| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 9, Number 6, December 2016, pages 99-102
Extramammary Paget’s Disease of Anal Canal Associated With Rectal Adenoma Without Invasive Carcinoma
Vaibhav Chumbalkara, Timothy A. Jenningsa, Sanaz Ainechia, Edward C. Leeb, Hwajeong Leea, c
aPathology and Laboratory Medicine, Albany Medical College, Albany, NY, USA
bGeneral Surgery, Albany Medical College, Albany, NY, USA
cCorresponding Author: Hwajeong Lee, Pathology and Laboratory Medicine, Albany Medical College, 47 New Scotland Ave., MC81, Albany, NY 12208, USA
Manuscript accepted for publication November 16, 2016
Short title: Anal Paget’s Disease Associated With Rectal Adenoma
doi: https://doi.org/10.14740/gr727e
| Abstract | ▴Top |
Extramammary Paget’s disease (EMPD) is a rare disease which is found in apocrine-rich locations such as anogenital region, axilla and rarely in other sites. Perianal EMPD is often reported as the involvement of perianal skin, but involvement of anal mucosa is very rare. Based on pathogenesis and association with either synchronous or metachronous malignancy, EMPD can be divided into primary and secondary types. Treatment approach for these two types of Paget’s disease and their prognosis is different, thus it is important to make the distinction. Secondary type of Paget’s disease is almost always described in association with invasive malignancy. While secondary Paget’s disease arising in association with ductal carcinoma in situ of the breast is common, secondary EMPD associated with precursor lesion of the rectum without invasion is exceedingly rare. We report a very rare case of secondary Paget’s disease of the anal canal in association with rectal tubular adenoma (precursor lesion) without malignancy.
Keywords: Paget’s disease; Adenoma; Anorectal; Anus; Secondary; Perianal
| Introduction | ▴Top |
Paget’s disease of breast was described in late 19th century by Sir James Paget [1] and extramammary Paget’s disease (EMPD) involving the scrotum and penis [2] was described shortly later. While EMPD has been recognized for many years, very few cases are reported, thus it is considered a rare disease. The most commonly affected demography comprises females and Caucasians between ages 50 and 80 [3]. The most common site is vulva, followed by perineal, perianal, scrotal and penile skin. Other rarer sites are axilla, buttocks, thigh, eyelids and external auditory canal [4]. EMPD manifests as an erythematous or eczema like skin lesion that is long standing and refractory to conservative local treatment based on the assumption that it is a benign condition [5]. Histologically, Paget’s disease is characterized by the presence of atypical large vacuolated cells within the epidermis. Based on suggested pathogenesis and histochemical markers, two types of EMPD - primary and secondary - are described [6-8]. Primary EMPD is mostly local and a relatively benign disease [9-11]. Secondary EMPD has a relatively poor prognosis and is almost always associated with synchronous or metachronous malignancy [6, 12-14]. Based on our review, EMPD is extremely rarely reported in association with tubular adenoma without evidence of underlying invasive carcinoma [15]. Herein we report such a case.
| Case Report | ▴Top |
A 68-year-old gentleman with past history of renal cell carcinoma, prostatic adenocarcinoma and metastatic thyroid cancer but with no history of colorectal carcinoma underwent hemorrhoidectomy for recurrent internal hemorrhoids. On histological examination of the hemorrhoidectomy specimen in addition to a hemorrhoidal lesion, large atypical cells with pale and vacuolated cytoplasm were noted infiltrating the squamous epithelium of the anal mucosa. The atypical cells were positive for mucicarmine, CK20, CEA and CK7 (focally) and negative for melan A and GCDFP15 (Fig. 1). CDX2 immunohistochemistry was not performed. A diagnosis of anal EMPD was rendered. In addition to this, a detached portion of colonic mucosa involved by adenoma was noted. There was no evidence of invasive malignancy in the specimen.
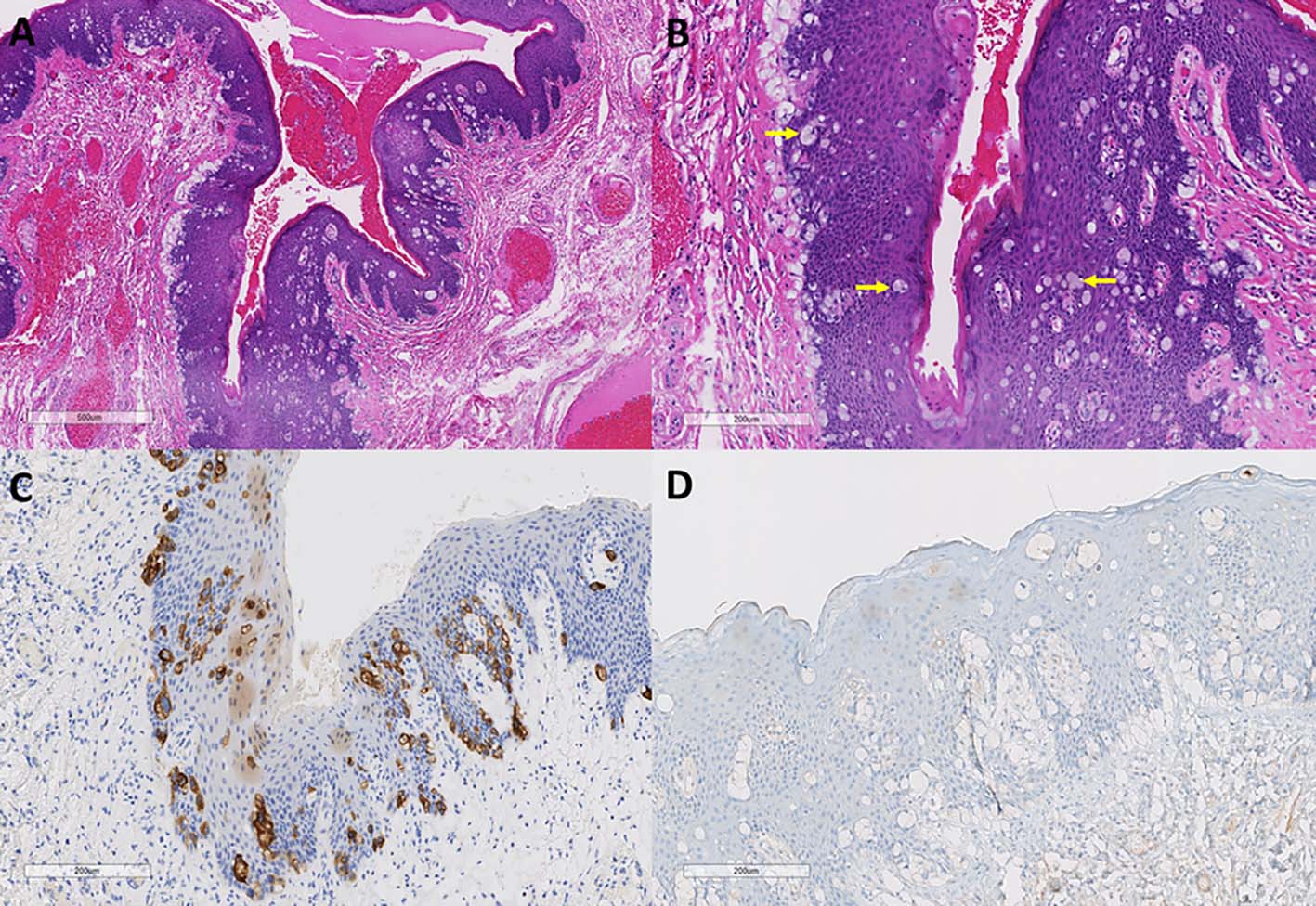 Click for large image | Figure 1. Paget’s disease of anal canal. (A, B) Anal mucosa from first resection specimen showing infiltration of large atypical vacuolated cells (Paget’s cells, arrows) with cytoplasmic mucin (hematoxylin and eosin stain, original magnification × 40 (A); × 100 (B)). (C, D) These cells are CK20 positive and GCDFP15 negative (immunoperoxidase stain, CK20, original magnification × 100 (C); GCDFP15, original magnification × 100 (D)). |
Given the strong association between EMPD and underlying malignancy, unsampled colorectal adenocarcinoma was suspected. To evaluate the possibility of underlying malignancy, a follow- up colonoscopy was performed within a month, which revealed a localized area of moderately congested and nodular mucosa in the distal rectum. The biopsy of this nodular mucosa revealed hyperplastic polyp.
The patient was referred for consultation to our institute at this time. Flexible sigmoidoscopy was performed under anesthesia at the time of wide excision of the lesion, which revealed old hemorrhoidectomy site and two small benign appearing nodules close to the anorectal junction in the rectum. Transanal wide excision of the mucosa to include the previous hemorrhoidectomy site and the additional rectal nodules was performed.
This transanal excision specimen consisted of two tan fragments of anorectal mucosa measuring 1.5 and 1.7 cm. The specimen was entirely submitted for microscopic examination.
Upon microscopic examination, the larger tissue fragment - previous hemorrhoidectomy site - showed residual Paget’s disease adjacent to residual adenomatous lesion. No high grade dysplasia or invasive malignancy was identified. The Paget cells stained positive for CK7 and CDX2 and were minimally positive for CK20 (Fig. 2). The Paget’s disease was noted close to the distal anal margin, but the margin was negative for the disease. The smaller tissue fragment revealed a small adenomatous lesion (Fig. 2D) without high grade dysplasia or invasion. No Paget’s disease was noted in this smaller tissue fragment.
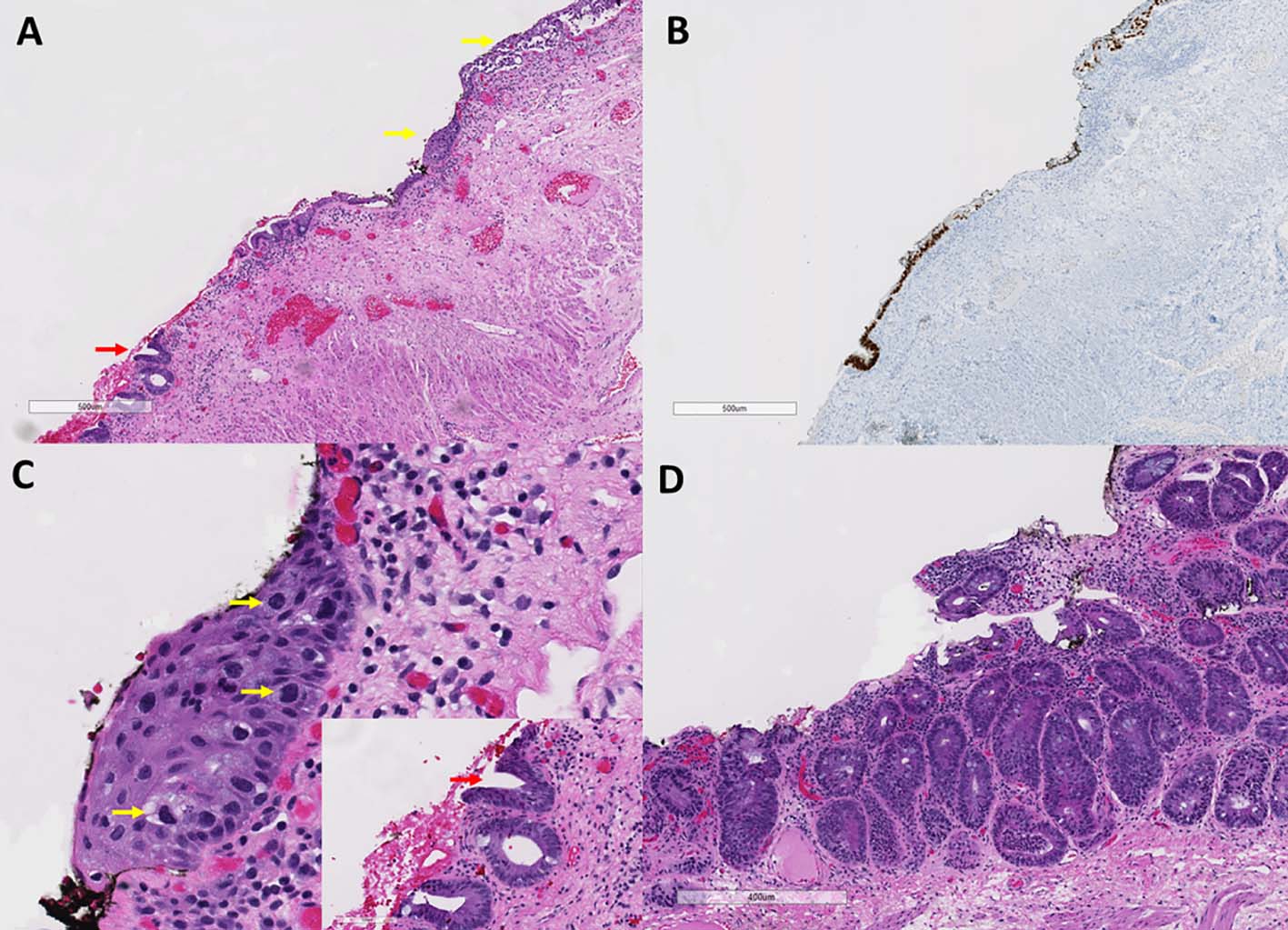 Click for large image | Figure 2. Residual Paget’s disease and rectal adenoma. (A) Anal mucosa from second resection specimen showing residual Paget’s disease (yellow arrows) adjacent to flat adenoma (red arrow) (hematoxylin and eosin stain, original magnification, × 40). (B) The Paget’s cells and adenomatous cells are CDX2 positive (immunoperoxidase stain, CDX2, original magnification, × 40). (C) Higher magnification view of residual Paget’s disease (yellow arrows) and adenomatous crypts (inset with red arrow) (hematoxylin and eosin stain, original magnification, × 200). (D) Adenoma in the smaller tissue fragment (hematoxylin and eosin, original magnification, × 62). |
The patient is being followed till submission of this manuscript (11 months after excision) by physical examination and anoscopy and there is no recurrence of the disease.
| Discussion | ▴Top |
Paget’s disease of anal canal is an extremely rare condition based on rare reports [16]. Some reports in the literature which discuss Paget’s disease of the anus indeed describe perianal Paget’s disease [13]. Given the anatomic continuity of the anal canal and perianal skin and the extreme rarity of anal canal lesions, these two entities can be discussed together.
EMPD is a very rare condition with variable histology amongst different cases. In some cases, Paget’s cells are predominant in basal layer while in others Paget’s cells are spread throughout the epidermis. Moreover, the appearance of Paget’s cell is different and is described as type A (classical) and type B (signet ring like) [7, 15, 17]. Two different hypotheses are proposed for the development of EMPD. According to the first hypothesis, it is a neoplasm of apocrine cells occurring within skin epidermis [18], while the other hypothesis suggests a “pagetoid” migration of malignant cells from an underlying malignancy such as anorectal adenocarcinoma [9].
Based on these hypotheses, two different types of perianal EMPD are described. Primary perianal EMPD is classically described as cutaneous adenocarcinoma commonly seen in apocrine rich zones with typical Paget cells [19]. In contrast, secondary EMPD will exhibit intraepidermal invasion of an underlying anorectal adenocarcinoma wherein the neoplastic cells demonstrate signet ring morphology or endodermal differentiation similar to colonic type mucosa [5]. Clinical presentation may be similar for both types. Even upon histological evaluation, these two types cannot always be distinguished. Distinguishing primary from secondary type is important as primary Paget’s disease has better prognosis and is treated differently than a secondary Paget’s disease. In addition, if secondary Paget’s disease is identified, a thorough effort should be made to identify associated malignant lesion [20, 21].
Immunohistochemical study can help in differentiating these two types [6, 21, 22]. CK7 is a sensitive marker for Paget cells, but not very specific [6]. Paget cells also stain positive for GCDFP15, which is a marker of apocrine cells and is used to identify primary EMPD [20]. CK20 is more typical for anorectal cancer and hence its positivity suggests secondary EMPD [6]. Similarly, CDX2, which is sensitive and specific marker for gastrointestinal mucosa, can be used to identify secondary type EMPD [22]. Overall, primary EMPD exhibits positivity for CK7 and GCDFP15 consistent with apocrine origin, while secondary EMPD is positive for CK20, CDX2 and variably positive for CK7 consistent with lower gastrointestinal origin. The staining pattern in our case is consistent with secondary EMPD.
Paget’s disease needs to be differentiated from other lesions with similar morphological features. Certain reactive changes such as pagetoid dyskeratosis are seen with hemorrhoidal disease [23]. Also, human papilloma virus (HPV) induced lesion with or without anal intraepithelial neoplasm, Bowen’s disease, and melanocytic lesions can mimic Paget’s disease [21]. Histochemical markers such as mucicarmine, periodic acid-Schiff and immunohistochemical markers such as CK7, CK20, GCDFP15, CDX2, S-100, p16, melan A, and CEA can be employed to establish the diagnosis among these differentials.
Till now, almost all the cases of secondary EMPD described in the literature showed invasive adenocarcinoma associated with it. The current case is a very rare instance in which precursor lesion - tubular adenoma - is present in association with EMPD. We found only two reports in the literature describing perianal Paget’s disease in association with adenoma of colon with no obvious invasive lesion [15, 24].
This may represent a phenomenon similar to Paget’s disease of breast where its association with ductal carcinoma in situ is relatively common [25]. One of the leading hypotheses for the pathogenesis of Paget’s disease of breast is secretion by keratinocytes of epidermal heregulin-α (neuregulin I) which induces chemotaxis of Her-2 positive malignant cells to epidermis [26]. Overexpression of Her-2 is noted on the membrane and in the cytoplasm of approximately 25% and 66% of colorectal cancer cases, respectively [27] and is also reported on colorectal adenomas [28]. Similar to breast, Her-2 positive malignant cells of colorectal adenocarcinoma or tubular adenoma may migrate toward the anal mucosa or perianal epidermis due to chemotaxis in perianal EMPD.
Conclusion
We report an exceedingly rare case of anal Paget’s disease associated with adjacent precursor lesion (tubular adenoma) with no overt invasive lesion. Awareness of this association may alert endoscopists and pathologists of the need to pay close attention to adjacent anal mucosa when an adenomatous lesion of the rectum is encountered. Immunohistochemistry can aid in rendering a correct diagnosis and in distinguishing primary from secondary EMPD, a distinction of important therapeutic and prognostic significance.
Grant Support
None.
Financial Disclosure
None.
| References | ▴Top |
- Paget J. On disease of mammary areola preceding cancer of the mammary gland. St Barth Hosp Rep. 1874;10:87-89.
- Crocker HR. Paget's disease affecting the scrotum and penis. Trans Pathol Soc Lond. 1889;40:187-191.
- Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget's disease. BJOG. 2005;112(3):273-279.
doi pubmed - Heymann WR. Extramammary Paget's disease. Clin Dermatol. 1993;11(1):83-87.
doi - Bontinck H, Bontinck J, Rondou T, Pattyn P, Lockefeer F. Perianal Paget's disease: case report and review of the literature. Acta Chir Belg. 2016:1-6.
doi - Ohnishi T, Watanabe S. The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget's disease. Br J Dermatol. 2000;142(2):243-247.
doi pubmed - Goldblum JR, Hart WR. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22(2):170-179.
doi - Ishibuchi H, Shimizu A, Negishi I, Ishikawa O. A perianal erythematous plaque: a quiz. Secondary extramammary Paget's disease from adenocarcinoma of the anorectal region. Acta Derm Venereol. 2014;94(4):492-493.
doi pubmed - Sarmiento JM, Wolff BG, Burgart LJ, Frizelle FA, Ilstrup DM. Paget's disease of the perianal region - an aggressive disease? Dis Colon Rectum. 1997;40(10):1187-1194.
doi pubmed - Moller MG, Lugo-Baruqui JA, Milikowski C, Salgado CJ. Staged marginal contoured and central excision technique in the surgical management of perianal Paget's disease. Am J Surg. 2014;207(4):485-492.
doi pubmed - Perez DR, Trakarnsanga A, Shia J, Nash GM, Temple LK, Paty PB, Guillem JG, et al. Management and outcome of perianal Paget's disease: a 6-decade institutional experience. Dis Colon Rectum. 2014;57(6):747-751.
doi pubmed - Beck DE, Fazio VW. Perianal Paget's disease. Dis Colon Rectum. 1987;30(4):263-266.
doi - Armitage NC, Jass JR, Richman PI, Thomson JP, Phillips RK. Paget's disease of the anus: a clinicopathological study. Br J Surg. 1989;76(1):60-63.
doi pubmed - Lian P, Gu WL, Zhang Z, Cai GX, Wang MH, Xu Y, Sheng WQ, et al. Retrospective analysis of perianal Paget's disease with underlying anorectal carcinoma. World J Gastroenterol. 2010;16(23):2943-2948.
doi pubmed - McCarter MD, Quan SH, Busam K, Paty PP, Wong D, Guillem JG. Long-term outcome of perianal Paget's disease. Dis Colon Rectum. 2003;46(5):612-616.
doi pubmed - Arminski TC, Pollard RJ. Paget's disease of the anus secondary to a malignant papillary adenoma of the rectum. Dis Colon Rectum. 1973;16(1):46-55.
doi - Carbotta G, Sallustio P, Prestera A, Laforgia R, Lobascio P, Palasciano N. Perineal Paget's disease: A rare disorder and review of literature. Ann Med Surg (Lond). 2016;9:50-52.
doi pubmed - Kyriazanos ID, Stamos NP, Miliadis L, Noussis G, Stoidis CN. Extra-mammary Paget's disease of the perianal region: a review of the literature emphasizing the operative management technique. Surg Oncol. 2011;20(2):e61-71.
doi pubmed - Minicozzi A, Borzellino G, Momo R, Steccanella F, Pitoni F, de Manzoni G. Perianal Paget's disease: presentation of six cases and literature review. Int J Colorectal Dis. 2010;25(1):1-7.
doi pubmed - Nowak MA, Guerriere-Kovach P, Pathan A, Campbell TE, Deppisch LM. Perianal Paget's disease: distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch Pathol Lab Med. 1998;122(12):1077-1081.
pubmed - Shepherd NA. Anal intraepithelial neoplasia and other neoplastic precursor lesions of the anal canal and perianal region. Gastroenterol Clin North Am. 2007;36(4):969-987, ix.
doi pubmed - De Nisi MC, D'Amuri A, Toscano M, Lalinga AV, Pirtoli L, Miracco C. Usefulness of CDX2 in the diagnosis of extramammary Paget disease associated with malignancies of intestinal type. Br J Dermatol. 2005;153(3):677-679.
doi pubmed - Val-Bernal JF, Pinto J. Pagetoid dyskeratosis is a frequent incidental finding in hemorrhoidal disease. Arch Pathol Lab Med. 2001;125(8):1058-1062.
pubmed - Yoon SN, Park IJ, Kim HC, Yu CS, Lee MW, Koh JK, Kim JW, et al. Extramammary Paget's disease in Korea: its association with gastrointestinal neoplasms. Int J Colorectal Dis. 2008;23(11):1125-1130.
doi pubmed - Dixon AR, Galea MH, Ellis IO, Elston CW, Blamey RW. Paget's disease of the nipple. Br J Surg. 1991;78(6):722-723.
doi pubmed - Schelfhout VR, Coene ED, Delaey B, Thys S, Page DL, De Potter CR. Pathogenesis of Paget's disease: epidermal heregulin-alpha, motility factor, and the HER receptor family. J Natl Cancer Inst. 2000;92(8):622-628.
doi pubmed - Kruszewski WJ, Rzepko R, Ciesielski M, Szefel J, Zielinski J, Szajewski M, Jasinski W, et al. Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers. 2010;29(5):207-212.
doi pubmed - Terzuoli L, Carlucci F, Martino AD, Frosi B, Porcelli B, Minacci C, Vernillo R, et al. Determination of p185 and adenylosuccinate lyase (ASL) activity in preneoplastic colon lesions and intestinal mucosa of human subjects. Clin Biochem. 1998;31(7):523-528.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


