Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 4, August 2024, pages 159-174
Effect of Pemafibrate on the Lipid Profile, Liver Function, and Liver Fibrosis Among Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease
Figures

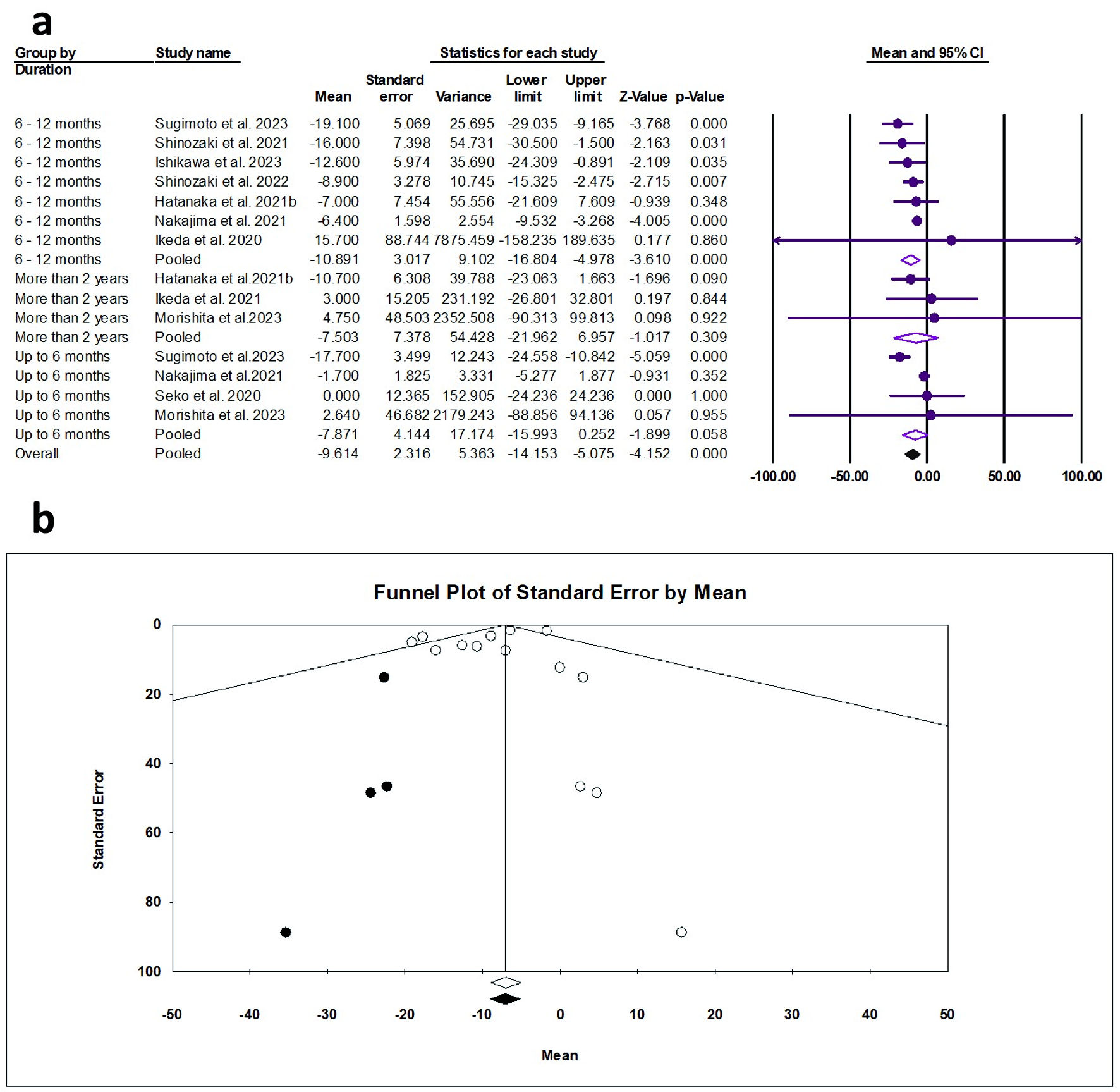
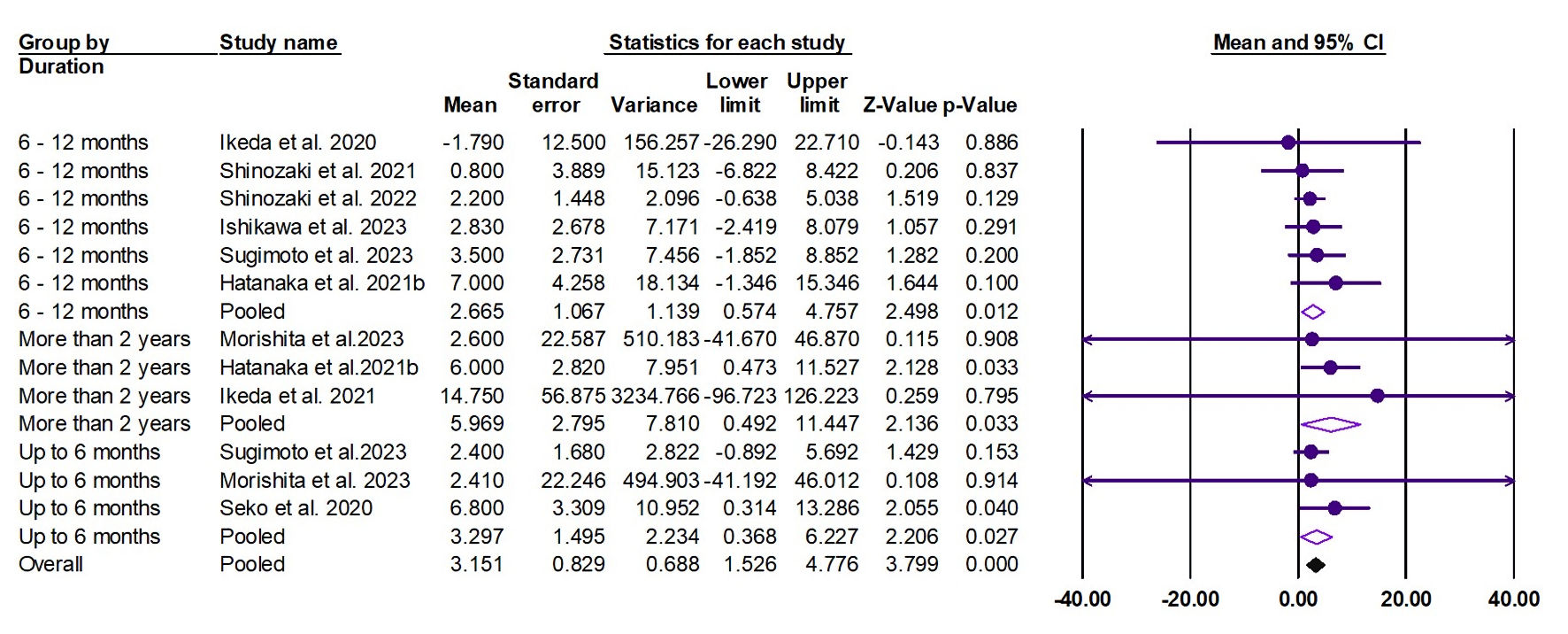
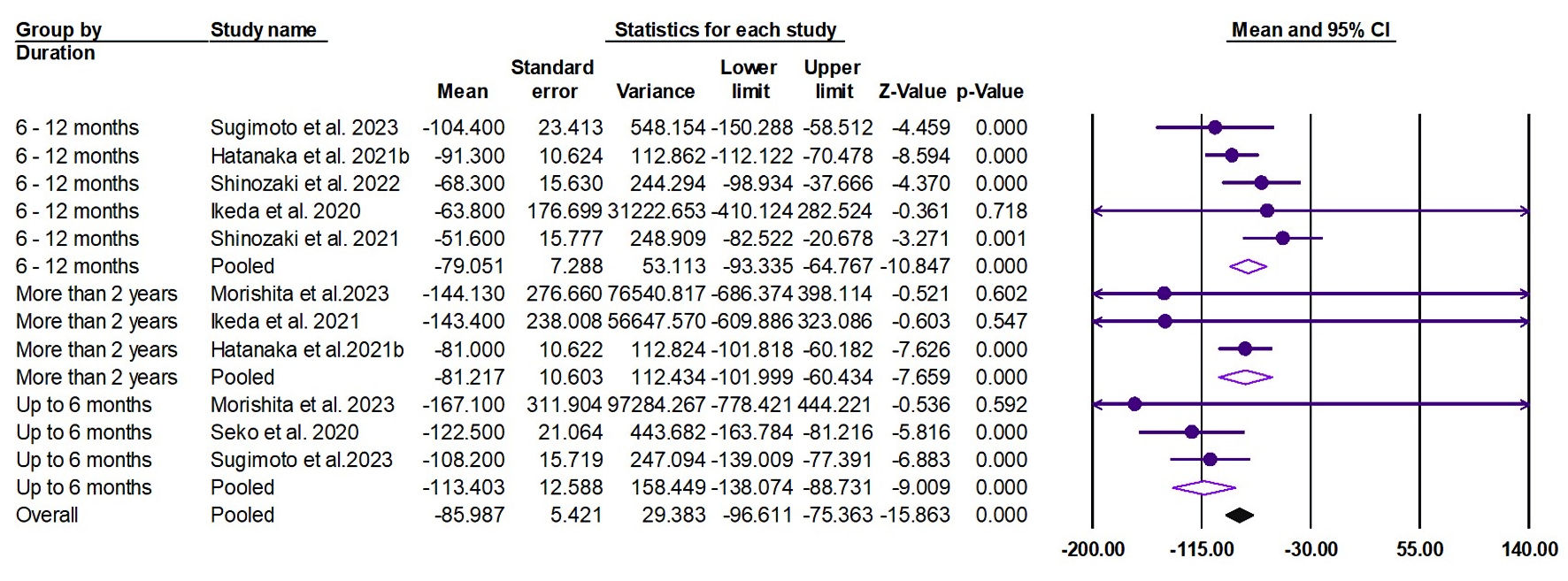
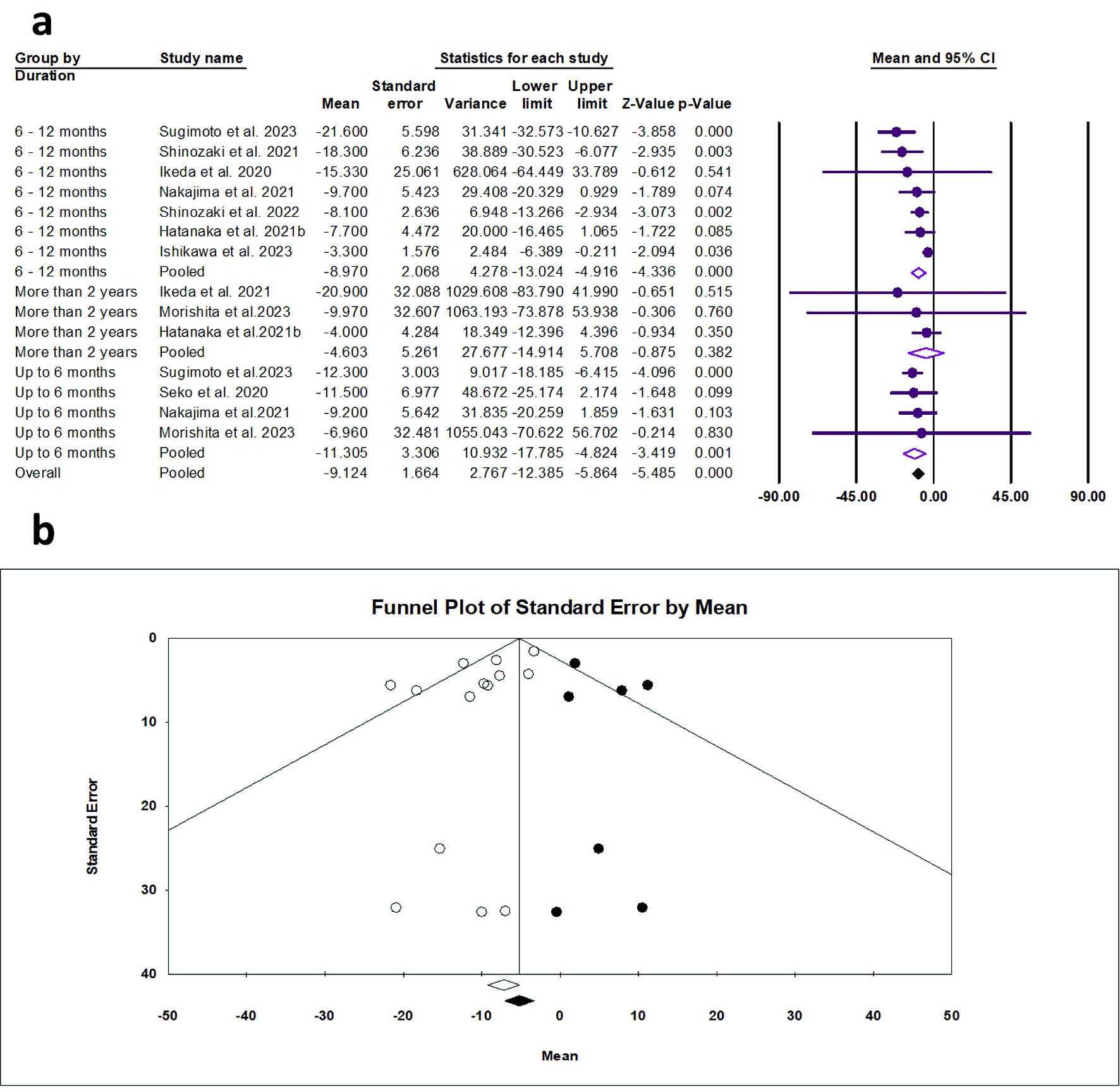
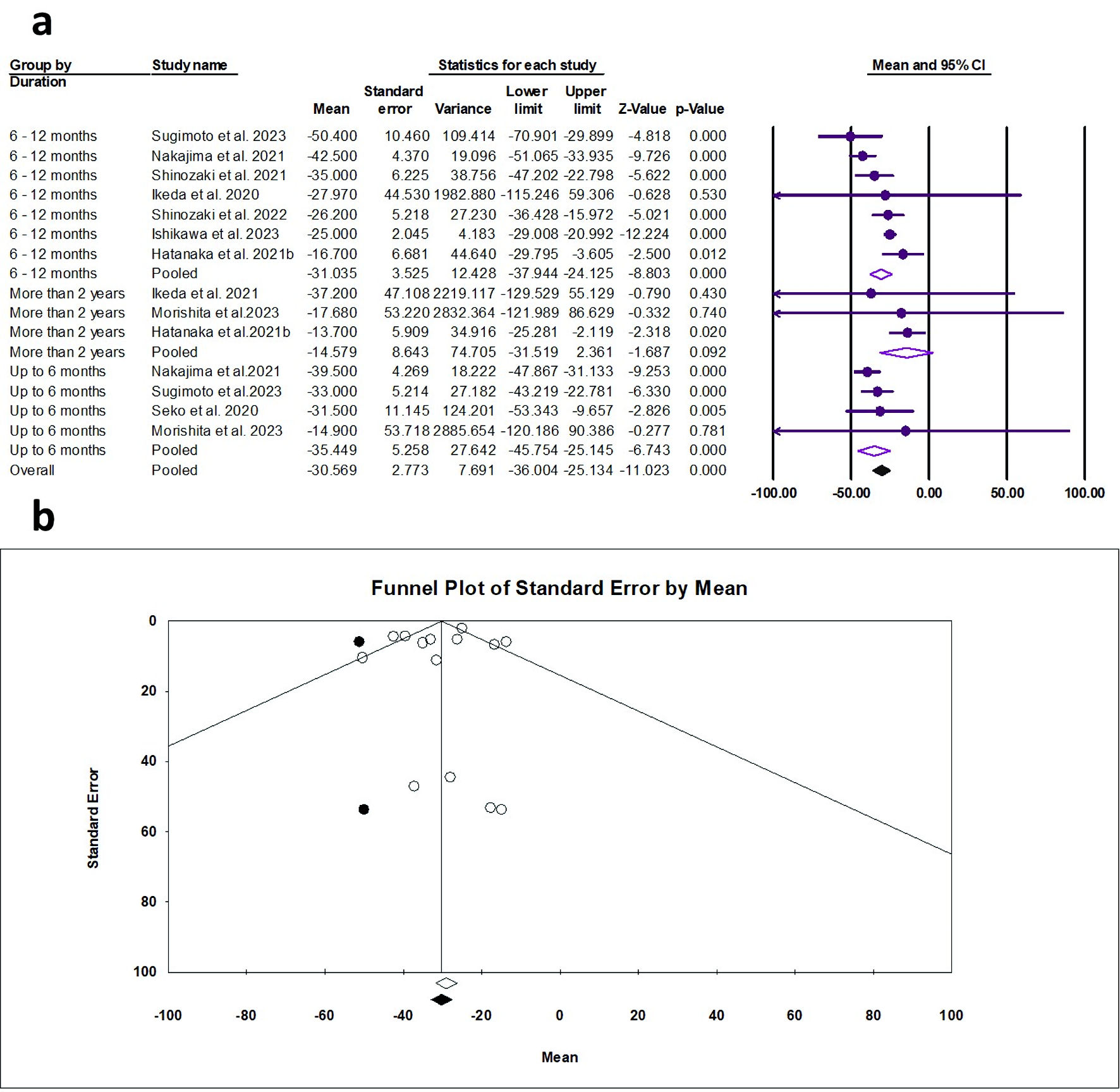
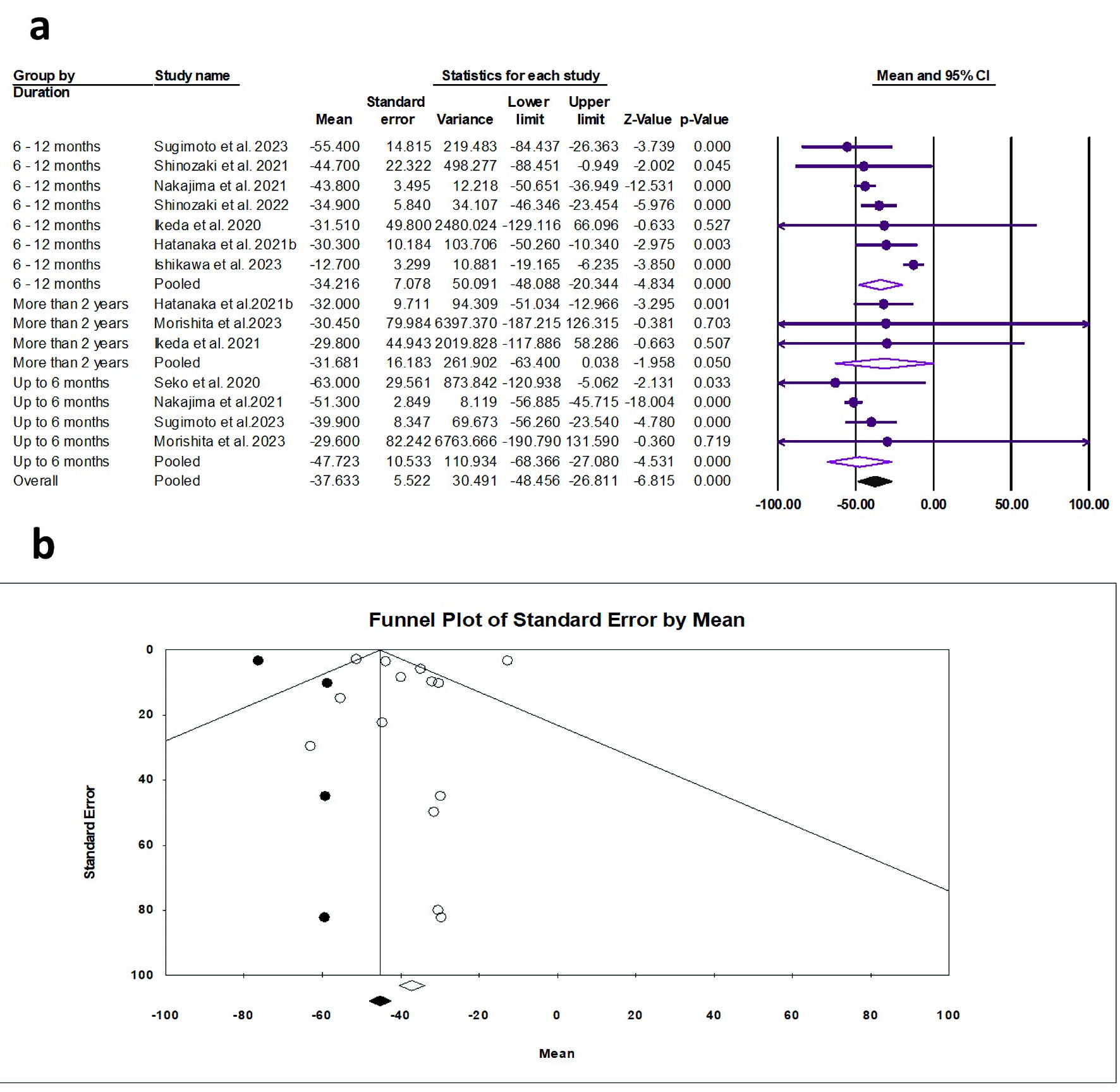
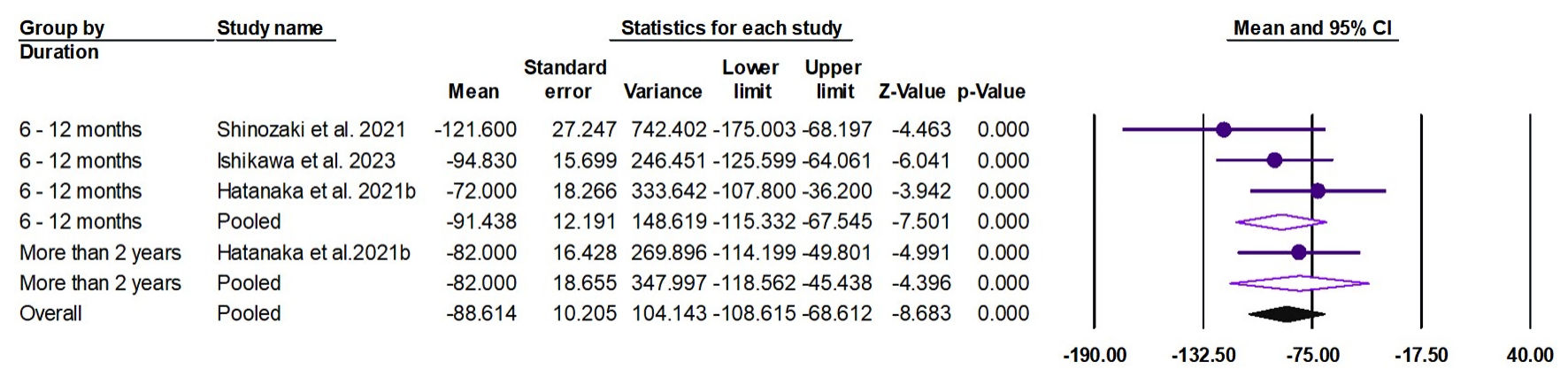
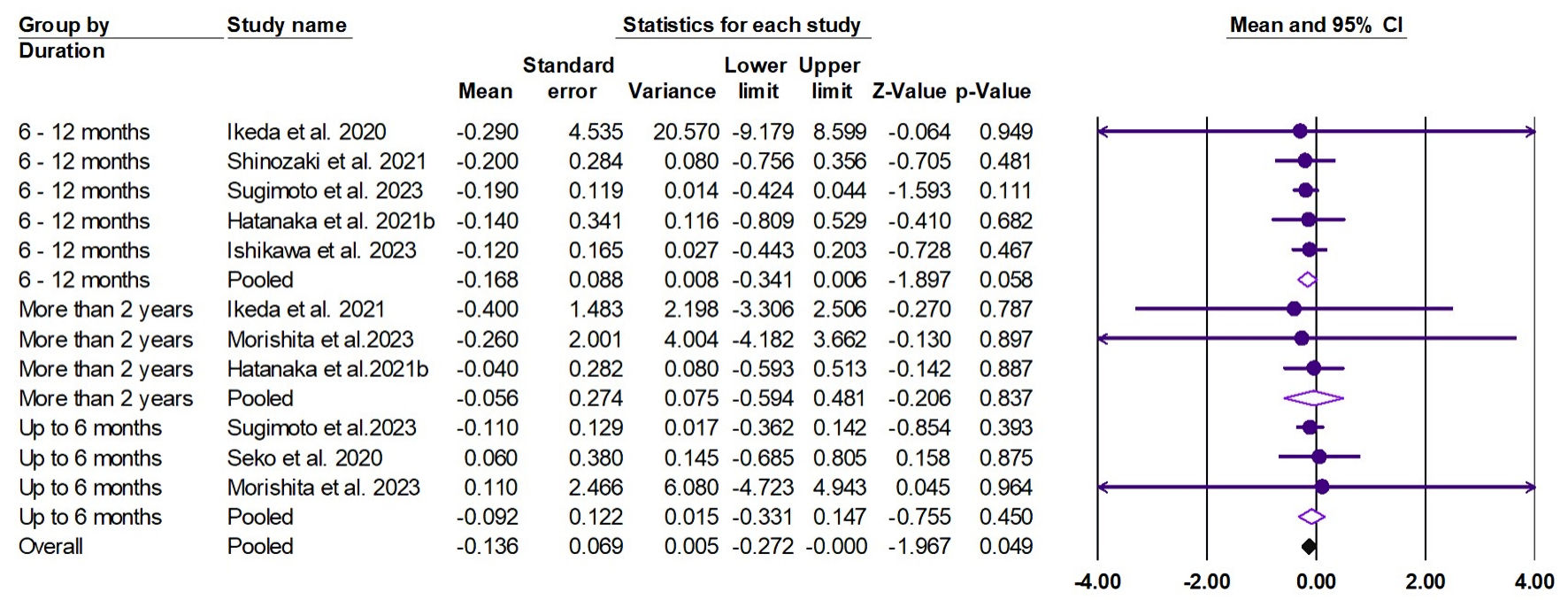
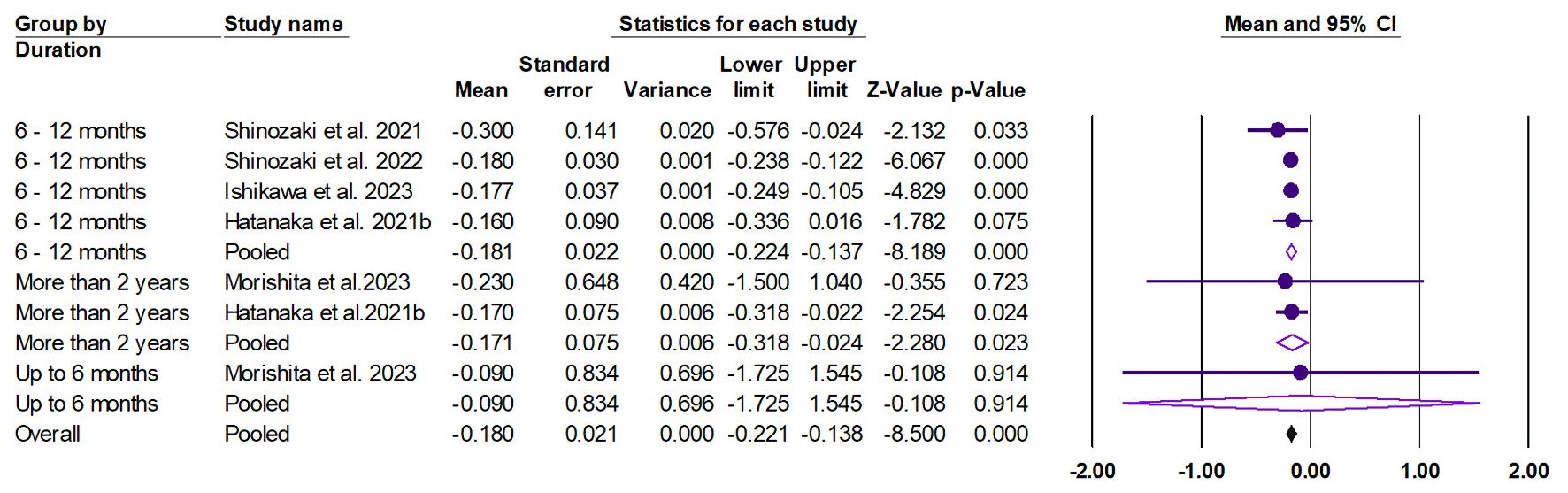
Tables
| Study ID | Study design | Site | Period | Patients’ criteria | Pemafibrate dose | Follow-up | Conclusion |
|---|---|---|---|---|---|---|---|
| BID: twice a day; AST: aspartate aminotransferase; FAST score: FibroScan-AST; NASH: non-alcoholic steatohepatitis; NAFLD: non-alcoholic fatty liver disease; ALT: alanine aminotransferase; ALP: alkaline phosphatase; US: ultrasonography; CT: computed tomography; MRI: magnetic resonance imaging; LDL: low-density lipoprotein; HDL: high-density lipoprotein; TG: triglycerides; FIB-4: fibrosis-4 index; APRI: aspartate aminotransferase to platelet ratio index; BMI: body mass index; PEM: pemafibrate; HTG: hypertriglyceridemia; LFT: liver function tests; MRE: magnetic resonance elastography; IgG: immunoglobulin G. | |||||||
| Hatanaka et al, 2021 [15] | Retrospective, single-arm study | Japan | 2018 - 2019 | The study included non-alcoholic patients with fatty liver, hypertriglyceridemia, and preserved liver function. | Oral, 0.1 mg, BID | 6 months | Pemafibrate’s anti-inflammatory effect improved FAST score, suggesting potential NASH progression prevention in hypertriglyceridemia patients. |
| Hatanaka et al, 2021 [19] | Retrospective, single-arm study | Japan | 2018 - 2020 | The study included non-alcoholic patients with fatty liver, hypertriglyceridemia, and preserved liver function. | Oral, 0.1 mg, BID | 48 weeks | Pemafibrate therapy showed promise as a safe and efficient option for individuals with NASH and hypertriglyceridemia. |
| Ikeda et al, 2020 [13] | Retrospective, single-arm study | Japan | 2018 - 2020 | The study included imaging-confirmed fatty liver patients (US, CT, MRI), excluding those with different hepatitis causes, alcohol history, or brief pemafibrate use (< 3 months). | Oral, 0.1 - 0.4 mg, BID | 6 months | Pemafibrate significantly improved liver function test values and APRI scores in NAFLD patients. |
| Ikeda et al, 2021 [14] | Retrospective, single-arm study | Japan | 2018 - 2021 | The study included imaging-confirmed fatty liver patients (US, CT, MRI), excluding those with different hepatitis causes, alcohol history, or brief pemafibrate use (< 3 months). | Oral, 0.1 - 0.3 mg, BID | Median 94 weeks | Pemafibrate notably enhanced TG, liver function, FIB-4 index, APRI, and fatty liver in hypertriglyceridemic NAFLD patients. |
| Ishikawa et al, 2023 [26] | Retrospective, single-arm study | Japan | 2019 - 2022 | Individuals diagnosed with dyslipidemia-linked NAFLD, verified through histological evidence, received ongoing pemafibrate treatment for ≥ 12 months targeting hypertriglyceridemia. | Oral, 0.1 mg, BID | 12 months | Pemafibrate enhanced dyslipidemia, liver function, and body composition, potentially linking body changes to improved NAFLD, necessitating extended treatment for conclusive results. |
| Iwadare et al, 2022 [25] | Retrospective, single-arm study | Japan | 2019 - 2022 | Patients included had hepatic contrast changes, echogenicity on ultrasound, limited alcohol intake (< 20 g/day), no other liver causes, TG > 150 mg/dL, preserved liver function. | Oral, 0.1 mg, BID | 6 months | Pemafibrate treatment could be prioritized for HTG-NAFLD women with elevated transaminases and fat mass. |
| Morishita et al, 2023 [27] | Retrospective, single-arm study | Japan | 2018 - 2021 | Patients with US-diagnosed fatty liver were included; those with other hepatitis causes were excluded. Hypertriglyceridemia was determined by elevated fasting or non-fasting TG levels. | Oral, 0.1 mg, BID | 72 weeks | Pemafibrate’s anti-inflammatory impact improved LFTs, fibrotic markers, and FAST score, indicating potential NAFLD progression prevention in hypertriglyceridemia patients. |
| Nakajima et al, 2021 [16] | RCT | Japan | 2017 - 2020 | Patients with MRI-estimated proton density fat fraction ≥ 10%, MRI-estimated proton density fat fraction-measured liver stiffness ≥ 2.5 kPa, and elevated ALT (> 40 U/L for men, > 30 U/L for women) were enrolled. | Oral 0.2 mg, BID | 96 weeks | Pemafibrate did not lower liver fat content, yet reduced MRE-based stiffness, promising for NAFLD/NASH treatment, possibly combined with agents targeting liver fat reduction. |
| Seko et al, 2020 [21] | Retrospective, single-arm study | Japan | 2019 - 2020 | NAFLD diagnosis used abdominal US with hepatic echogenicity changes. Inclusion criteria: ALT > 40 IU/L, TG ≥ 150 mg/dL, age ≥ 20 and < 75, alcohol < 30 g/day for men and < 20 g/day for women. | Oral, 0.1 mg, BID | 12 weeks | Selective peroxisome proliferator-activated receptor α (SPPARMα) shows potential for NAFLD/DL treatment by modulating fatty acid composition. |
| Shinozaki et al, 2020 [20] | Retrospective, single-arm study | Japan | 2019 - 2020 | Inclusion criteria encompassed ultrasound-diagnosed fatty liver, pemafibrate-treated dyslipidemia, sustained ALT elevation (> 30) for ≥ 3 months, negative hepatitis markers, normal IgG, and limited alcohol intake. | Oral, 0.1 mg, BID | 3 months | Pemafibrate treatment for 3 months enhances hepatic inflammation, function, and fibrosis markers in NAFLD patients. |
| Shinozaki et al, 2021 [22] | Retrospective, single-arm study | Japan | 2019 - 2020 | Inclusion criteria encompassed ultrasound-diagnosed fatty liver, pemafibrate-treated dyslipidemia, sustained ALT elevation (> 30) for ≥ 3 months, negative hepatitis markers, normal IgG, and limited alcohol intake. | Oral, 0.1 mg, BID | 12 months | A year of pemafibrate treatment benefits non-diabetic NAFLD patients, enhanced inflammation, function, and fibrosis markers. Improved fibrosis relates to better inflammation and TG levels. |
| Shinozaki et al, 2022 [23] | Retrospective, single-arm study | Japan | 2019 - 2021 | Inclusion criteria encompassed ultrasound-diagnosed fatty liver, pemafibrate-treated dyslipidemia, sustained ALT elevation (> 30) for ≥ 3 months, negative hepatitis markers, normal IgG, and limited alcohol intake. | Oral, 0.1 mg, BID | 6 months | Pemafibrate treatment enhanced hepatic inflammation and fibrosis markers, irrespective of BMI. |
| Sugimoto et al, 2023 [24] | Retrospective, single-arm study | Japan | 2018 - 2021 | Patients were included based on the abdominal US-diagnosed fatty liver (increased echogenicity, contrast, poor hepatic visualization), pemafibrate-treated dyslipidemia (0.1 mg twice daily), and limited alcohol intake (< 30 g/day males, < 20 g/day females). | Oral, 0.1 mg, BID | 48 weeks | Pemafibrate notably enhances liver function, serum TG, and liver stiffness in individuals with NAFLD. |
| Study ID | Study arms | Sample | Age (years), median (IQR) | Males, n (%) | BMI (kg/m2), median (IQR) | LFTS | Lipid profile | Liver stiffness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AST (U/L), median (IQR) | ALT (U/L), median (IQR) | ALP (U/L), median (IQR) | γ-GTP (U/L), median (IQR) | LDL-C (mg/dL), median (IQR) | HDL-C (mg/dL), median (IQR) | TG (mg/dL), median (IQR) | FIB-4, median (IQR) | APRI, median (IQR) | ||||||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; LDL: low-density lipoprotein; HDL: high-density lipoprotein; TG: triglycerides; FIB-4: fibrosis-4 index; APRI: aspartate aminotransferase to platelet ratio index; BMI: body mass index; LFT: liver function tests; γ-GTP: gamma-glutamyl transferase; IQR: interquartile range. | ||||||||||||||
| Hatanaka et al, 2021 [15] | Pemafibrate | 10 | 66.0 (53.8, 74.8) | 5 (50.0) | 27.3 (24.6, 30.0) | 43.5 (24.0, 55.0) | 51.5 (27.0, 65.3) | 285 (224, 429) | 40.0 (35.0, 84.0) | 107 (81, 135) | 46 (36, 60) | 175 (149, 247) | 2.26 (1.07, 3.12) | 0.58 (0.43, 1.01) |
| Hatanaka et al, 2021 [19] | Pemafibrate | 31 | 64.0 (55.0, 75.0) | 14 (45.2) | 26.8 (23.8, 28.8) | 41 (24, 53) | 49 (25, 66) | 242 (181, 296) | 55 (32, 104) | 114 (88, 134) | 50 (43, 63) | 172 (153, 227 | 1.62 (1.03, 2.95) | 4.4 (4.1, 4.6) |
| Ikeda et al, 2020 [13] | Pemafibrate | 17 | 63 (27, 81) | 10 (59) | 26.8 (19.2, 33.8) | 43.8 ± 5.4 | 57.5 ± 8.8 | - | 63.9 ± 10.3 | 109.5 ± 10.6 | 46.5 ± 2.4 | 300.5 ± 22.5 | 1.7 ± 0.2 | 0.7 ± 0.1 |
| Ikeda et al, 2021 [14] | Pemafibrate | 16 | 59 (27, 81) | 11 (69) | 26.8 (19.2, 33.8) | 49.6 ± 7.0 | 65.1 ± 10.8 | - | 68.9 ± 10.9 | 113.5 ± 10.0 | 47.3 ± 2.4 | 342.3 ± 54.0 | 1.8 ± 0.3 | 0.8 ± 0.1 |
| Ishikawa et al, 2023 [26] | Pemafibrate | 67 | 65.7 (58.4, 73.7) | 29 (43.2) | 24.2 (22.5, 26.9) | 29.5 (23.0, 36.8) | 42.0 (34.5, 55.0) | 251.5 (194.3, 339.8) | 31.0 (17.0, 49.5) | 128.0 (94.0, 140.8) | 50.5 (39.5, 56.8) | 168.5 (127.5, 213.0) | 1.42 (1.14, 2.33) | -3.02 (-3.13, -2.88) |
| Iwadare et al, 2022 [25] | Pemafibrate | 88 | 57 (46, 66) | 35 (39.8) | 27.2 (25.2, 30.3) | 43 (30, 61) | 56 (37, 85) | 4.4 (4.2, 4.6) | 59 (40, 95) | 124 (99, 150) | 43 (36, 656) | 197 (153, 288) | 1.21 (0.89, 2.45) | 0.7 (0.4, 1.1) |
| Morishita et al, 2023 [27] | Pemafibrate | 60 | 57.1 (24, 82) | 36 (60) | 27.8 (18.3, 38.2) | 52.7 ± 3.9 | 74.3 ± 6.1 | - | 98.7 ± 11.4 | 120.5 ± 4.7 | 48.9 ± 1.6 | 272.1 ± 30.7 | 2.0 ± 0.2 | 0.7 ± 0.07 |
| Nakajima et al, 2021 [16] | Pemafibrate | 58 | 53.2 (12.5) | 31 (53.4) | 29.5 (4.9) | 29.5 (4.9) | 82.8 (36.6) | 260 (76) | 85.3 (73.4) | 131 (29) | 49.0 (8.9) | 166 (63) | 1.61 (1.00) | - |
| Placebo | 60 | 53.3 (16.6) | 37 (61.7) | 29.8 (6.5) | 29.8 (6.5) | 94.6 (49.4) | 254 (74) | 78.0 (54.1) | 122 (29) | 48.4 (11.3) | 190 (148) | 1.62 (1.15) | - | |
| Seko et al, 2020 [21] | Pemafibrate | 20 | 59.6 (12.0) | 11 (55) | 26.9 (3.5) | 55.5 (25.9) | 75.1 (42.4) | - | 111 (126.3) | 117.4 (43.1) | 52.0 (13.3) | 236.6 (84) | 1.89 (1.17) | - |
| Shinozaki et al, 2020 [20] | Pemafibrate | 38 | 57.1 (2.2) | 22 (58) | 28.1 (0.6) | 49.1 (3.7) | 63.9 (3.6) | 301 (23) | 76.8 (11.8) | 98.3 (4.4) | 51.7 (2.3) | 171 (34) | 1.51 (0.16) | -2.9 (0.04) |
| Shinozaki et al, 2021 [22] | Pemafibrate | 22 | 60.4 (2.5) | 12 (54) | 27.0 (0.7) | 53.4 (6) | 64.7 (5.7) | 291.6 (25.3) | 87.6 (18.8) | 98.3 (6.3) | 54.0 (2.8) | 125.9 (14.2) | 1.1 (0.2) | -2.8 (0.1) |
| Shinozaki et al, 2022 [23] | Pemafibrate | 71 | 50.7 (1.6) | 48 (68) | 28.3 (0.4) | 49.2 (2.8) | 78.2 (5.5) | 74.5 (7.4) | - | 104.2 (3.4) | 50.2 (1.6) | 179.4 (20.7) | - | -3.00 (0.03) |
| Sugimoto et al, 2023 [24] | Pemafibrate | 132 | 48.5 (14.0) | 100 (75.7) | - | 48.7 (28.4) | 81.0 (50.0) | - | 89.8 (80.8) | 131.7 (28.1) | 50.0 (14.4) | 235.2 (165.4) | 1.24 (1.14) | - |