an 18-Year-Old Patient: A Diagnostic Dilemma
Hassan Tariqa, Muhammad Umar Kamala, d, Vamshidhar Vootlaa, Mohamed ElZaeedib, Masooma Niazic, Brian Gilchristb, Ariyo Ihimoyana, Anil Deva, Sridhar Chilimuria
aDepartment of Medicine, Bronx Lebanon Hospital Center, Bronx, NY 10457, USA
bDepartment of Surgery, Bronx Lebanon Hospital Center, Bronx, NY 10457, USA
cDepartment of Pathology, Bronx Lebanon Hospital Center, Bronx, NY 10457, USA
dCorresponding Author: Muhammad Umar Kamal, Department of Medicine, Bronx Lebanon Hospital Center, 1650 Selwyn Ave, Suit #10C, Bronx, NY 10457, USA
Manuscript submitted December 30, 2017, accepted January 23, 2018
Short title: Rare Cause of Abdominal Pain in a Young Male
doi: https://doi.org/10.14740/gr955w
| Abstract | ▴Top |
We present a case of an 18-year-old male who presented with complains of abdominal pain, nausea and vomiting for 2 years. An esophagogastroduodenoscopy (EGD) revealed a 3 mm nodule on the lesser curvature of the stomach and prominent gastric folds. Biopsy of the nodule revealed a well-differentiated neuroendocrine tumor (NET) in lamina prop with focal extension into muscularis mucosa consistent with a gastric carcinoid. Tumor cells stained with neuron-specific enolase (NSE), chromogranin and synaptophysin only. The prominent gastric fold biopsy revealed gastric fundic mucosa with mucosal edema and focal mild chronic inflammation. Serum gastrin level was found to be 2,083 pg/mL. Abdomen CT and endoscopic ultrasound (EUS) revealed a mass near the pancreatic neck. These findings were consistent with a functional gastrin producing well-differentiated grade 1 neuroendocrine neoplasm (gastrinoma). The patient underwent exploratory laparotomy with resection of the mass and resulting in normalization of gastrin levels.
Keywords: Gastrinoma; Gastric carcinoids; Neuroendocrine tumor; Zollinger-Ellison syndrome; Primary peripancreatic gastrinoma
| Introduction | ▴Top |
Gastroenteropancreatic neuroendocrine tumors (NETs), also called carcinoid, originate from diffuse neuroendocrine cells that are dispersed throughout the gastrointestinal tract and islets of Langerhans in the pancreas [1]. These tumors are characterized by their ability to secrete different kinds of peptides that can cause a wide spectrum of clinical manifestations. The incidence of NETs has increased about fivefold over the last four decades, likely secondary to advances in diagnostic modalities [2]. Gastrinomas are associated with increased production of gastrin and have varied presentations such as peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD), diarrhea, or abdominal pain [3]. Gastric carcinoids (GCs) are rare tumors, the percentage of these tumors among all gastric malignancies is 1.8% and the proportion of these carcinoids among all gastrointestinal carcinoids is 8.7% [4]. The rarity of these tumors presents a problem for timely diagnosis and management. Here we present the clinical manifestations, diagnostic workup and treatment of a gastric NET in a patient who presented with vague complains of abdominal pain, nausea and vomiting.
| Case Report | ▴Top |
An 18-year-old man was referred to our gastroenterology clinic with complains of abdominal pain, nausea and vomiting for 2 years. The abdominal pain was epigastric, burning, intermittent, occurring 1 - 2 times per week and was relieved by proton pump inhibitors (PPIs). It was associated with watery diarrhea, nausea, vomiting, acid reflux symptoms and postprandial bloating. He reported that the episodes started with pain, and then progressed to multiple episodes of emesis and diarrhea for a few days. He denied any loss of appetite, weight loss, hematochezia or hematemesis and never underwent esophagogastroduodenoscopy (EGD) or colonoscopy.
His comorbidities included mild persistent asthma with no surgical history. He was taking albuterol, Montelukast and omeprazole with good compliance. Family history was significant for stomach cancer in grandmother. He was actively using cannabinoids.
On examination, vitals were normal and cardio-respiratory exam was unremarkable. Abdomen was soft, not tender, without any palpable masses or audible bowel sounds. A complete blood count, serum chemistry and liver function panel were within normal limits. The patient underwent an EGD that revealed a small 3 mm nodule on the lesser curvature of the stomach and prominent gastric folds (Fig. 1). The examined esophagus, duodenal bulb and second part of the duodenum were normal. Biopsy revealed well-differentiated NET in lamina propria with focal extension into muscularis mucosa consistent with a GC (Fig. 2). Tumor cells stained with neuron-specific enolase (NSE), chromogranin and synaptophysin only. The prominent gastric fold biopsy revealed gastric fundic mucosa with mucosal edema and focal mild chronic inflammation. Serum gastrin level was found to be 2,083 pg/mL (normal < 100 pg/mL). Vitamin B 12 level was 1,151 pg/mL (243 - 894 pg/mL), and methylmalonic acid level was 94 nmol/L (87 - 318 nmol/L). Considering elevated gastrin levels associated with prominent gastric folds, our impression was that the patient likely had a type 2 GC and needed further evaluation for the presence of a possible gastrinoma and/or multiple endocrine neoplasia (MEN 1 syndrome). An octreotide scan showed abnormal uptake in the stomach consistent with a large GC without any evidence of metastatic disease (Fig. 3). A computed tomography (CT) scan of abdomen and pelvis with contrast showed a lobulated soft tissue mass in the mid upper abdomen inseparable from inferior part of the liver and lesser curvature of the stomach (Fig. 3). An endoscopic ultrasound (ESU) showed a 5 × 4 cm hypoechoic mass in the vicinity of pancreatic neck.
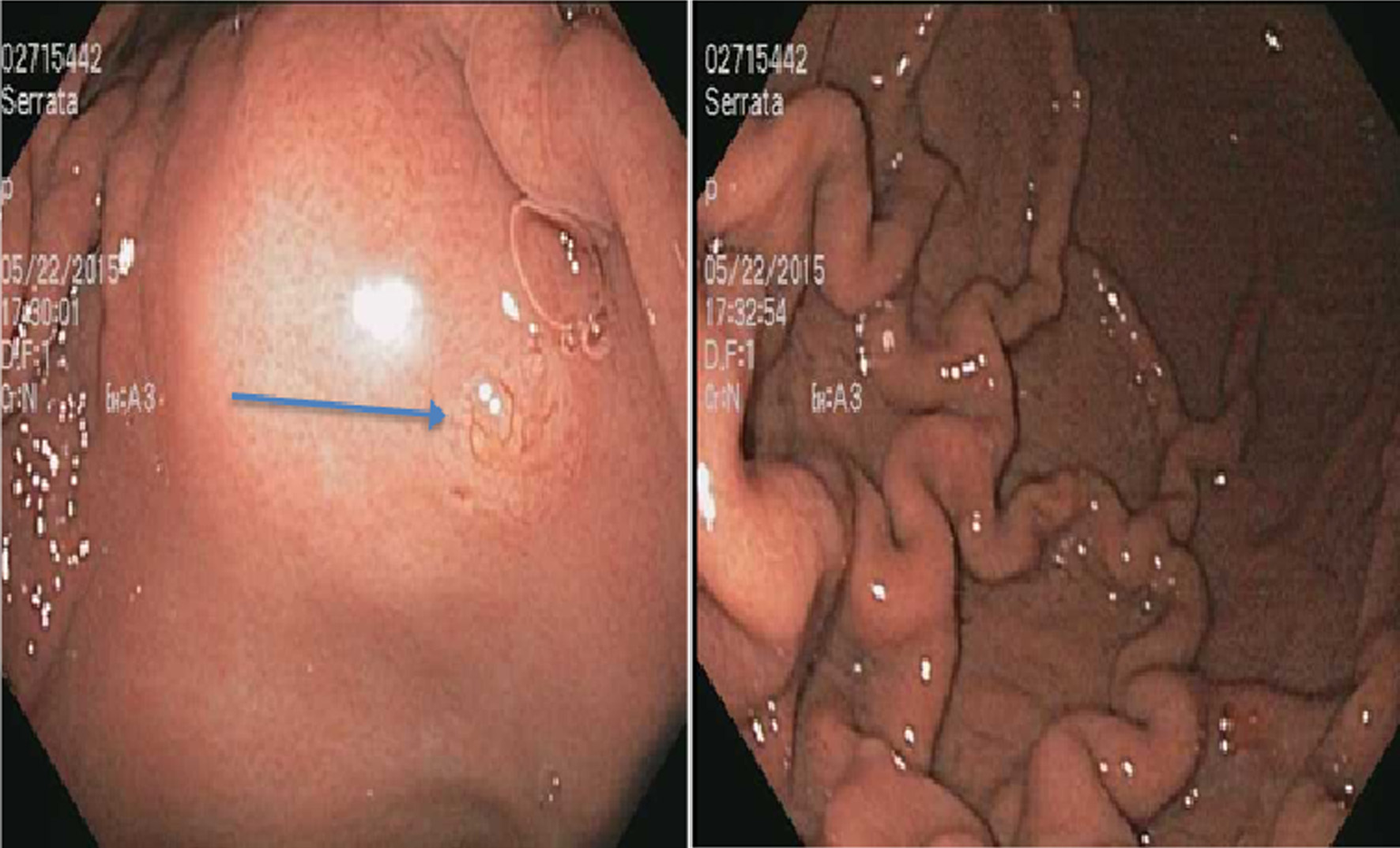 Click for large image | Figure 1. A 3 mm nodule on the lesser curvature of the stomach (arrow) (left) and prominent gastric folds (right). |
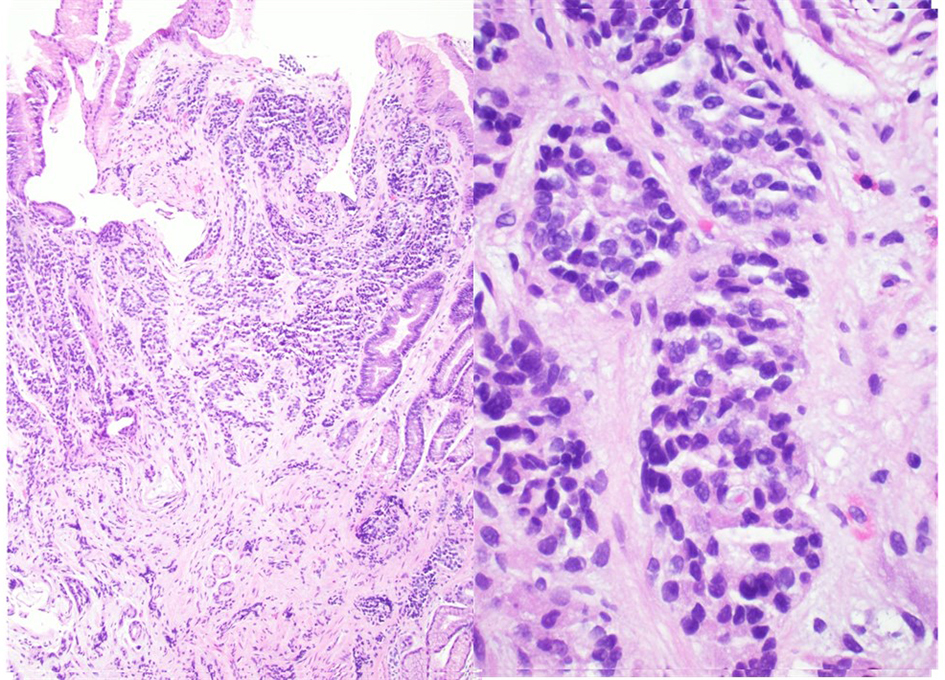 Click for large image | Figure 2. A well-differentiated neuroendocrine tumor in lamina propria with focal extension into muscularis mucosa consistent with a gastric carcinoid (hematoxylin and eosin stain, magnification: left × 40, right × 100). |
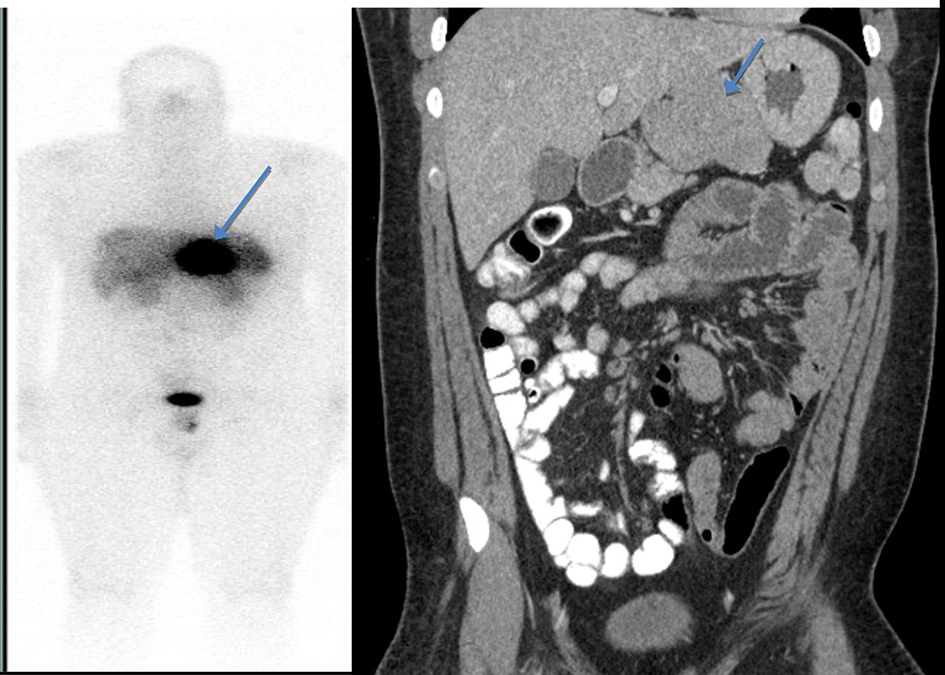 Click for large image | Figure 3. Octreotide scan showing abnormal uptake in the stomach consistent with a large gastric carcinoid without any evidence of metastatic disease (left). A computed tomography (CT) scan of abdomen and pelvis with contrast showed a lobulated soft tissue mass in the mid upper abdomen inseparable from inferior part of the liver and lesser curvature of the stomach (right). |
The patient underwent exploratory laparotomy with resection of the mass. The lobulated mass was adherent to the lesser curve of stomach and anterior surface of pancreas (Fig. 4). No infiltration into stomach or pancreas was identified. Immunohistochemistry was positive for chromogranin, synaptophysin and gastrin but negative for insulin. Immunostain showed < 2% of tumor cells to be positive for Ki-67 (Fig. 5). These findings, in conjunction with high serum gastrin levels, were consistent with a functional gastrin producing well-differentiated grade 1 NET (gastrinoma).
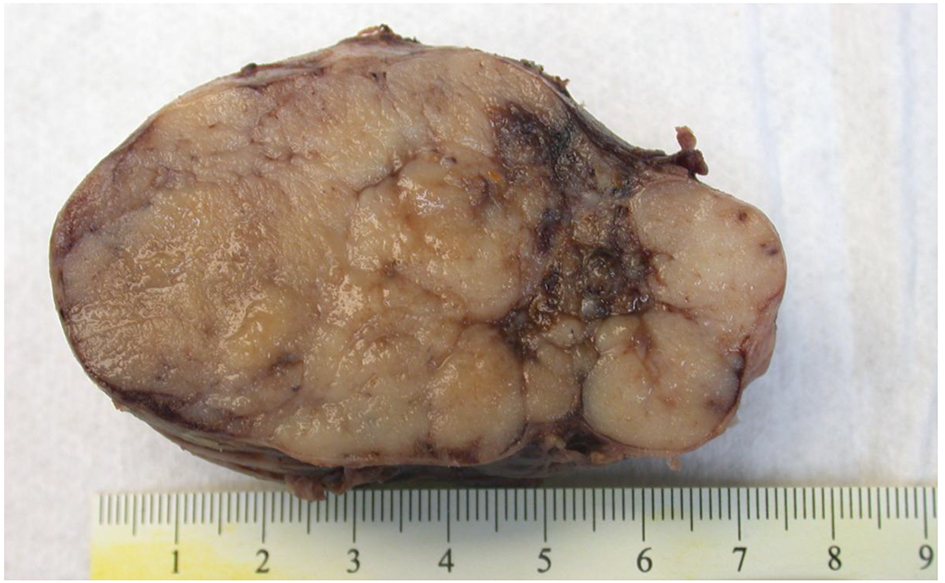 Click for large image | Figure 4. Macroscopic picture of peripancreatic mass with a lobulated cut surface. The mass was adherent to the lesser curve of stomach and anterior surface of pancreas. |
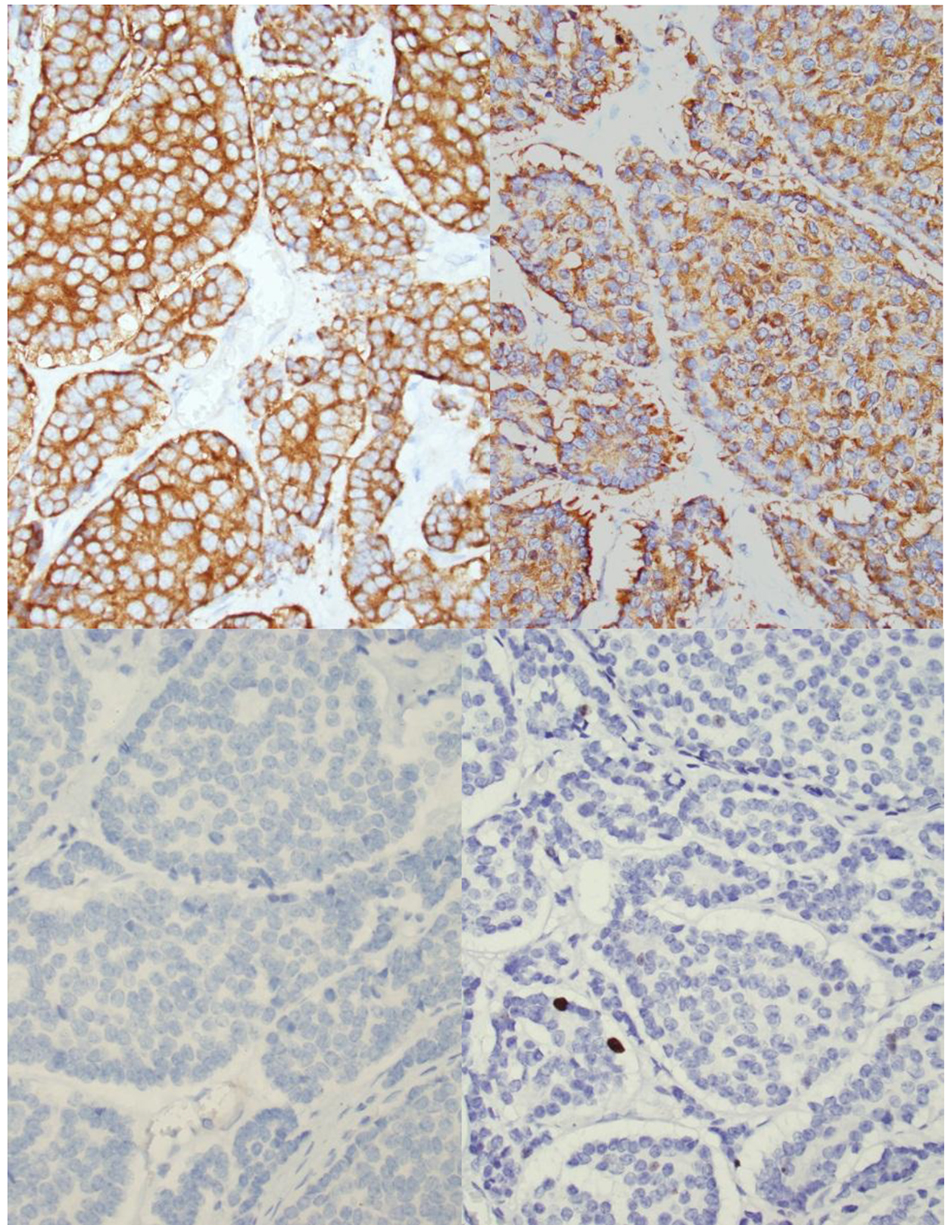 Click for large image | Figure 5. Histology of peripancreatic gastrinoma. Upper left (hematoxylin and eosin stain, please provide magnification). Immunohistochemistry revealed that the tumor cells are positive for chromogranin (upper mid), synaptophysin upper right) and gastrin (lower right) but negative for insulin (lower mid). Immunostain showed < 2% of tumor cells to be positive for Ki-67 (lower right). |
To evaluate for the presence of MEN 1 syndrome, workup showed a serum parathormone level (37.4 pg/mL) as well as anterior pituitary hormone levels to be within normal limits. A 24-h urine calcium/creatinine excretion ratio was 0.007. Magnetic resonance imaging (MRI) of brain was done that showed a normal pituitary gland without any hypo-enhancement to suggest a pituitary adenoma. The absence of clinical, hormonal and radiological evidence was not consistent with MEN 1 syndrome. However, considering that a few cases of patients with no clinical features of MEN syndrome were found to have MEN syndromes on genetic testing, we referred him for genetics counseling and further testing for MEN1 syndrome.
Our impression was that the patient had a sporadic primary peripancreatic gastrinoma associated with type II GC. A repeat EGD for further evaluation of duodenum was performed with biopsies taken from stomach and duodenum. No evidence of NET was seen on pathology. A repeat gastrin level 1 week and 2 months after surgery was 69 and 58 pg/mL, respectively.
| Discussion | ▴Top |
In 1956, Dr. Robert M. Zollinger and Dr. Edwin H. Ellison described two cases, at the annual meeting of the American Surgical Association in Philadelphia, of patients with multiple, recurrent and severe ulcerations in the proximal gastrointestinal tract controlled only by total gastrectomy associated with tumors in pancreas. They first proposed the diagnostic triad of mucosal ulcerations in unusual locations such as distal duodenum or proximal jejunum, gastric acid hypersecretion, and presence of non-β-cell pancreatic tumors that was eventually known as Zollinger-Ellison syndrome (ZES). In the 1960s, gastrin was first discovered as a key hormone in the pathogenesis of the Zollinger-Ellison syndrome [5].
Gastrinomas are NETs derived from multipotential stem cells of endodermal origin. There are three types of GCs: type 1 GCs (70-80%) are associated with chronic atrophic gastritis and pernicious anemia, typically seen in women 50 - 70 years of age. They are associated with an elevated gastrin level and gastric pH. Type 2 GCs (5-10%) are associated with MEN 1 syndrome and ZES. They are associated with a high gastrin level but unlike type 1, the gastric pH is low. Type 3 GCs (< 20%) have a male preponderance with a mean age of 55. Unlike type 1 and 2, the gastrin level and pH are normal [6]. Type 1 and type 2 GCs develop because of hypergastrinemia causing hyperplasia of precursor ECL cells. Excessive gastrin secretion from a gastrinoma results in high gastric acid output due to the trophic action of gastrin on parietal cells and histamine-secreting enterochromaffin-like (ECL) cells. There is no evidence of hypergastrinemia or abnormal gastric acidity in type 3 GCs. There is an absence of ECL hyperplasia in the corpus mucosa that is evident in type 1 and 2 GCs [6].
The most common symptoms at the time of presentation include abdominal pain (75%), diarrhea (73%), heartburn (44%), weight loss (17%), and gastrointestinal bleeding (25%). Patients rarely present with only one symptom (11%): pain and diarrhea being the most frequent combination (55%).
Over 90% of patients with ZES develop peptic ulcers, usually less than 1 cm in diameter. Ulcers are more likely to be refractory to PPIs and to recur as compared with patients with sporadic ulcer disease. An important presenting sign that should suggest ZES is prominent gastric body folds, which may be present on EGD in 94% of patients. Patients may also have evidence of reflux esophagitis. However, strictures of the esophagus, pylorus, or duodenum are present in less than 10% of patients [7, 8].
ZES should be suspected in patients with multiple or refractory peptic ulcers; ulcers distal to the duodenum; peptic ulcer disease and diarrhea, enlarged gastric folds, or multiple endocrine neoplasia type 1. Initial evaluation in a patient with suspected gastrinoma should begin with measuring a gastrin level. If gastrin levels are not diagnostic, a secretin stimulation test should be considered [9]. Any patient diagnosed with ZES should also be screened for MEN 1 syndrome [10]. Tumor localization should begin with an EGD. Radiologic imaging (CT, MRI) is recommended before undergoing any surgical exploration to localize the lesion and identify or rule out metastatic disease; however, both frequently miss tumors smaller than 2 cm. Somatostatin receptor scintigraphy (SRS) has a higher sensitivity than conventional imaging and is the imaging study of choice for identifying primary tumors and metastatic lesions in ZES. In recent years, ESU has also become an important tool for localization of endocrine tumors located in the pancreas, as it allows good visualization of sub-centimeter tumors. If CT/MRI and SRS are negative, and surgery is being considered, an EUS should be performed because of its greater sensitivity in detecting small tumors and a fine-needle aspiration for histological identification can also be performed [5].
In patients with ZES as part of the MEN 1 syndrome, medical therapy with PPIs is the standard of care, whereas in patients with a sporadic gastrinoma, without evidence of metastatic spread of disease, they should be treated with exploratory laparotomy with curative intent [8, 11, 12]. More than 80 of sporadic gastrinomas are located in the gastrinoma triangle created by confluence of the cystic and common bile duct superiorly, the second and third portions of the duodenum inferiorly, and the neck and body of the pancreas medially, both dorsally and ventrally [8]. Our case was unique as the peripancreatic gastrinoma was diagnosed in an 18-year-old patient.
In conclusion, gastrinoma diagnosis should be prompted by high index of suspicion based on clinical presentation, and confirmatory biochemical testing should be performed. Sporadic and MEN 1 syndrome associated variants should be distinguished. All patients without contraindication to surgery should undergo surgical exploration following radiological and nuclear localization studies.
Disclosures
None.
Author Contributions
All authors have made contributions to the article and have reviewed it before submission.
Institutional Review Board Statement
This case report was exempted from our Institutional Review Board as per its policy.
Informed Consent
Informed consent for participation was obtained from this patient.
Conflict of Interest
None of the authors have any financial conflict of interest.
| References | ▴Top |
- Fraenkel M, Kim MK, Faggiano A and Valk GD. Epidemiology of astroenteropancreatic neuroendocrine tumours. Best practice & research Clinical gastroenterology. 2012;26:691-703.
doi pubmed - Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50(1):58-64.
doi pubmed - Huang SF, Kuo IM, Lee CW, Pan KT, Chen TC, Lin CJ, Hwang TL, et al. Comparison study of gastrinomas between gastric and non-gastric origins. World J Surg Oncol. 2015;13:202.
doi pubmed - Borch K, Ahren B, Ahlman H, Falkmer S, Granerus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005;242(1):64-73.
doi pubmed - Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014;19(1):44-50.
doi pubmed - Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39(10):1071-1084.
doi pubmed - Roy PK, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore). 2000;79(6):379-411.
doi - Meko JB, Norton JA. Management of patients with Zollinger-Ellison syndrome. Annu Rev Med. 1995;46:395-411.
doi pubmed - Berna MJ, Hoffmann KM, Long SH, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore). 2006;85(6):331-364.
doi pubmed - Mignon M, Cadiot G, Marmuse JP, Lewin MJ. Is gastrinoma a medical disease? Yale J Biol Med. 1996;69(3):289-300.
pubmed - Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, Jensen RT. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244(3):410-419.
doi - Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341(9):635-644.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


