| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 4, August 2024, pages 159-174
Effect of Pemafibrate on the Lipid Profile, Liver Function, and Liver Fibrosis Among Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease
Mona Hassana, Hasan Al-Obaidib, Megan Karricka, Nooraldin Merzaa, Yusuf Nawrasc, Omar Saabd, Ahmed Dheyaa Al-Obaidie, l, Fatima Merzaf, Hashim Talib Hashimg, Khalid Al Zubaidih, Daniah Al-Sabbaghi, Rand Matbachie, Zainab Noorih, Hajra Amatul-Raheemj, Sarmad Mansura, Omer Al Najafik, Marwah Algodii, Tamarah Al Hamdanyf, Abdallah Kobeissya
aGastroenterology and Hepatology Department, The University of Toledo, Toledo, OH, USA
bInternal Medicine Department, Jamaica Hospital Medicine Center, Queens, NY, USA
cUniversity of Toledo College of Medicine and Life Science, Toledo, OH, USA
dInternal Medicine Department, Cleveland Clinic, Cleveland, OH, USA
eInternal Medicine Department, College of Medicine, University of Baghdad, Baghdad, Iraq
fDepartment of Health and Human Services, University of Michigan, Dearborn, MI, USA
gCollege of Medicine, University of Warith Al-Anbiyaa, Karbala, Iraq
hInternal Medicine Department, University of Al-Mostansiryah College of Medicine, Baghdad, Iraq
iInternal Medicine Department, Al-Kindy College of Medicine, University of Baghdad, Baghdad, Iraq
jDeccan College of Medical Sciences, Hyderabad, Telangana, India
kInternal Medicine Department, Zucker School of Medicine, Northwell Health at Mather Hospital, Hempstead, NY, USA
lCorresponding Author: Ahmed Dheyaa Al-Obaidi, Internal Medicine Department, College of Medicine, University of Baghdad, Baghdad 10053, Iraq
Manuscript submitted June 20, 2024, accepted July 29, 2024, published online August 31, 2024
Short title: Impact of Pemafibrate in NAFLD Patients
doi: https://doi.org/10.14740/gr1750
| Abstract | ▴Top |
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) are prevalent conditions linked to obesity and metabolic disturbances, with potential complications such as cirrhosis and cardiovascular risks. This systematic review and meta-analysis aimed to evaluate the efficacy of pemafibrate, a drug targeting fat and sugar metabolism genes, in treating patients with MASLD/MASH.
Methods: Databases such as MEDLINE, Web of Science, Cochrane Library, and Scopus were searched until September 2023 to identify relevant studies. Selected studies underwent a thorough quality assessment using tools like Risk of Bias 2 tool (ROB-2) and the National Institutes of Health (NIH) Quality Assessment Tools. Comprehensive meta-analysis software was used for statistical evaluations, with a focus on lipid profiles, liver function tests, and fibrosis measurements.
Results: A total of 13 studies were included; 10 of them were included in the quantitative analysis. Our findings showed that pemafibrate significantly decreased low-density lipoprotein cholesterol (LDL-C) (effect size (ES) = -9.61 mg/dL, 95% confidence interval (CI): -14.15 to -5.08), increased high-density lipoprotein cholesterol (HDL-C) (ES = 3.15 mg/dL, 95% CI: 1.53 to 4.78), and reduced triglycerides (TG) (ES = -85.98 mg/dL, 95% CI: -96.61 to -75.36). Additionally, pemafibrate showed a marked reduction in liver enzyme levels, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), and alkaline phosphatase (ALP), with significant effect sizes and P values. For liver stiffness outcomes, pemafibrate decreased AST to platelet ratio index (APRI) (ES = -0.180, 95% CI: -0.221 to -0.138).
Conclusions: Pemafibrate, with its enhanced efficacy and safety profile, presents as a pivotal agent in MASLD/MASH treatment. Its lipid-regulating properties, coupled with its beneficial effects on liver inflammation markers, position it as a potentially invaluable therapeutic option.
Keywords: MASLD; MASH; Pemafibrate; PPAR; Lipid profile; Liver fibrosis; Liver stiffness
| Introduction | ▴Top |
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a condition where excess fat accumulates in the liver, which is detectable via tissue biopsy or imaging. This hepatic fat accumulation is not due to other factors like heavy alcohol intake, hepatitis B/C, or drug use [1]. MASLD can be divided into two categories: one, where there is only fat accumulation without any liver damage, and the other, metabolic dysfunction-associated steatohepatitis (MASH), which is defined by fat accumulation, inflammation, and liver cell damage [2, 3]. Commonly linked to conditions like obesity, diabetes, high cholesterol, and high blood pressure, MASLD is also a recognized component of metabolic syndrome [2, 4]. With obesity on the rise globally, the number of MASLD/MASH cases is growing, with roughly 20-30% and 2-6% of the global population being affected, respectively [5]. Serious complications can arise from MASLD/MASH, such as cirrhosis, liver cancer, and even a heightened risk of heart-related incidents [4, 6]. Addressing MASLD/MASH primarily involves lifestyle changes emphasizing diet and exercise for weight reduction [4]. Yet, sustaining such changes can be challenging for many.
While vitamin E and pioglitazone show promise in treating some aspects of MASH, they are not officially approved for its treatment [7]. The potential in treating MASH may lie with peroxisome proliferator-activated receptors (PPARs), which play a role in managing fat and cholesterol in the bloodstream [8]. PPARα’s role, in particular, has shown potential therapeutic relevance in MASH [9]. One drug, pemafibrate, a selective PPARα modulator, already approved in Japan for treating high triglycerides (TG) [10, 11], is under investigation in a vast international trial named PROMINENT (ClinicalTrials.gov identifier: NCT03071692) to determine its impact on cardiovascular events. Pemafibrate acts on certain genes affecting liver fat and sugar metabolism. It has been shown to positively influence energy metabolism and improve various MASH indicators in animal studies [12]. In prior clinical trials involving dyslipidemia patients, pemafibrate not only effectively decreased TG levels but also benefited other liver markers, including alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) [8]. However, the specific effect of pemafibrate on MASLD/MASH, especially when compared to a placebo using advanced imaging measures beyond standard lab tests, remains inadequately explored. A few studies have investigated the effect of pemafibrate in patients with MASLD/MASH [8, 13-16]; however, some of them reported conflicting results. Therefore, in this systematic review and meta-analysis, we aimed to summarize the current evidence regarding the efficacy of pemafibrate in patients with MASLD/MASH.
| Materials and Methods | ▴Top |
Institutional Review Board approval and ethical compliance with human study regulations are not applicable to this research.
Inclusion criteria
Inclusion criteria were: 1) Population: patients with MASLD and/or liver dysfunction (studies including patients diagnosed with MASLD or non-alcoholic fatty liver disease (NAFLD); additionally, studies involving patients with liver dysfunction where MASLD is a confirmed or likely contributing factor); 2) Exposure: studies that include patients who received pemafibrate; 3) Outcomes: studies that assessed liver function tests, lipid profiles, and/or fibrosis measurements, and studies that assessed liver function tests, lipid profile, and/or fibrosis measurements, specifically including tests such as AST to platelet ratio index (APRI), fibrosis-4 index (FIB-4), ALP, low-density lipoprotein cholesterol (LDL-C), AST, ALT, and GGT; 4) Study design: randomized controlled trials (RCTs) and observational studies, including prospective and retrospective cohort studies, case-control studies, and cross-sectional studies.
Exclusion criteria
Exclusion criteria were: 1) Non-MASLD liver dysfunction: studies where liver dysfunction is attributed to causes other than MASLD, such as viral hepatitis, alcoholic liver disease, or drug-induced liver injury; 2) Study type: non-English studies, reviews, animal studies, abstracts, and case reports; 3) Lack of treatment evaluation: studies that do not evaluate or provide data on treatments specifically aimed at MASLD or NAFLD.
Information sources and search strategy
Historically, “NAFLD” has been the standard term used to describe liver fat accumulation not attributable to alcohol consumption. This includes a spectrum from simple steatosis to more severe forms like non-alcoholic steatohepatitis (NASH). Recently, there has been a shift towards using “MASLD” to better reflect the metabolic underpinnings of liver fat accumulation. This term emphasizes that liver steatosis is closely linked with metabolic dysfunctions such as obesity, type 2 diabetes, and dyslipidemia. The search strategy was designed accordingly.
A computerized search from inception to September 2023 was conducted on MEDLINE via PubMed, Web of Science, Cochrane Library, and Scopus. We used the following keywords to identify the relevant citations: ((“pemafibrate” OR “K-877” OR “pemafibrate sodium” OR “fenofibrate derivative”) AND (“metabolic associated steatotic liver disease” OR “MASLD” OR “metabolic associated steatohepatitis” OR “MASH” OR “hepatic steatosis” OR “fatty liver” OR “metabolic liver disease”)).
Selection process
Following the database searches, all citations were imported into the EndNote X9 Windows version. Duplicate references resulting from the overlap of database content were identified and removed. Two independent reviewers (XX and YY) screened the titles and abstracts of all unique citations according to the predefined inclusion and exclusion criteria. Any disagreements between the two reviewers at this stage were resolved through discussion, or, if necessary, a third reviewer (XY) was consulted. Studies that appeared to meet the inclusion criteria, or for which there was insufficient information in the title and abstract to make a clear decision, were advanced to full-text review. Again, two independent reviewers (XX and YY) assessed each full-text article to determine its eligibility. Disagreements at this stage were resolved through consultation with a third reviewer (YY). The reference lists of all included studies were scanned to identify additional studies that might have been missed during the initial database searches. Any potentially relevant studies identified through this process were subjected to full-text review and included if they met the criteria.
Data collection process and data items
For studies that met the inclusion criteria, relevant data were extracted using a standardized data extraction form. This form was piloted on a subset of included studies and refined as needed. We extracted data regarding the study characteristics (study ID, duration, sample size, inclusion criteria, and conclusion), patient characteristics (age and gender, body mass index, and baseline lipids and liver function tests), and outcomes, including lipid profile (LDL-C, high-density lipoprotein cholesterol (HDL-C), and TG), liver function tests (AST, ALT, ALP, GGT), and fibrosis measurements (FIB-4 and APRI score).
Quality assessment
The evaluation of study quality and potential bias was conducted using the Risk of Bias 2 tool (ROB-2) developed by the Cochrane Collaboration [17]. The domains studied involved a randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was meticulously evaluated to determine the extent of bias that could potentially influence study outcomes. A clear and structured approach was adopted to rate the risk of bias as either “low”, “some concerns”, or “high” for each individual domain, subsequently contributing to an overall judgment on the study’s risk of bias. For cross-sectional studies, we employed the National Institutes of Health (NIH) Quality Assessment Tools [18].
Data synthesis
Statistical analyses were performed using the Comprehensive Meta-Analysis (CMA) software version 4. We performed a single-arm meta-analysis using the DerSimonian-Laird random-effects model to estimate the effect size (ES) of the studied outcomes. Data were reported as pooled mean with the corresponding 95% confidence intervals (CIs). Publication bias was meticulously examined using funnel plots, Egger’s test, and the Begg-Mazumdar test.
| Results | ▴Top |
The comprehensive literature search across multiple databases yielded a total of 188 citations. Upon deduplicating these entries, 120 studies were retained for title and abstract assessment. This initial screening led to the exclusion of 104 studies. A subsequent in-depth review of the full texts was conducted for 16 articles, resulting in the final selection of 13 studies for qualitative synthesis [8, 13-16, 19-25]. Out of these, 10 studies were deemed suitable for quantitative synthesis [8, 13-16, 21-24, 26]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is presented in Figure 1.
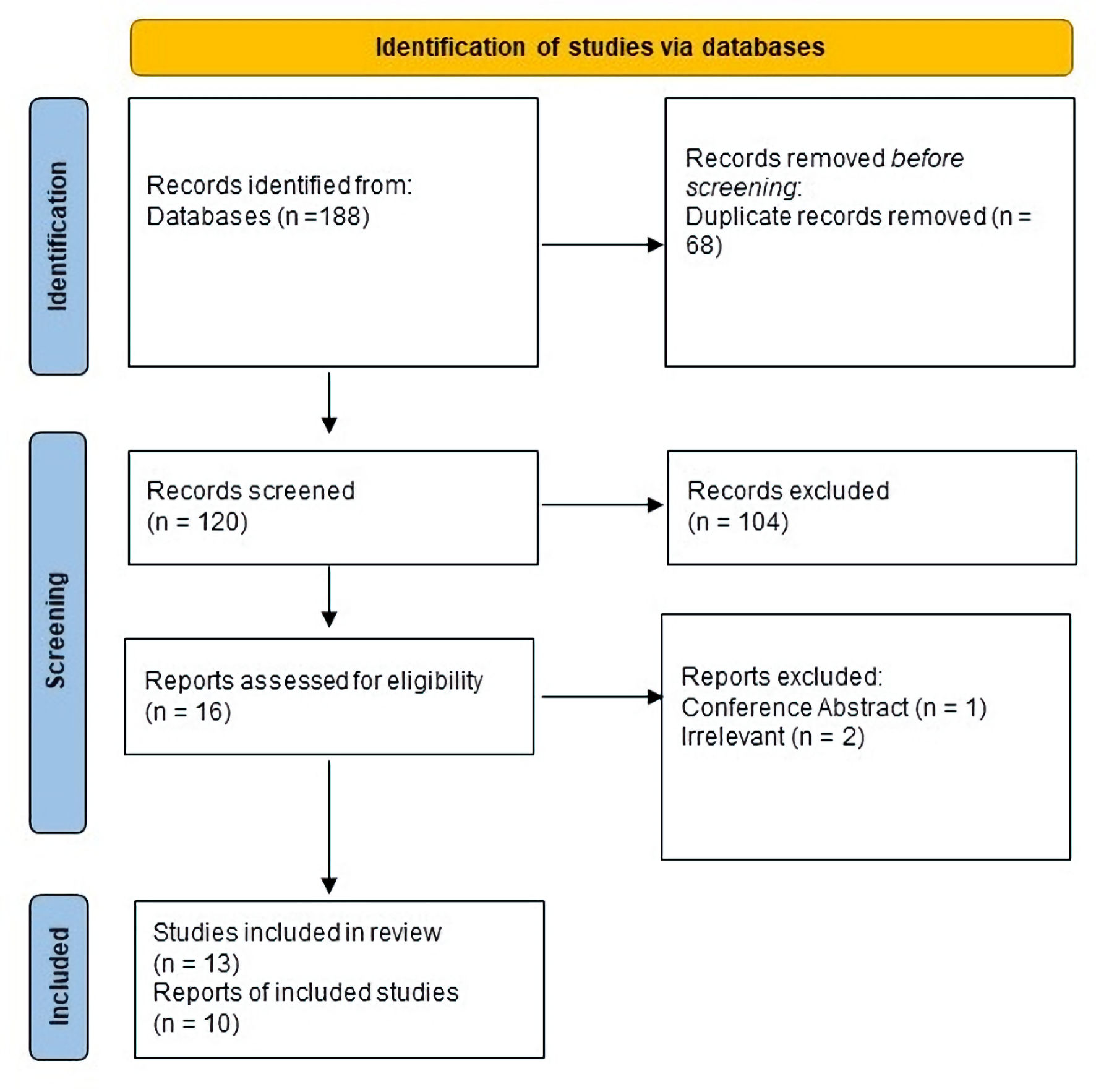 Click for large image | Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Study characteristics
Out of the included 13 studies (n = 690 patients), 12 studies were retrospective [13-15, 19-27], and only one study was a RCT [16]. The retrospective studies studied pemafibrate as a single arm, while the RCT study compared it with a placebo group. All the included studies were conducted in Japan between 2017 and 2022. Most of the included patients had MASLD and dyslipidemia, with a male predominance (60%) and a mean age of 57.93 years. The used dose for pemafibrate was 0.1 - 0.2 mg oral twice a day (BID). The average duration of follow-up was 12 months. Tables 1 and 2 [13-16, 19-27] summarize the characteristics of the included studies and patients’ baseline, respectively.
 Click to view | Table 1. Summary of Included Studies [13-16, 19-27] |
 Click to view | Table 2. Baseline Characteristics of Included Studies |
Risk of bias in studies
Based on the NIH tool for the risk of bias in observational studies, only four studies had good quality, and eight studies had fair quality. In terms of the ROB-2 tool, the risk of bias was deemed as low in the study of Nakajima et al [16]. The details of the risk of bias assessment are shown here (Supplementary Material 1, www.gastrores.org).
Lipid profile outcomes
LDL-C
The random effects estimate of 10 studies showed that pemafibrate significantly reduced the LDL-C (ES = -9.61 mg/dL, 95% CI: -14.15 to -5.08, P < 0.001). A significant reduction was observed from 6-12 months of pemafibrate (ES = -10.89 mg/dL, 95% CI: -16.80 to -4.98, P < 0.001). In terms of 0 - 6 months and ≥ 2 years, the reduction was not significant, as shown in Figure 2a. The pooled data were moderately heterogeneous (Q = 27.58, I2 = 52.86%, P = 0.010). The visualization of the funnel plot showed no effect of small studies (Fig. 2b). The Egger’s regression and Begg-Mazumdar test showed no evidence of publication bias (P = 0.360 and P = 0.228, respectively).
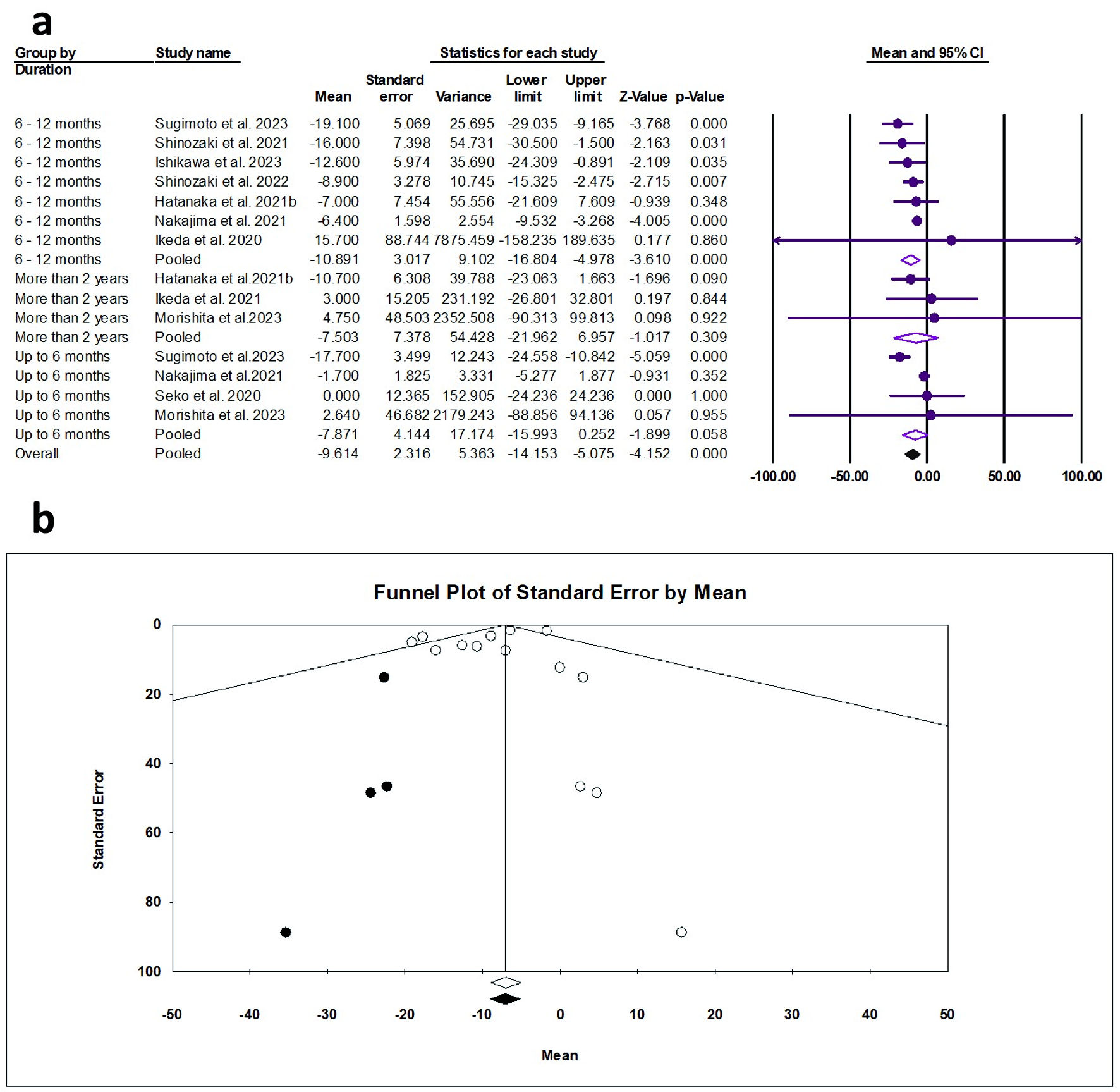 Click for large image | Figure 2. (a) Forest plot of LDL-C. (b) Funnel plot. LDL-C: low-density lipoprotein cholesterol; CI: confidence interval. |
HDL-C
The random effects estimate of nine studies showed that pemafibrate significantly increased the HDL-C (ES = 3.15 mg/dL, 95% CI: 1.53 to 4.78, P < 0.001). A significant elevation was observed at the duration of 0 - 6 months (ES = 3.30 mg/dL, 95% CI: 0.39 to 6.23, P = 0.027), 6 - 12 months (ES = 2.67 mg/dL, 95% CI: 0.57 to 4.76, P = 0.012), and ≥ 2 years (ES = 5.96 mg/dL, 95% CI: 0.49 to 11.47, P = 0.033), as shown in Figure 3. The pooled data were homogenous (Q = 4.28, I2 = 0.00%, P = 0.961).
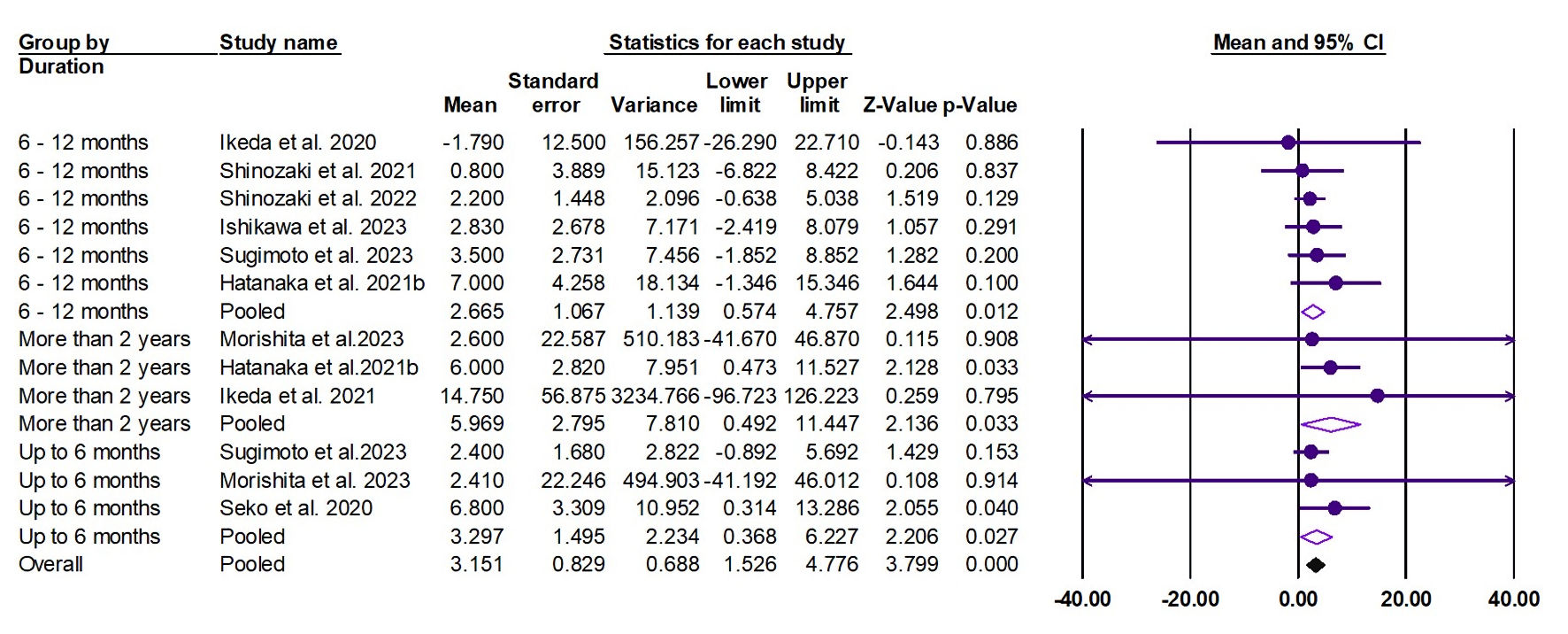 Click for large image | Figure 3. Forest plot of HDL-C. HDL-C: high-density lipoprotein cholesterol; CI: confidence interval. |
TG
The random effects estimate of eight studies showed that pemafibrate significantly reduced the TG (ES = -85.98 mg/dL, 95% CI: -96.61 to -75.36, P < 0.001). A significant reduction was observed at the duration of 0 - 6 months (ES = -113.40 mg/dL, 95% CI: -138.07 to -88.73, P < 0.001), 6 - 12 months (ES = -79.05 mg/dL, 95% CI: -93.33 to -64.76, P < 0.001), and ≥ 2 years (ES = -81.22 mg/dL, 95% CI: -101.99 to -60.43, P < 0.001), as shown in Figure 4. The pooled data were homogenous (Q = 12.31, I2 = 18.75%, P = 0.26).
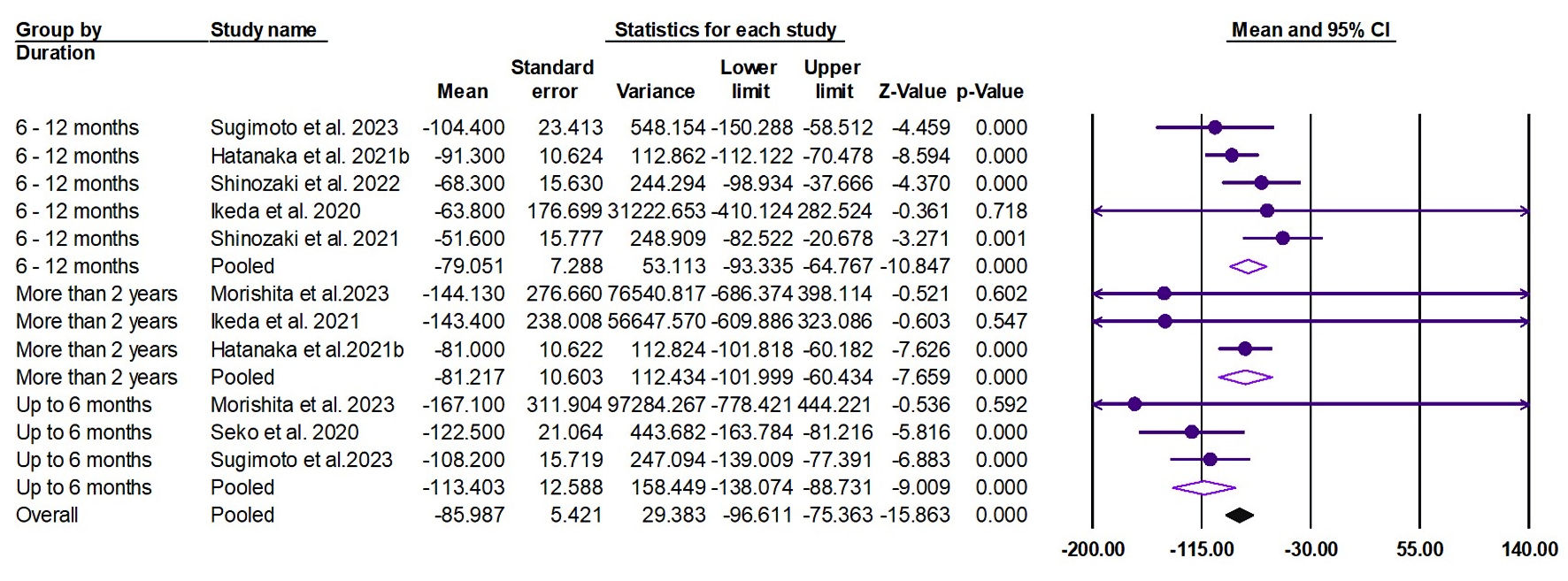 Click for large image | Figure 4. Forest plot of TG. CI: TG: triglycerides; confidence interval. |
Liver function outcomes
AST
The random effects estimate of 10 studies showed that pemafibrate significantly reduced the AST (ES = -9.12 U/L, 95% CI: -12.39 to -5.86, P < 0.001). A significant reduction was observed at the duration of 0 - 6 months (ES = -11.31 U/L, 95% CI: -17.79 to -4.82, P = 0.001) and 6 - 12 months (ES = -8.97 U/L, 95% CI: -13.02 to -4.92, P < 0.001), as shown in Figure 5a. The pooled data showed mild heterogeneity (Q = 20.49, I2 = 36.56%, P = 0.084). The funnel plot showed significant asymmetry (Fig. 5b); however, the Egger’s regression and Begg-Mazumdar test showed no evidence of publication bias (P = 0.053 and P = 1.00, respectively).
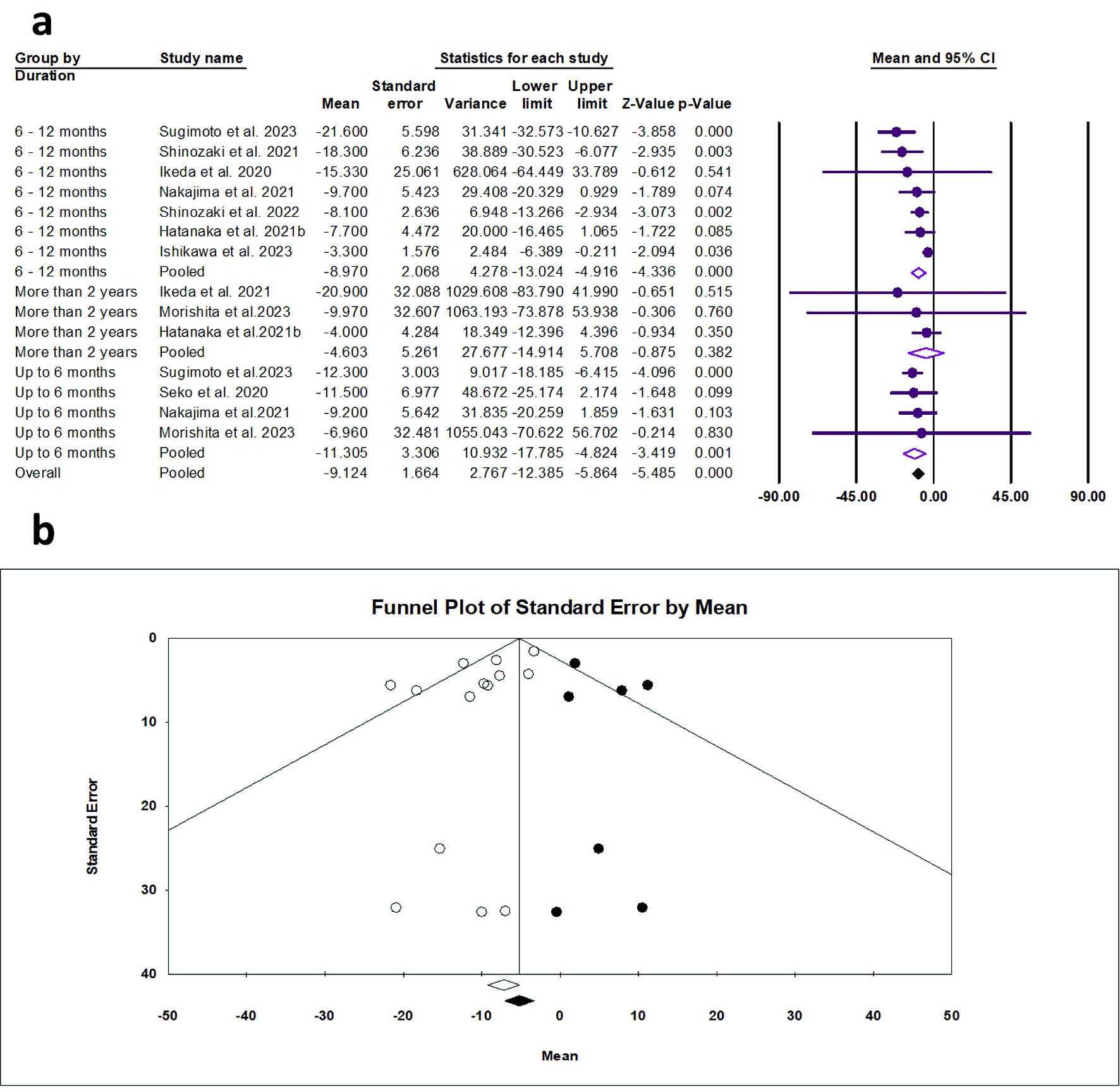 Click for large image | Figure 5. (a) Forest plot of AST. (b) Funnel plot. AST: aspartate aminotransferase; CI: confidence interval. |
ALT
The random effects estimate of 10 studies showed that pemafibrate significantly reduced the ALT (ES = -30.57 U/L, 95% CI: -36.00 to -25.13, P < 0.001). A significant reduction was observed at the duration of 0 - 6 months (ES = -35.45 U/L, 95% CI: -45.75 to -25.15, P = 0.005) and 6 - 12 months (ES = -31.04 U/L, 95% CI: -37.94 to -24.13, P < 0.001), as shown in Figure 6a. The pooled data showed substantial heterogeneity (Q = 35.69, I2 = 63.58%, P = 0.001). The funnel plot showed a mild asymmetry (Fig. 6b); however, the Egger’s regression and Begg-Mazumdar test showed no evidence of publication bias (P = 0.592 and P = 0.511, respectively).
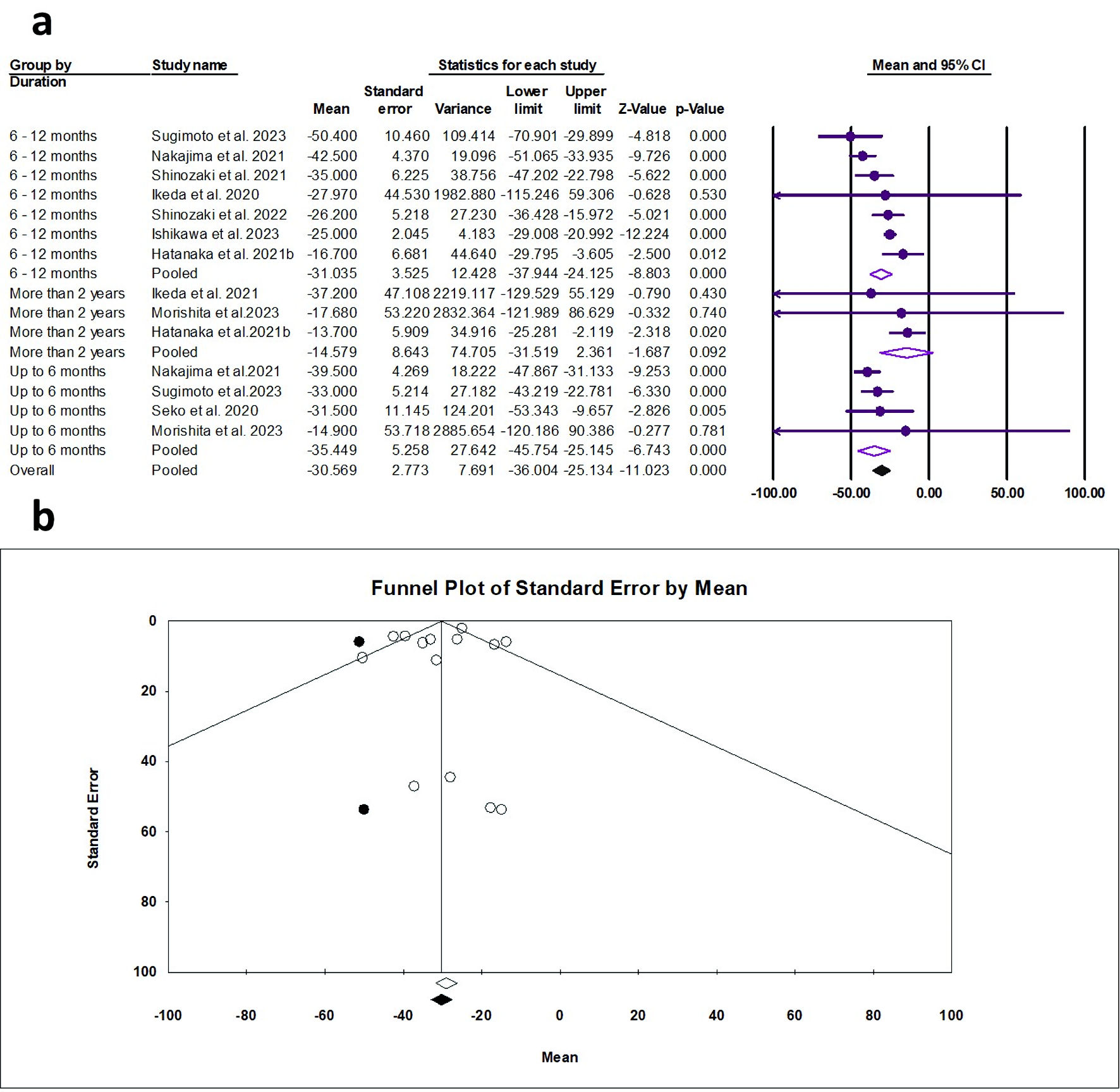 Click for large image | Figure 6. (a) Forest plot of ALT. (b) Funnel plot. ALT: alanine aminotransferase; CI: confidence interval. |
GGT
The random effects estimate of 10 studies showed that pemafibrate significantly reduced the ALT (ES = -37.63 U/L, 95% CI: -48.46 to -26.81, P < 0.001). A significant reduction was observed at the duration of 0 - 6 months (ES = -47.72 U/L, 95% CI: -68.37 to -27.08, P < 0.001) and 6 - 12 months (ES = -34.22 U/L, 95% CI: -48.46 to -26.81, P < 0.001), as shown in Figure 7a. The pooled data showed substantial heterogeneity (Q = 35.69, I2 = 85.00%, P = 0.001). The funnel plot showed a moderate asymmetry (Fig. 7b); however, the Egger’s regression and Begg-Mazumdar test showed no evidence of publication bias (P = 0.985 and P = 0.827, respectively).
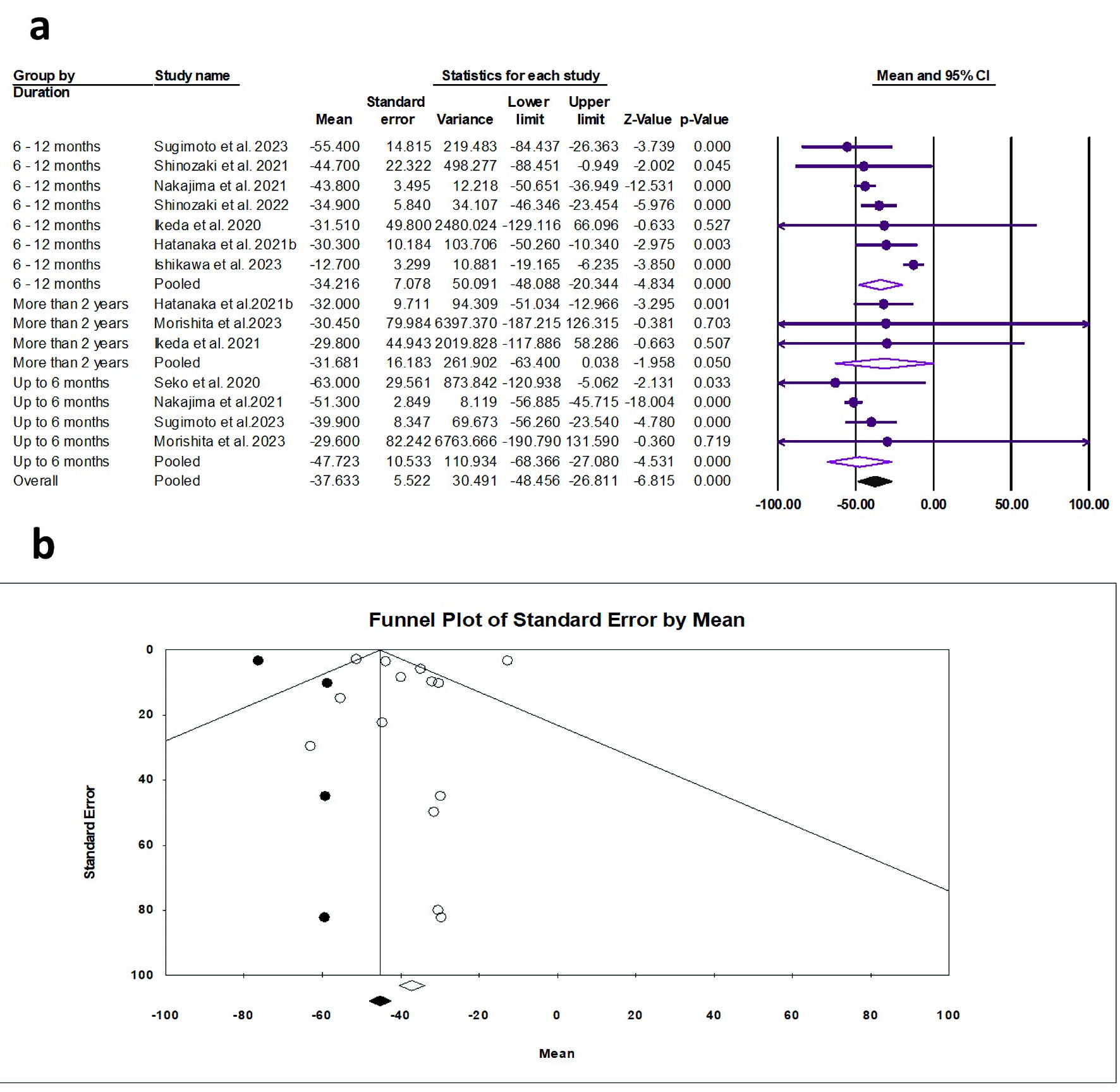 Click for large image | Figure 7. (a) Forest plot of γ-GTP. (b) Funnel plot. γ-GTP: gamma-glutamyl transferase; CI: confidence interval. |
ALP
The random effects estimate of four studies showed that pemafibrate significantly reduced the ALP (ES = -88.61 U/L, 95% CI: -108.62 to -68.61, P < 0.001), as shown in Figure 8. The pooled data were homogenous (Q = 2.61, I2 = 0.0%, P = 0.456).
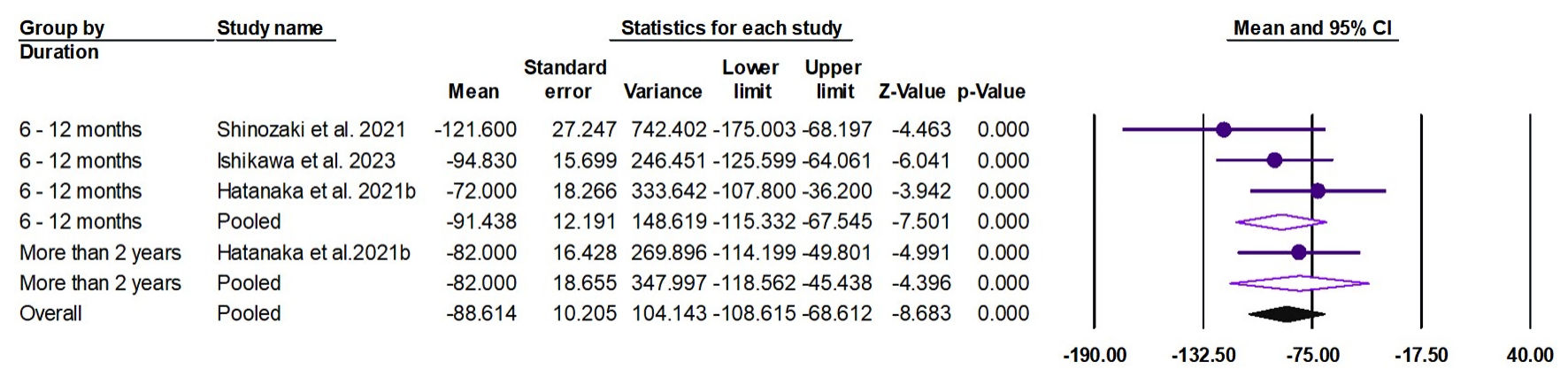 Click for large image | Figure 8. Forest plot of ALP. ALP: alkaline phosphatase; CI: confidence interval. |
Liver stiffness outcomes
FIB-4
The random effects estimate of nine studies showed that pemafibrate reduced the FIB-4 (ES = -0.136, 95% CI: -0.272 to 0.00, P = 0.049), as shown in Figure 9. The pooled data were homogenous (Q = 0.735, I2 = 0.0%, P = 1.00).
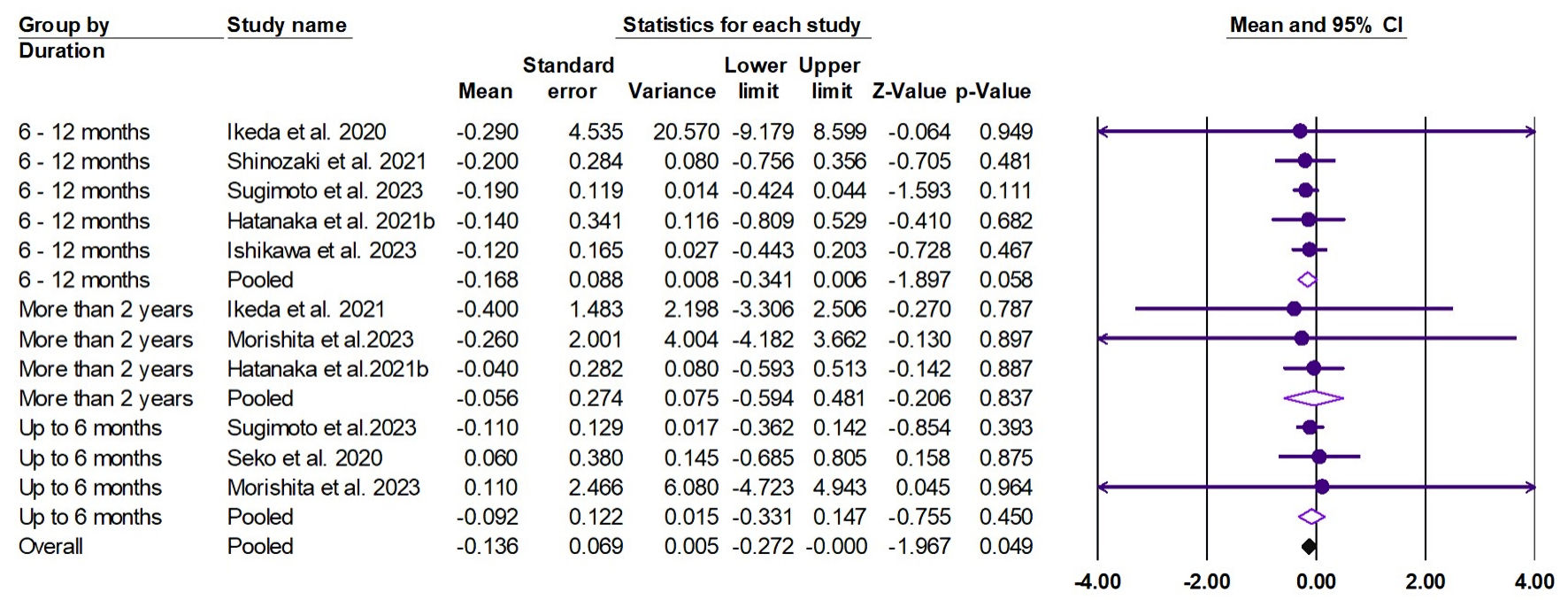 Click for large image | Figure 9. Forest plot of FIB-4. FIB-4: fibrosis-4 index; CI: confidence interval. |
APRI
The random effects estimate of five studies showed that pemafibrate significantly reduced the APRI (ES = -0.180, 95% CI: -0.221 to -0.138, P < 0.001). A significant reduction was observed at the duration of 6 - 12 months (ES = -0.181, 95% CI: -0.224 to -0.137, P < 0.001) and more than 2 years (ES = -0.171, 95% CI: -0.318 to -0.024, P = 0.023), as shown in Figure 10. The pooled data were homogenous (Q = 0.810, I2 = 0.0%, P = 0.992).
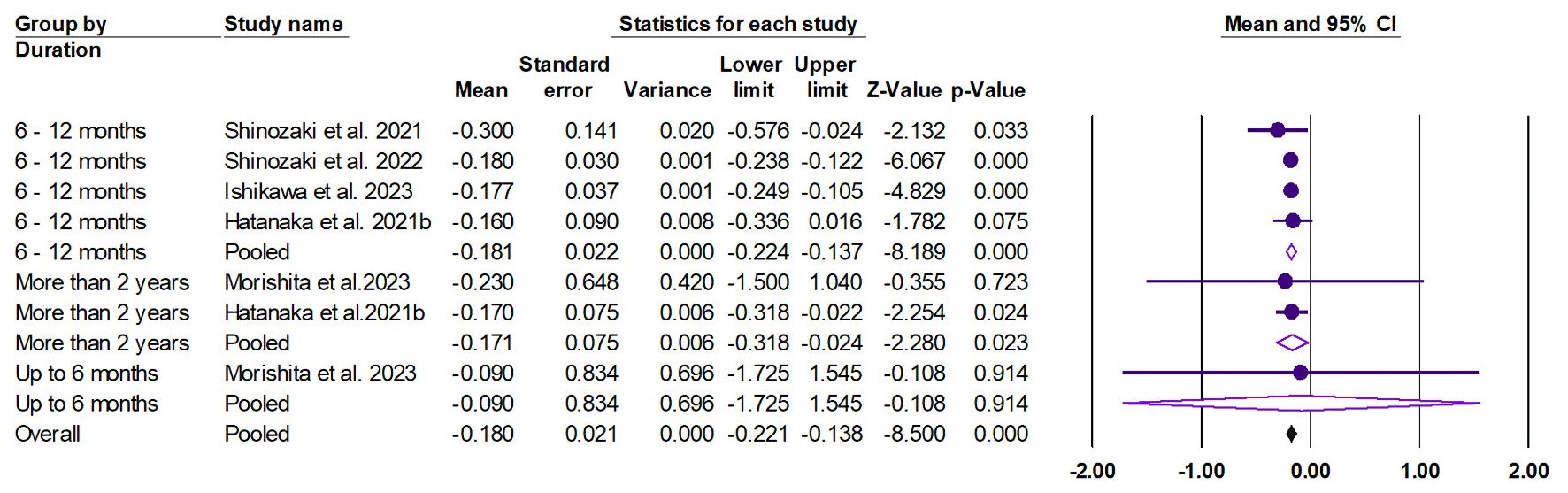 Click for large image | Figure 10. Forest plot of APRI. APRI: aspartate aminotransferase to platelet ratio index; CI: confidence interval. |
| Discussion | ▴Top |
Pemafibrate, designed as a selective PPARα modulator, boasts enhanced selectivity, a more potent TG-reducing effect, and a superior safety profile, showcasing fewer side effects such as liver dysfunction and increased creatine kinase compared to traditional fibrate formulations [8, 10, 11, 28]. While it is particularly potent in addressing hypertriglyceridemia, a deeper dive into clinical trial data indicates its beneficial role in liver function enhancement. Interestingly, pemafibrate has been linked to improved liver elasticity when assessed using magnetic resonance elastography (MRE). However, a local phase II trial focusing on MASLD revealed that pemafibrate did not significantly reduce liver fat content [16]. Nonetheless, when tested on a diet-induced rodent model of MASH, pemafibrate demonstrated its potential to ameliorate MASH’s pathogenesis, altering lipid metabolism and energy processing in the liver more effectively than fenofibrate [12]. In this systematic review and meta-analysis, our findings showed that pemafibrate showed a significant improvement in patients with MASLD/MASH in lipid profile by reducing LDL-C and TG and increasing HDL-C. Additionally, the liver function tests showed a significant improvement in terms of ALT, AST, GGT, and ALP. In terms of FIB-4, the improvement was marginal; however, the APRI score showed substantial improvement. These findings underscore the potential of pemafibrate as an efficacious treatment option for patients with MASLD/MASH.
Pemafibrate selectively regulates target genes involved in lipid metabolism among these PPARα-regulated genes [10]. It also upregulates the expression of uncoupling protein 3 (UCP3) in the liver. UCP3 plays a role in energy metabolism and lipid turnover, contributing to improved lipid profiles. Moreover, pemafibrate also increases acyl-coenzyme A oxidase-1 level [29], further stimulating lipid turnover and energy utilization. While the majority of the included studies noted a rise in HDL-C due to pemafibrate [15, 19-24, 26, 27], Nakajima et al observed a decline in HDL-C [16]. This outcome might be attributed to a simultaneous decrease in cholesterol in larger HDL particles and an increase in smaller HDL particles. Such a shift is potentially beneficial given the pivotal role smaller HDL particles have in the reverse cholesterol transport system [30]. Prior foundational and clinical studies have explored the positive impacts of pemafibrate on this transport system [31, 32]. For patients with MASLD/MASH, these lipid-regulating properties of pemafibrate offer substantial clinical benefits. MASLD and MASH, which are intrinsically associated with metabolic dysfunctions, often present with elevated LDL-C and TG levels and decreased HDL-C, all of which contribute to progressive liver damage and increased cardiovascular risk [6]. By normalizing these lipid levels, pemafibrate not only addresses the underlying metabolic disturbances but also potentially slows the progression of liver disease and reduces the associated cardiovascular risk in this population.
In patients with MASLD/MASH, reducing levels of AST, ALT, GGT, and ALP has substantial clinical implications [33]. Elevated levels of these enzymes often signal liver damage or inflammation, with ALT and AST being directly related to liver cell injury, GGT indicating possible bile duct damage, and ALP reflecting potential blockages in the bile ducts or damage to the liver cells [34]. Lowering these enzyme levels can not only indicate a reduction in liver inflammation and damage but also decrease the risk of disease progression to more severe stages, including cirrhosis or liver cancer [35]. Clinically, maintaining these enzymes within a normal range can enhance patient outcomes, prolong liver function, and reduce associated complications, emphasizing the importance of therapeutic interventions that target these markers in MASLD/MASH management [36].
Typically, a decrease in serum ALT and AST levels serves as an indicator of improved histological inflammation in patients diagnosed with MASH through biopsy [37]. Further, Argo et al found that the only predictive factor for fibrosis progression in subsequent biopsies was the presence of histological inflammation [38]. This evidence points towards the idea that a drop in serum ALT can be viewed as a representative marker for positive histological changes, encompassing both liver inflammation and fibrosis. In research conducted using MASH model mice, pemafibrate led to notable improvements in liver fibrosis, highlighted by a decrease in collagen 1α1 mRNA expression in the liver. Concurrently, there was a reduction in both the ALT level and the expression of genes linked to inflammation [12]. This suggests pemafibrate might boost liver health by mitigating inflammation and/or directly curbing liver fibrosis. Echoing findings from earlier clinical trials involving dyslipidemia patients [8], pemafibrate in the current study remarkably lowered serum levels of AST, ALT, GGT, and ALP. Given these outcomes, it is plausible to anticipate that pemafibrate might offer a more potent therapeutic effect against inflammation in MASLD/MASH.
In this study, we observed a decrease in the average values of both the APRI and FIB-4 index. Both of these measures incorporate platelet counts. Notably, several studies found that pemafibrate treatment notably elevated platelet counts [14, 20-22]. Beyond their role in hemostasis, platelets are also pivotal in inflammatory responses, angiogenesis, wound repair, and resolving inflammation [39]. They are understood to be instrumental in liver inflammation, significantly influencing the transition from simple fatty liver to MASH [40, 41]. Given the trends in other liver-related metrics, the rise in platelet counts is interpreted as a sign of liver inflammation resolution. This likely contributes to the substantial decrease seen in APRI and FIB-4 index values. The beneficial impact of pemafibrate on liver fibrosis has been corroborated by another research. Nakajima et al showed that pemafibrate demonstrated a significant reduction in liver stiffness assessed by MRE. In addition, a significant reduction was observed in fibrosis markers such as mac-2-binding protein glycosylation isomer (M2BPGi), hyaluronic acid, 7S domain of type IV collagen, and enhanced liver fibrosis (ELF) test [16]. These findings further confirm the clinical benefits of pemafibrate in terms of liver fibrosis.
Clinical implications
Pemafibrate emerges as a promising therapeutic agent for MASLD/MASH patients, addressing both lipid profile irregularities and liver function markers. Its ability to reduce LDL-C and TG and raise HDL-C, coupled with the normalization of liver enzyme levels, holds clinical significance. By addressing the inherent metabolic disturbances seen in MASLD/MASH, pemafibrate may not only mitigate liver disease progression but also counteract the heightened cardiovascular risk associated with these disorders. Its effect on platelet counts and liver inflammation underscores its multifaceted role in MASLD/MASH management.
PPAR modulators are metabolized primarily in the liver. In patients with decreased liver function, there is a potential risk of altered drug metabolism, leading to increased drug levels and possible adverse effects. We recommend cautious use of selective PPAR modulators in patients with liver impairment. Regular monitoring of liver function and potential adjustments in dosing are advised to mitigate risks. Future research should focus on understanding the safety profile of these agents in populations with liver dysfunction.
Future directions
Future studies should delve deeper into understanding the mechanisms underpinning pemafibrate’s lipid-regulating properties, particularly its interaction with smaller HDL particles and their role in the reverse cholesterol transport system. Moreover, extended-duration trials could elucidate any long-term impacts and potential unforeseen side effects of the drug. Investigations could also explore the combined effects of pemafibrate with other therapeutic agents to enhance its efficacy and address the broader spectrum of MASLD/MASH symptoms.
Limitations
This systematic review and meta-analysis, though comprehensive, has its constraints. The variation in trial durations, differences in study populations, and heterogeneity in the outcome measures across studies could introduce bias. Additionally, while pemafibrate’s positive impact on liver function is evident, the lack of significant reduction in liver fat content in certain trials warrants further exploration. The reliance on surrogate markers, like APRI and FIB-4 index, though indicative, does not replace the gold standard of liver biopsies in ascertaining histological improvements. A significant limitation of this study is the predominance of Japanese studies, which may not capture global variations in MASLD. Genetic differences among populations can affect disease prevalence and progression, highlighting the need for research across diverse geographic regions to better understand the global impact of MASLD.
Conclusions
Pemafibrate, with its enhanced selectivity and safety profile, presents as a pivotal agent in MASLD/MASH treatment. Its lipid-regulating properties, coupled with its beneficial effects on liver inflammation markers, position it as a potentially invaluable therapeutic option. While the findings are promising, more extended and diverse studies are essential to solidify its role in MASLD/MASH management and to further explore its long-term safety and efficacy.
| Supplementary Material | ▴Top |
Suppl 1. Risk of bias assessment.
Acknowledgments
None to declare
Financial Disclosure
This research did not receive any grant in the public, commercial or non-profit sector.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
Yusuf Nawras: writing - review and editing, project administration, data curation. Hasan Al-Obaidi: writing - review and editing, project administration, data curation. Nooraldin Merza: supervision, formal analysis, validation, investigation. Omar Saab: investigation, visualization, project administration, supervision. Khalid Al Zubaidi: investigation, visualization, project administration, supervision. Daniah Al-Sabbagh: writing - review and editing, writing abstract. Sarmed Mansur: writing - review and editing. Marwah Algodi: editing and data extraction. Omer Al Najafi: writing - review and editing. Rand Matbachi: writing - review and editing. Tamarah Al Hamdany: conceptualization, funding acquisition, writing - review and editing. Zainab Noori: conceptualization, funding acquisition, writing - review and editing. Abdallah Kobeissy: supervision, investigation, writing - original draft. Mona Hassan: supervision, investigation, writing - original draft. Megan Karrick: topic search, data extraction, writing discussion, and supervising the project. Ahmed Dheyaa Al-Obaidi: data extraction, writing the introduction, and editing the abstract. Fatima Merza: data extraction, drafting results, and abstract writing. Hashim Talib Hashim: data extraction, statistical analysis, and results writing. Hajra Amatul-Raheem: data extraction and method writing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22(1):63.
doi pubmed pmc - Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005-2023.
doi pubmed - Sharma B, John S. Non-alcoholic Steatohepatitis (NASH). Treasure Island (FL). 2023.
- Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le MD, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17(4):630-637.e638.
doi pubmed pmc - Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
doi pubmed - Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 2018;68(2):335-352.
doi pubmed - Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362-376.
doi pubmed pmc - Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new selective PPARalpha modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep. 2020;22(1):5.
doi pubmed pmc - Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, Lefebvre P, et al. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63(1):164-173.
doi pubmed - Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol. 2017;16(1):124.
doi pubmed pmc - Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720-733.
doi pubmed - Honda Y, Kessoku T, Ogawa Y, Tomeno W, Imajo K, Fujita K, Yoneda M, et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep. 2017;7:42477.
doi pubmed pmc - Ikeda S, Sugihara T, Hoshino Y, Matsuki Y, Nagahara T, Okano JI, Kitao S, et al. Pemafibrate dramatically ameliorated the values of liver function tests and fibrosis marker in patients with non-alcoholic fatty liver disease. Yonago Acta Med. 2020;63(3):188-197.
doi pubmed pmc - Ikeda S, Sugihara T, Kihara T, Matsuki Y, Nagahara T, Takata T, Kitao S, et al. Pemafibrate ameliorates liver dysfunction and fatty liver in patients with non-alcoholic fatty liver disease with hypertriglyceridemia: a retrospective study with the outcome after a mid-term follow-up. Diagnostics (Basel). 2021;11(12).
doi pubmed pmc - Hatanaka T, Kosone T, Saito N, Takakusagi S, Tojima H, Naganuma A, Takagi H, et al. Effect of 48-week pemafibrate on non-alcoholic fatty liver disease with hypertriglyceridemia, as evaluated by the FibroScan-aspartate aminotransferase score. JGH Open. 2021;5(10):1183-1189.
doi pubmed pmc - Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54(10):1263-1277.
doi pubmed pmc - Jorgensen L, Paludan-Muller AS, Laursen DR, Savovic J, Boutron I, Sterne JA, Higgins JP, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016;5:80.
doi pubmed pmc - Han S, Olonisakin TF, Pribis JP, Zupetic J, Yoon JH, Holleran KM, Jeong K, et al. A checklist is associated with increased quality of reporting preclinical biomedical research: A systematic review. PLoS One. 2017;12(9):e0183591.
doi pubmed pmc - Hatanaka T, Kakizaki S, Saito N, Nakano Y, Nakano S, Hazama Y, Yoshida S, et al. Impact of pemafibrate in patients with hypertriglyceridemia and metabolic dysfunction-associated fatty liver disease pathologically diagnosed with non-alcoholic steatohepatitis: a retrospective, single-arm study. Intern Med. 2021;60(14):2167-2174.
doi pubmed pmc - Shinozaki S, Tahara T, Lefor AK, Ogura M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin Exp Hepatol. 2020;6(3):270-274.
doi pubmed pmc - Seko Y, Yamaguchi K, Umemura A, Yano K, Takahashi A, Okishio S, Kataoka S, et al. Effect of pemafibrate on fatty acid levels and liver enzymes in non-alcoholic fatty liver disease patients with dyslipidemia: A single-arm, pilot study. Hepatol Res. 2020;50(12):1328-1336.
doi pubmed - Shinozaki S, Tahara T, Lefor AK, Ogura M. Pemafibrate improves hepatic inflammation, function and fibrosis in patients with non-alcoholic fatty liver disease: a one-year observational study. Clin Exp Hepatol. 2021;7(2):172-177.
doi pubmed pmc - Shinozaki S, Tahara T, Miura K, Lefor AK, Yamamoto H. Pemafibrate therapy for non-alcoholic fatty liver disease is more effective in lean patients than obese patients. Clin Exp Hepatol. 2022;8(4):278-283.
doi pubmed pmc - Sugimoto R, Iwasa M, Eguchi A, Tamai Y, Shigefuku R, Fujiwara N, Tanaka H, et al. Effect of pemafibrate on liver enzymes and shear wave velocity in non-alcoholic fatty liver disease patients. Front Med (Lausanne). 2023;10:1073025.
doi pubmed pmc - Iwadare T, Kimura T, Kunimoto H, Tanaka N, Wakabayashi SI, Yamazaki T, Okumura T, et al. Higher responsiveness for women, high transaminase levels, and fat percentage to pemafibrate treatment for NAFLD. Biomedicines. 2022;10(11):2-13.
doi pubmed pmc - Ishikawa T, Terai N, Igarashi T, Yamazaki S, Kobayashi T, Sato T, Iwanaga A, et al. Effects of body composition and liver function after long-term pemafibrate treatment on dyslipidemia-associated non-alcoholic fatty liver disease. Clin Exp Hepatol. 2023;9(2):172-178.
doi pubmed pmc - Morishita A, Oura K, Takuma K, Nakahara M, Tadokoro T, Fujita K, Tani J, et al. Pemafibrate improves liver dysfunction and non-invasive surrogates for liver fibrosis in patients with non-alcoholic fatty liver disease with hypertriglyceridemia: a multicenter study. Hepatol Int. 2023;17(3):606-614.
doi pubmed pmc - Sahebkar A, Chew GT, Watts GF. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother. 2014;15(4):493-503.
doi pubmed - Priya Pulipati V, Brinton EA. 23 - Pemafibrate: A New Selective Peroxisome Proliferator-Activated Receptor-α Modulator for Hypertriglyceridemia Management. In: Ballantyne CMBT-CL (Third E (ed) Companion to Braunwald's Heart Disease. Elsevier, New Delhi. 2024; p. 214-223.e2
- Camont L, Lhomme M, Rached F, Le Goff W, Negre-Salvayre A, Salvayre R, Calzada C, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33(12):2715-2723.
doi pubmed - Yamashita S, Arai H, Yokote K, Araki E, Suganami H, Ishibashi S, K-877 Study GroupShow less. Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol. 2018;12(5):1267-1279 e1264.
doi pubmed - Hennuyer N, Duplan I, Paquet C, Vanhoutte J, Woitrain E, Touche V, Colin S, et al. The novel selective PPARalpha modulator (SPPARMalpha) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200-208.
doi pubmed - Sanyal D, Mukherjee P, Raychaudhuri M, Ghosh S, Mukherjee S, Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab. 2015;19(5):597-601.
doi pubmed pmc - Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367-379.
doi pubmed pmc - Sharma A, Nagalli S. Chronic liver disease. In: StatPearls. Treasure Island (FL) ineligible companies. 2024.
pubmed - Sebastiani G, Patel K, Ratziu V, Feld JJ, Neuschwander-Tetri BA, Pinzani M, Petta S, et al. Current considerations for clinical management and care of non-alcoholic fatty liver disease: Insights from the 1st International Workshop of the Canadian NASH Network (CanNASH). Can Liver J. 2022;5(1):61-90.
doi pubmed pmc - Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, et al. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatol Res. 2015;45(10):E53-61.
doi pubmed - Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51(2):371-379.
doi pubmed - Margraf A, Zarbock A. Platelets in inflammation and resolution. J Immunol. 2019;203(9):2357-2367.
doi pubmed - Lisman T, Luyendyk JP. Platelets as modulators of liver diseases. Semin Thromb Hemost. 2018;44(2):114-125.
doi pubmed pmc - Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol. 2017;23(18):3228-3239.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


