| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 3, June 2024, pages 116-125
The Influence of Urbanization on the Patterns of Hepatocellular Carcinoma Mortality From 1999 to 2020
Alexander Kusnika, d, Mostafa Najima, Keerthi Mannumbeth Renjitha, Charmee Vyasb, Sarath Lal Mannumbeth Renjithlalc, Richard Alweisa
aDepartment of Internal Medicine, Unity Hospital, Rochester, NY, USA
bDivision of Palliative Care, University of Kentucky, Lexington, KY, USA
cDepartment of Hospital Medicine, Tufts Medical Center, Boston, MA, USA
dCorresponding Author: Alexander Kusnik, Department of Internal Medicine, Unity Hospital, Rochester, NY 14626, USA
Manuscript submitted May 20, 2024, accepted June 15, 2024, published online June 29, 2024
Short title: Urbanization on HCC Mortality 1999 - 2020
doi: https://doi.org/10.14740/gr1743
| Abstract | ▴Top |
Background: Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related fatalities despite early diagnosis and treatment progress, creating a significant public health issue in the United States. This investigation utilized death certificate data from the Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) database to investigate HCC mortality patterns and death locations from 1999 to 2020. The objective was to analyze trends in HCC mortality across different population groups, considering the impact of urbanicity.
Methods: In this study, death certificate data obtained from the CDC WONDER database were utilized to investigate the trends in HCC mortality and location of death between 1999 and 2020. The annual percent change (APC) method was applied to estimate the average annual rate of change during the specified timeframe for the relevant health outcome. Furthermore, including data on the location of death and geographic areas allowed us to gain deeper insights into the patterns and characteristics of HCC and its impact on different regions.
Results: Between 1999 and 2020, there were 184,073 reported deaths attributed to HCC, and data on the location of death were available for all cases. Most deaths occurred during inpatient admissions (34.93%) or at home (41.19%). The study also found that the highest age-adjusted mortality rate (AAMR) for HCC was observed among male patients, particularly among those identified as Asian or Pacific Islander. Variations in AAMR were determined based on the level of urbanization or rurality of the area, with higher rates observed in more densely populated and urbanized regions. In contrast, less urbanized and populated areas experienced a profound increase in AAMR over the past two decades.
Conclusion: The HCC-related AAMRs have worsened over time for most ethnic groups, except for Asian or Pacific Islanders, which showed a reduction in APC despite having the worst AAMR. Although rural and less densely populated areas have substantially increased AAMR over the past two decades, more urbanized areas continued to have higher AAMR rates.
Keywords: Hepatocellular carcinoma; Urbanicity; Rurality; Mortality
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) poses a significant public health challenge in the United States, and it is projected to experience one of the most significant increases in prevalence by 2030 [1]. Worldwide, liver cancer will impact over 1 million people annually by 2025 and will likely surpass 1,300,000 cases by 2040 [1, 2]. Its relevant importance is further emphasized by its rank as the second most common cause of cancer-related deaths [3]. Cirrhosis represents a significant risk factor for both the incidence and mortality of HCC, as the majority of patients with HCC have underlying chronic liver disease [3, 4]. Even in the absence of cirrhosis, conditions such as hepatitis B and metabolic dysfunction-associated steatohepatitis (formerly known as non-alcoholic steatohepatitis or NASH) can lead to the development of HCC [5]. Despite significant advancements in the treatment and prevention of viral-related liver diseases, such as the implementation of neonatal immunization [6] and the availability of direct-acting antivirals (DAAs) to cure chronic infections [7], there remains a concerning upward trend in the incidence of HCC. This suggests that while there has been notable progress in managing viral liver diseases, other factors or mechanisms may contribute to the persistent rise in HCC. The understanding of urbanicity and its influences on developing HCC is currently limited. Wong et al found that rural areas exhibited the steepest rise in HCC incidence among US adults between 2004 and 2017 [8]. The causes and effects are likely multifactorial, but the suboptimal access to healthcare poses a major concern. Rural populations usually have higher rates of poverty, more uninsured or underinsured patients, and longer distances to specialized medical centers [9]. The distance to a liver transplant center has been shown to negatively impact the chances of getting on the waitlist, receiving a transplant, and, hence survival [10]. Similar observations have been made in other types of cancer, including colon and prostate cancer [9, 11]. Overall, identifying and addressing the unique challenges rural populations face can help improve access to preventive care, early diagnosis, and timely treatment. Furthermore, studying these trends can provide valuable insights into the broader implications of healthcare inequality and guide efforts to achieve more equitable health outcomes across diverse populations.
| Materials and Methods | ▴Top |
This study utilized the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database [12]. The CDC WONDER data set encompasses cause of death from death certificates from the 50 states and the District of Columbia. It has been previously used in several studies to determine trends in mortality from various pathologies. The study collected data from 1999 to 2020, specifically due to HCC as the primary cause of death. The cause of death was identified using specific codes from the International Statistical Classification of Diseases and Related Health Problems-10th Revision (ICD-10)-C22.0. The ICD-10 codes utilized for HCC have consistently exhibited a positive predictive value (PPV) and negative predictive value (NPV) of approximately 98% in previous studies, establishing them as a reliable and robust tool for assessing this patient population [13].
This retrospective observational study was exempt from local institutional review board review. The study uses a deidentified government-issued public use dataset and follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
The study gathered data on various mortality-related factors, such as population size, year, location of death, demographics, urban-rural classification, region, and states. Demographic information included age and race/ethnicity. At the same time, the area of death was categorized into medical facilities (outpatient, emergency room, inpatient, death on arrival, or status unknown), home, hospice, and nursing home/long-term care facility. Race/ethnicity was classified into Hispanic and non-Hispanic White, African American, Asian, or Pacific Islanders. The data were obtained from death certificates and have been used in previous analyses of the WONDER database [14, 15].
The National Center for Health Statistics Urban-Rural Classification Scheme assessment of the population was used to define urban (large metropolitan area (population 1 million), medium/small metropolitan area (population 50,000 - 999,999)), and rural (population < 50,000) counties per the 2013 US census classification, for the reporting the place of death. According to the US Census Bureau definitions, regions were classified into Northeast, Midwest, South, and West.
HCC-related crude and age-adjusted mortality rates (AAMRs) per 100,000 persons were determined. The calculations were done for persons of all age groups. Crude mortality rates were determined by dividing the number of HCC-related deaths by the corresponding US population of that year. As previously done, AAMR was calculated by standardizing the HCC-related deaths to the 2000 US population, with 95% confidence intervals. To quantify annual national trends in HCC-related mortality, the Joinpoint Regression Program (Joinpoint V 4.9.0.0, National Cancer Institute, Bethesda, MD, USA) was used to determine the annual percent change (APC) with 95% CI in AAMR. This method identifies significant differences in AAMR over time by fitting log-linear regression models where temporal variation occurred.
The precise formula for calculating the age-adjusted rate is as follows: Age-adjusted death rate = (Age-specific death rate × Standard population weight) × 100,000
The age-specific death rate is the number of deaths for a given age group divided by the population of that age group: Age-specific death rate = Number of deaths in age group/Population of age group
The “standard population weight” for an age group is calculated by dividing the population by the sum of the populations for all of the age groups in the query: Standard population weight = Population for age group/Sum of age group populations for all age groups in query
| Results | ▴Top |
Annual trends for HCC-related AAMR
The AAMR per 100,000 population for HCC-related deaths was observed to be 1.9 (95% CI: 1.8 - 1.9) in the year 1999 and 2.8 (95% CI: 2.7 - 2.8) in the year 2020. Over the entire period from 1999 to 2020, the overall AAMR showed a statistically significant APC increase of 1.92 (95% CI: 1.7-2.1) (Fig. 1).
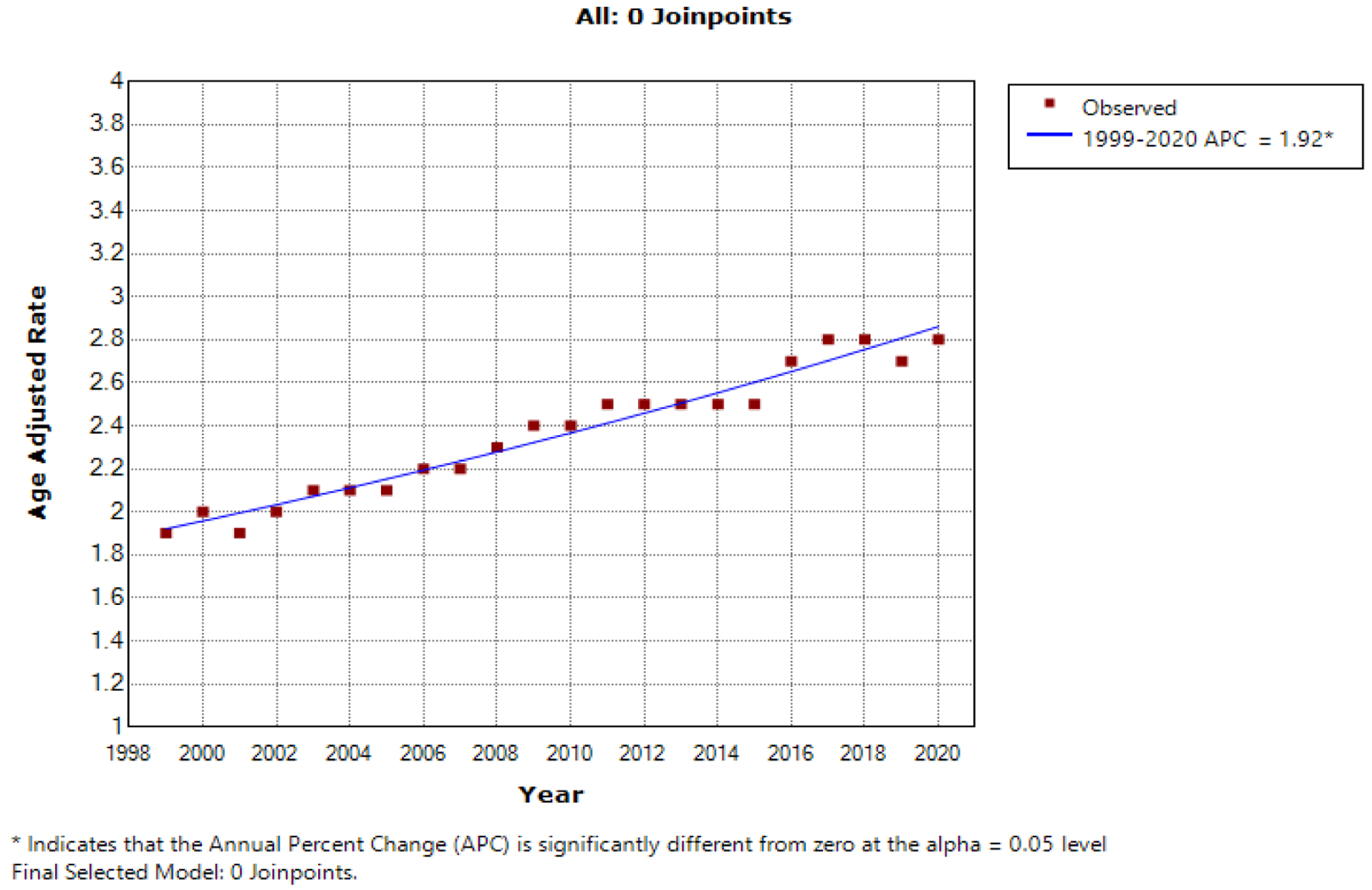 Click for large image | Figure 1. The age-adjusted mortality rate (AAMR) for hepatocellular carcinoma (HCC) per 100,000 population exhibited a significant upward trend over the two-decade study period in the USA for the general population, with the x-axis representing the year and race, and the y-axis indicating the AAMR. |
HCC-related AAMR stratified by sex
Throughout the study period, men consistently exhibited higher AAMRs than women. The overall AAMR for men was 4.1 (95% CI: 4.0 - 4.1), while for women, it was 1.0 (95% CI: 1.0 - 1.0). In 1999, the AAMR for men was 3.1 (95% CI: 3.0 - 3.2), which increased to 4.5 (95% CI: 4.4 - 4.6) in 2020 (APC: 1.6; 95% CI: 1.1 - 2.0). Similarly, the AAMR for women in 1999 was 0.9 (95% CI: 0.8 - 0.9), which worsened to 1.2 (95% CI: 1.2 - 1.3) in 2020 (APC: 1.45; 95% CI: 1.2 - 1.7) (Fig. 2).
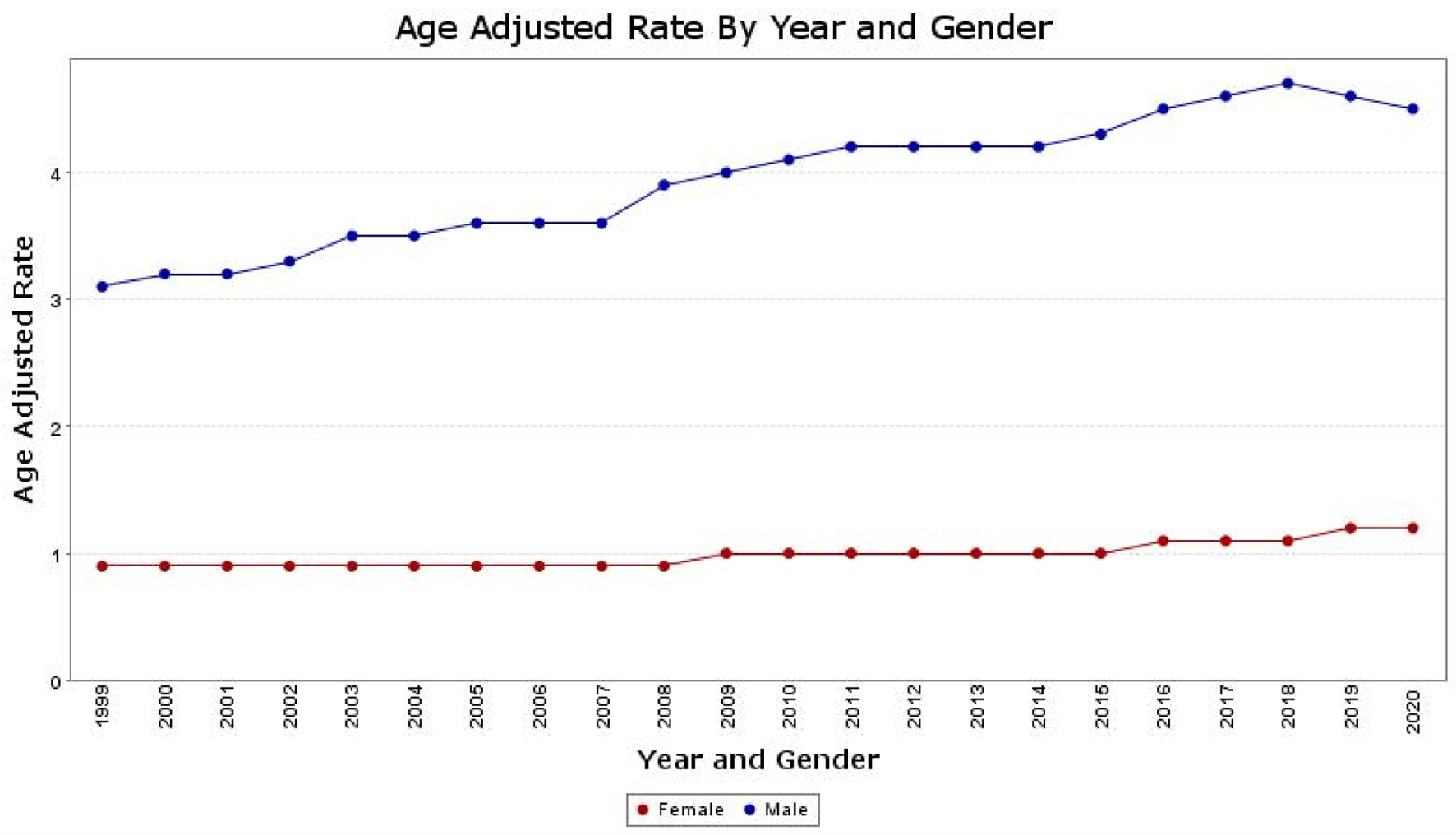 Click for large image | Figure 2. The age-adjusted mortality rate (AAMR) for hepatocellular carcinoma (HCC) is depicted separately for males and females on the graph. The y-axis represents the AAMR values, while the x-axis indicates the years from 1999 to 2020. There is an observed increase in AAMR for men, rising from 3.1 in 1999 to 4.5 in 2020. Similarly, for women, the AAMR increased from 0.9 in 1999 to 1.2 in 2020, indicating an overall worsening trend in the AAMRs for both sexes. |
HCC-related AAMR stratified by race/ethnicity
The AAMR for HCC, stratified by race/ethnicity, revealed distinct patterns among different population groups. Among these groups, the highest AAMR was observed among Asian or Pacific Islander patients, with a rate of 4.5 (95% CI: 4.5 - 4.6) (Fig. 3b). The next highest AAMR was for Black patients at 3.5 (95% CI: 3.5 - 3.5) (Fig. 3d), then American Indian or Alaska Native patients at 3.3 (95% CI: 3.1 - 3.4) (Fig. 3a), and finally, White patients at 2.1 (95% CI: 2.1 - 2.2) (Fig. 3e).
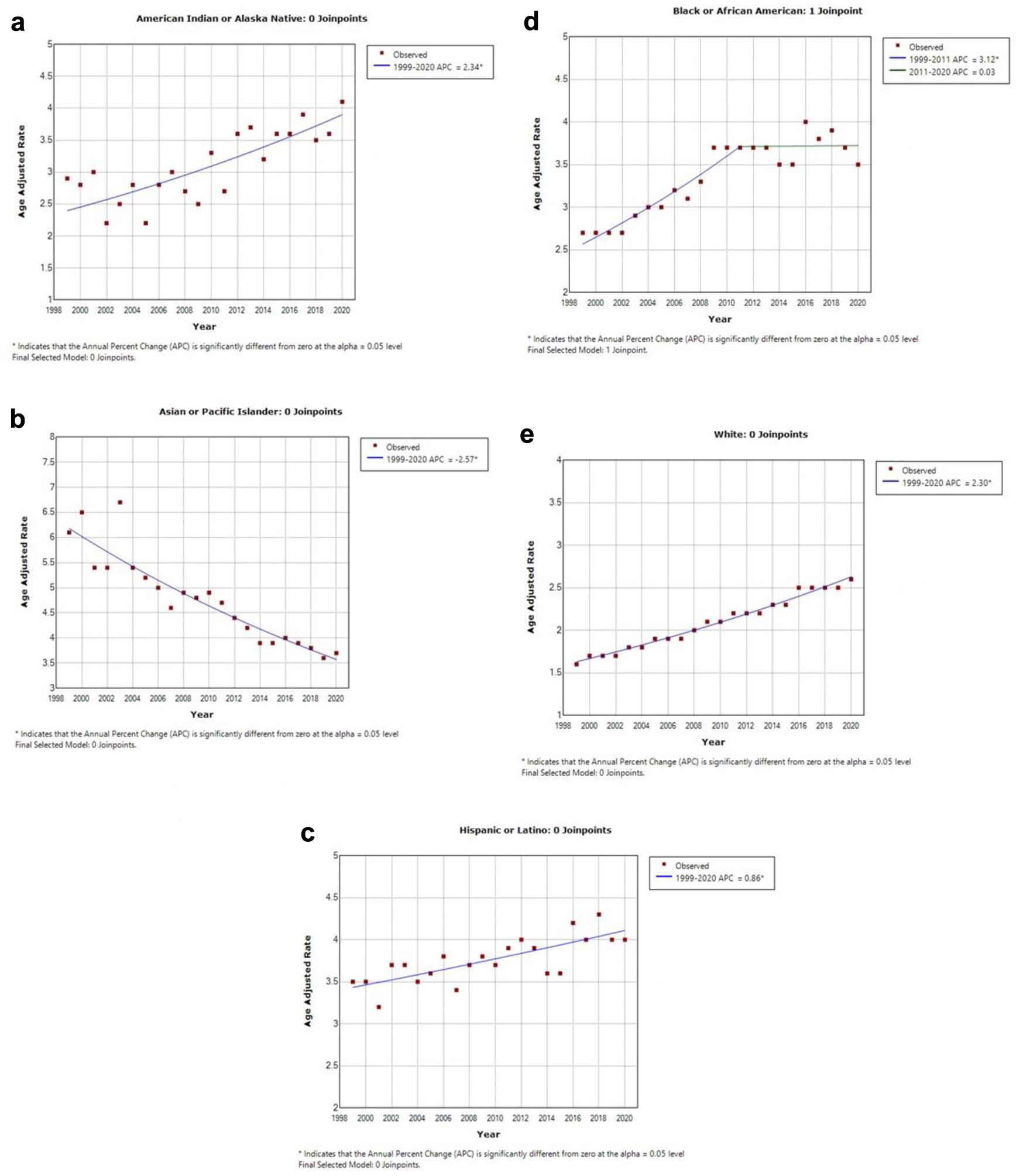 Click for large image | Figure 3. This illustration contains five graphs depicting the age-adjusted mortality rate (AAMR) within different ethnic groups. The y-axis represents the AAMR values, while the x-axis indicates the years from 1999 to 2020. (a) AAMR for American Indian or Alaska natives, which was 2.9 in 1999 and increased to 4.1 in 2020. (b) AAMR for Asian or Pacific Islanders, which declined from 6.1 in 1999 to 3.7 in 2020. (c) AAMR for Hispanic or Latino individuals, which increased from 3.5 in 1999 to 4.0 in 2020. (d) AAMR for Black or African American individuals, demonstrating an increase from 2.7 in 1999 to 3.5 in 2020. (e) AAMR for White individuals, which increased from 1.6 in 1999 to 2.6 in 2020. |
The AAMR for Asian or Pacific Islanders demonstrated improvement over the study period, decreasing from 6.1 (95% CI: 5.5 - 6.6) in 1999 to 3.7 (95% CI: 3.5 - 4.0) in 2020, with a significant APC of -2.6 (95% CI: -2.9 to -2.2). Conversely, Black patients experienced a worsening AAMR, increasing from 2.7 (95% CI: 2.5 - 2.9) in 1999 to 3.5 (95% CI: 3.4 - 3.7) in 2020, with an APC of 1.7 (95% CI: 1.3 - 2.2). American Indians or Alaskan Natives had an AAMR of 2.9 (95% CI: 2.0 - 4.0) in 1999, which rose to 4.1 (95% CI: 3.5 - 4.7) in 2020, showing an APC of 2.34 (95% CI: 1.6 - 3.1). For White patients, the AAMR worsened from 1.6 (95% CI: 1.6 - 1.7) in 1999 to 2.6 (95% CI: 2.5 - 2.6) in 2020, with an APC of 2.3 (95% CI: 2.3 - 2.5). Similarly, the Hispanic population experienced a worsening AAMR, increasing from 3.5 (95% CI: 3.2 - 3.8) in 1999 to 4.0 (95% CI: 3.8 - 4.2) in 2020 (Fig. 3c), with an APC of 0.9 (95% CI: 0.5 - 1.2). These results demonstrate significant variations in the AAMRs for HCC among different racial and ethnic groups over the study period.
HCC-related AAMR stratified by geographic region
The AAMR was found to be higher in large metro and urban areas compared to more rural areas. The 2006 National Center for Health Statistics (NCHS) Urban-Rural Classification was utilized, categorizing the 3,141 US counties and county equivalents into six groups, consisting of four metropolitan and two nonmetropolitan categories (Table 1) [16].
 Click to view | Table 1. Classification Rules Used to Assign Counties to the Six Urbanization Levels of the 2006 NCHS Urban-Rural Classification Adapted From Centers for Disease Control and Prevention (CDC) WONDER [16] |
The non-core metro area had an AAMR of 1.8 (95% CI: 1.8 - 1.9), the micropolitan area had an AAMR of 2.1 (95% CI: 2.0 - 2.1), the small metro area had an AAMR of 2.2 (95% CI: 2.2 - 2.2), the medium size metro had an AAMR of 2.5 (95% CI: 2.5 - 2.5), the large fringe metro had an AAMR of 2.2 (95% CI: 2.2 - 2.2), and the large central metro had the highest AAMR at 2.9 (95% CI: 2.9 - 3.0) (Figs. 4, 5). There is an observed increase in AAMR in all geographic regions, with the medium metropolitan, small metropolitan, and micropolitan demonstrating the greatest increment over time, approaching the large central metropolitan rates.
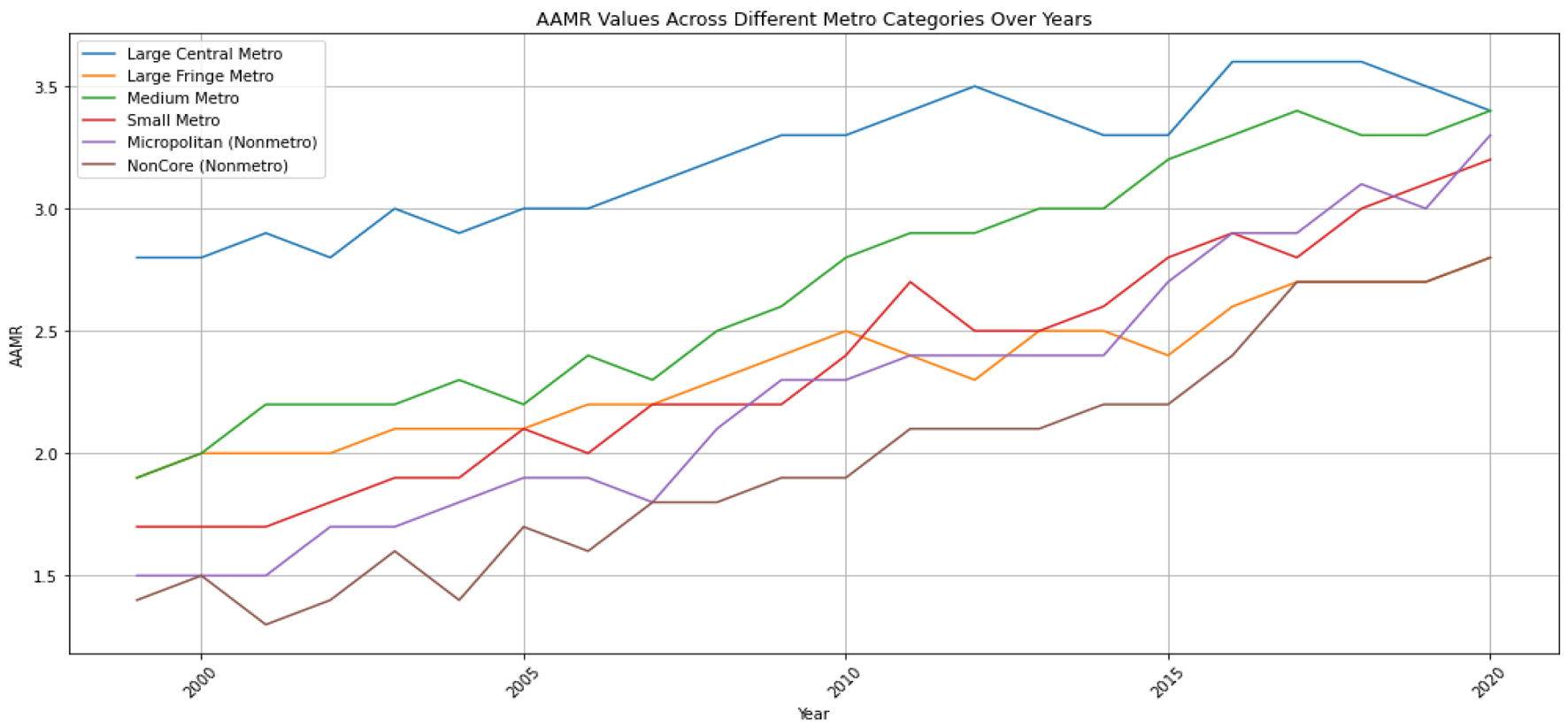 Click for large image | Figure 4. The age-adjusted mortality rate (AAMR) for hepatocellular carcinoma (HCC) is illustrated as a trend over time for the degree of urbanization. The y-axis represents the AAMR values per 100,000, while the x-axis indicates the years from 1999 to 2020. |
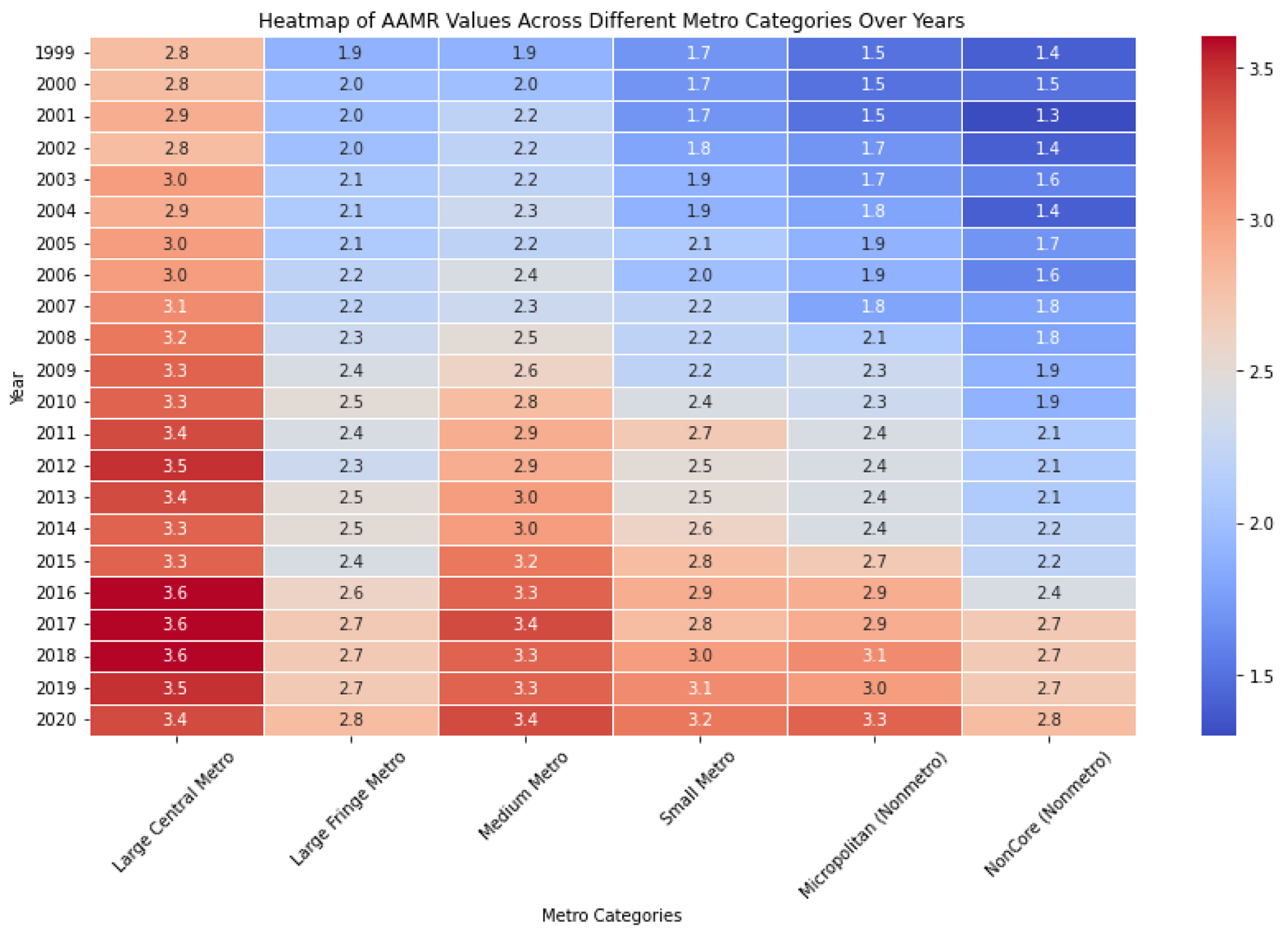 Click for large image | Figure 5. The heat map employs color intensity to depict the magnitude of age-adjusted mortality rate (AAMR) values, offering a concise visual summary of the data. Deeper hues signify elevated AAMR values, whereas lighter shades denote lower values. This visualization facilitates the quick comparison of AAMR across various metropolitan categories and years with a single glance. |
| Discussion | ▴Top |
In previous research, the Centers for Disease Control and Prevention (CDC) WONDER database was employed to scrutinize national trends in gender-specific and age-specific mortality related to HCC in a cohort from 1999 to 2015 [17]. Subsequently, an additional investigation was introduced to encompass an updated analysis, explicitly focusing on assessing trends in mortality related to hepatitis C virus (HCV) and non-HCV causes from 1999 to 2018 [18]. Our study encompasses the most recent discoveries regarding the AAMR for various ethnic groups, with a particular emphasis on investigating the influence of urbanicity on the 20-year mortality data extracted from the CDC WONDER database. The evaluation of the impact of urbanicity on HCC has been previously explored in studies. These investigations utilized various databases, such as the National Inpatient Sample (NIS) database or Surveillance, Epidemiology, and End Results (SEER) data to assess this relationship [14, 15]. However, it is essential to note that the periods covered in these studies were shorter, warranting a more comprehensive and extended analysis to gain a more thorough understanding of the trends [18].
Firstly, from 1999 to 2020, the AAMR per 100,000 population for all HCC-related deaths increased significantly from 1.9 to 2.8, with an APC increase of 1.92. This rise was primarily driven by the increase in AAMR among men, which increased from 3.1 to 4.5. However, a statistically significant increase was also observed among women, with the AAMR rising from 0.9 to 1.2 over the 20 years. These findings have been consistent with previously reported studies [19, 20]. In general, males are more prone to have metabolic (dysfunction)-associated fatty liver disease (MASLD)/metabolic dysfunction-associated steatotic liver disease (MASH), formerly known as non-alcoholic fatty liver disease (NAFLD) and NASH [21], than females. However, there is a notable shift in overall prevalence after the age of 60 years, where females become significantly more likely to suffer from MAFLD/MASH [22]. Although there was no significant difference in survival between older males and females [23], among younger individuals, women demonstrated a survival advantage over men.
Males tend to exhibit a higher prevalence of viral hepatitis and related cirrhosis when compared to females, partly due to androgen-related factors that can lead to increased viral production and inflammation [24]. Conversely, estrogen has been found to decrease interleukin-6 (IL-6)-mediated hepatic inflammation and viral production, which may contribute to the lower HCC incidence in females [25, 26]. Additional protection arises from the liver-specific gene CYP39A1, which demonstrates hepatoprotective properties and is more prevalent in females [27]. Overall, the factors contributing to the difference in HCC development between sexes are not fully understood and are likely a result of a complex interplay involving epigenetic influences, lifestyle, diet, behavioral factors, and hormonal differences [2, 28].
Secondly, among different racial and ethnic groups, Asian or Pacific Islander patients had the highest AAMR at 4.5 (95% CI: 4.5 - 4.6), followed by Black patients at 3.5 (95% CI: 3.5 - 3.5), American Indian or Alaska Native patients at 3.3 (95% CI: 3.1 - 3.4), and White patients at 2.1 (95% CI: 2.1 - 2.2). Over the past two decades, the general trend has shown a deterioration in AAMRs across all subpopulations except for Asian or Pacific Islanders. This observation aligns with earlier research indicating that incidence rates are expected to keep rising until at least 2030 in these subgroups [29]. The notable decline in the incidence of HCC within the Asian/Pacific Islander subpopulation can be predominantly attributed to the substantial advancements made in hepatitis B virus (HBV) vaccination programs and the overall improvement in antiviral treatments, mainly nucleos(t)ide analogs (NAs), for hepatitis B. The high AAMR previously observed in this subpopulation was primarily driven by a greater prevalence of HBV, which has now been addressed through these proactive measures [30, 31]. As mentioned earlier, the findings were substantiated by a study investigating the effects of a national hepatitis B immunization program in Taiwan [32]. The study demonstrated a notable decrease in HCC incidence and a significant reduction in mortality following the implementation of the vaccination program. This observation carries profound significance as Asian Americans constitute over 60% of the country’s chronic HBV infections [33] despite their total population representing only 5% of the overall population.
Regarding other ethnic populations, the American Indian or Alaska Native subgroup had the highest AAMR in 2020, reaching 4.1, with an APC of 2.34. The second-highest AAMR of 4.0 was observed in Hispanic or Latino individuals, although this subgroup displayed a milder increase in APC at 0.86 over the 20 years. African Americans experienced a notable surge in mortality until 2011, with an APC of 3.12, which subsequently plateaued significantly over the last decade, with an APC of 0.03, resulting in an AAMR of 3.5 in 2020. Lastly, the White subgroup had an APC of 2.3, reaching an AAMR of 2.6 in 2020. The observed stagnation in the last decade can be partly linked to the introduction of DAAs and better accessibility to HCC therapies among the population [34, 35]. Additionally, the aging of the cohort of HCV-infected individuals, coupled with other causes of death, has also contributed to this plateauing phenomenon [36-38].
Thirdly, pertaining to the aspects of urbanicity and rurality, our study corroborates the findings of previous investigations that have examined the association between urbanicity and HCC [8]. The CDC WONDER database entails the NCHS Urban-Rural Classification Scheme for Counties to examine the relationship between the level of urbanization in residents’ locales and health indicators. For a considerable period, geographic location has been acknowledged as a significant barrier to accessing healthcare, particularly in terms of specialized medical services, which tend to be more concentrated in urban settings [39]. Prior research found that residents of rural areas have higher rates of obesity, physical inactivity, and cigarette smoking, which leads to overall poorer health outcomes [40]. Other factors, such as social isolation and poverty, which may be more prevalent in rural areas, were also associated with increased cancer-related mortality [41]. This situation has had a noteworthy impact, particularly in cancer care, due to the inherent challenges in reaching surgical and medical resources, especially in rural areas [42]. In the case of rural patients with HCC, the longer distance to a liver transplant center is negatively linked to the probability of undergoing a liver transplant and, consequently, affects survival [43].
Our investigation has elucidated that the AAMR for HCC witnessed a significant increase across all distinct categories throughout the years. Particularly noteworthy was the substantial surge in AAMR observed within the nonmetro categories over a 20-year span, where the AAMR in the noncore and micropolitan areas rose from 1.4 to 2.8 and 1.5 to 3.3, respectively, between 1999 and 2020. Comparable albeit smaller increases were identified in the other categories, notably within the large central and large fringe metro areas, showing an elevation from 2.8 to 3.4 and 1.9 to 2.8 over the same two-decade period, respectively (Figs. 4, 5).
These findings indicate a pronounced increase in AAMR, particularly prominent in the micropolitan and noncore areas, consistent with previous studies [8].
These findings illustrate that less urbanized areas (small-medium metropolitan and micropolitan) are witnessing a narrowing of the gap in the AAMR compared to their large central metropolitan counterparts over time. Such an observation is also accurate for other types of cancer. In the period 1999 - 2014, the AAMR for the five leading causes of death in the United States (cancer is the second) were all higher in rural population compared to urban areas [44].
A conceivable explanation for these findings may be linked to the impact of residing in rural areas and the utilization of healthcare services. Those living in rural areas face unique challenges in accessing health information from primary care and specialist providers, coupled with an overall diminished access to specialists compared to urban counterparts [45, 46]. Similarly, Rongey et al, in a study encompassing more than 150,000 veterans, indicated that patients with HCV residing in rural and highly rural areas were significantly less likely to access HCV specialty care [47]. This can translate to more advanced stages of the disease at the time of diagnosis, significantly reducing survival rates. Beyond the evident structural differences, heightened rates of uninsurance and cost burden, combined with limited health literacy leading to reduced utilization of online health information, likely contribute to this issue [45, 46]. The phenomenon, identified as the “knowledge gap hypothesis”, postulates that individuals with a higher socioeconomic status, more prevalent in urban areas, tend to benefit more from newly disseminated health information. This accentuates a discernible gap between them and individuals with low socioeconomic statuses, a demographic more frequently encountered in rural areas [48, 49]. This hypothesis found validation in a study of over 200,000 individuals diagnosed with hepatitis C in which patients residing in rural areas exhibited a lower likelihood of adhering to DAA agents for hepatitis C treatment when compared to individuals in urban areas [50].
To conclude, people residing in rural areas have seen a noteworthy increase in AAMR related to HCC, reaching a level comparable to that of their counterparts in large central metropolitan areas over time. Addressing these trends requires a comprehensive approach that fills knowledge gaps and enhances healthcare utilization and health literacy. Additionally, healthcare providers must play a role in improving HCC surveillance efforts [51].
Limitation
The available dataset relies on information provided by healthcare providers, which could be prone to errors or inaccuracies, particularly regarding critical variables like race and ethnicity. Additionally, the limited availability of detailed information on individual risk factors or potential confounding variables that could impact the development or progression of HCC may restrict the comprehensive exploration of intricate relationships between variables. There are challenges associated with categorizing diverse populations, such as the Pacific and Asian Islander groups, as distinct subgroups within this classification, as these subgroups might display varying outcomes in mortality rates that cannot be captured.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Dr. Alweis supervised the entire project, including the design and the analysis. Dr. Kusnik, Dr. Najim, Dr. Vyas and Dr. Renjithlal designed the study, performed data analysis, and wrote the final manuscript. Dr. Kusnik, Dr. Renjith, and Dr. Renjithlal were responsible for the interpretation of data for the work. All authors critically revised the manuscript for important intellectual content. Final approval of the version to be published was collected from each author before submission. All authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The CDC WONDER database, created by the Centers for Disease Control and Prevention (CDC) in the United States, is an online platform that is publicly accessible. It provides an extensive array of public health data, which can be utilized to monitor disease outbreaks, identify patterns, and assess the effectiveness of public health initiatives. The database is freely available; users can access the information through the CDC WONDER website.
| References | ▴Top |
- Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67(2):600-611.
doi pubmed pmc - Kusnik A, Hunter N, Rasbach E, Miethke T, Reissfelder C, Ebert MP, Teufel A. Co-medication and nutrition in hepatocellular carcinoma: potentially preventative strategies in hepatocellular carcinoma. Dig Dis. 2021;39(5):526-533.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
doi pubmed - Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol. 2019;11(1):1-18.
doi pubmed pmc - Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598-1606.
doi pubmed pmc - Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017;152(1):142-156.e142.
doi pubmed - Wong RJ, Saab S, Konyn P, Sundaram V, Khalili M. Rural-urban geographical disparities in hepatocellular carcinoma incidence among us adults, 2004-2017. Am J Gastroenterol. 2021;116(2):401-406.
doi pubmed pmc - Han L, Sullivan R, Tree A, Lewis D, Price P, Sangar V, van der Meulen J, et al. The impact of transportation mode, socioeconomic deprivation and rurality on travel times to radiotherapy and surgical services for patients with prostate cancer: a national population-based evaluation. Radiother Oncol. 2024;192:110092.
doi pubmed - Goldberg DS, Newcomb C, Gilroy R, Sahota G, Wallace AE, Lewis JD, Halpern SD. Increased distance to a liver transplant center is associated with higher mortality for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2017;15(6):958-960.
doi pubmed pmc - Selvakumar T, Mu SZ, Prasath V, Arjani S, Chokshi RJ, Kra J. Colon cancer epidemiology, race and socioeconomic status: comparing trends in counties served by an urban hospital in Newark, NJ with overall NJ-state and nation-wide patterns. Cancer Epidemiol. 2023;86:102412.
doi pubmed - Available from: https://wonder.cdc.gov/wonder/help/mcd.html#.
- Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive predictive value of International Classification of Diseases, 10th revision, codes for cirrhosis and its related complications. Clin Gastroenterol Hepatol. 2018;16(10):1677-1678.
doi pubmed - Kusnik A, Renjithlal SLM, Chodos A, Shanmukhappa SC, Eid MM, Renjith KM, Alweis R. Trends in colorectal cancer mortality in the United States, 1999 - 2020. Gastroenterology Res. 2023;16(4):217-225.
doi pubmed pmc - Pierce JB, Shah NS, Petito LC, Pool L, Lloyd-Jones DM, Feinglass J, Khan SS. Trends in heart failure-related cardiovascular mortality in rural versus urban United States counties, 2011-2018: a cross-sectional study. PLoS One. 2021;16(3):e0246813.
doi pubmed pmc - 2006 NCHS Urban-Rural Classification Scheme for Counties 2024. Available from: https://wonder.cdc.gov/wonder/help/cmf/urbanization-methodology.html.
- Beal EW, Tumin D, Kabir A, Moris D, Zhang XF, Chakedis J, Washburn K, et al. Trends in the mortality of hepatocellular carcinoma in the United States. J Gastrointest Surg. 2017;21(12):2033-2038.
doi pubmed - Ramani A, Tapper EB, Griffin C, Shankar N, Parikh ND, Asrani SK. Hepatocellular carcinoma-related mortality in the USA, 1999-2018. Dig Dis Sci. 2022;67(8):4100-4111.
doi pubmed - Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5-S16.
doi pubmed - Wu EM, Wong LL, Hernandez BY, Ji JF, Jia W, Kwee SA, Kalathil S. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4:66.
doi pubmed pmc - Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80(2):e54-e56.
doi pubmed - Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46(Suppl 1):63-69.
doi pubmed - Rich NE, Murphy CC, Yopp AC, Tiro J, Marrero JA, Singal AG. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;52(4):701-709.
doi pubmed pmc - White DL, Tavakoli-Tabasi S, Kuzniarek J, Pascua R, Ramsey DJ, El-Serag HB. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2012;55(3):759-768.
doi pubmed pmc - Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121-124.
doi pubmed - Liu WC, Liu QY. Molecular mechanisms of gender disparity in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2014;20(20):6252-6261.
doi pubmed pmc - Ji F, Zhang J, Liu N, Gu Y, Zhang Y, Huang P, Zhang N, et al. Blocking hepatocarcinogenesis by a cytochrome P450 family member with female-preferential expression. Gut. 2022;71(11):2313-2324.
doi pubmed - Kusnik A, Penmetsa A, Chaudhary F, Renjith K, Ramaraju G, Laryea M, Allard JP. Clinical overview of sarcopenia, frailty, and malnutrition in patients with liver cirrhosis. Gastroenterology Res. 2024;17(2):53-63.
doi pubmed pmc - Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787-1794.
doi pubmed pmc - Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, Bell B. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202(2):192-201.
doi pubmed - McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4-13.
doi pubmed pmc - Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310(9):974-976.
doi pubmed - Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56(2):422-433.
doi pubmed - Han JE, Cho HJ, Cheong JY, Lim SG, Yang MJ, Noh CK, Lee GH, et al. Impact of guideline adherence on the prognosis of Barcelona clinic liver cancer stage B hepatocellular carcinoma. World J Gastroenterol. 2023;29(47):6122-6137.
doi pubmed pmc - Shah S, Alavi M, Hajarizadeh B, Matthews GV, Martinello M, Danta M, Amin J, et al. Trends in decompensated cirrhosis and hepatocellular carcinoma among people with a hepatitis B notification in New South Wales. JHEP Rep. 2022;4(10):100552.
doi pubmed pmc - Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191-199.
doi pubmed pmc - Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2-S6.
doi pubmed pmc - Estevez J, Yang JD, Leong J, Nguyen P, Giama NH, Zhang N, Ali HA, et al. Clinical features associated with survival outcome in African-American patients with hepatocellular carcinoma. Am J Gastroenterol. 2019;114(1):80-88.
doi pubmed - Reschovsky JD, Staiti AB. Access and quality: does rural America lag behind? Health Aff (Millwood). 2005;24(4):1128-1139.
doi pubmed - Eberhardt MS, Pamuk ER. The importance of place of residence: examining health in rural and nonrural areas. Am J Public Health. 2004;94(10):1682-1686.
doi pubmed pmc - Fleisch Marcus A, Illescas AH, Hohl BC, Llanos AA. Relationships between social isolation, neighborhood poverty, and cancer mortality in a population-based study of US adults. PLoS One. 2017;12(3):e0173370.
doi pubmed pmc - Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146.
doi pubmed - Goldberg DS, French B, Forde KA, Groeneveld PW, Bittermann T, Backus L, Halpern SD, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234-1243.
doi pubmed pmc - Moy E, Garcia MC, Bastian B, Rossen LM, Ingram DD, Faul M, Massetti GM, et al. Leading causes of death in nonmetropolitan and metropolitan areas - United States, 1999-2014. MMWR Surveill Summ. 2017;66(1):1-8.
doi pubmed pmc - Chen X, Orom H, Hay JL, Waters EA, Schofield E, Li Y, Kiviniemi MT. Differences in rural and urban health information access and use. J Rural Health. 2019;35(3):405-417.
doi pubmed pmc - Lustria ML, Smith SA, Hinnant CC. Exploring digital divides: an examination of eHealth technology use in health information seeking, communication and personal health information management in the USA. Health Informatics J. 2011;17(3):224-243.
doi pubmed - Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PLoS One. 2013;8(12):e84826.
doi pubmed pmc - Meit M, Knudson A, Gilbert T, Yu AT-C, Tanenbaum E, Ormson E, TenBroeck S, et al. The 2014 Update of the Rural-Urban Chartbook. Bethesda, MD: Rural Health Reform Policy Research Center; 2014.
- Tichenor PJ, Donohue GA, Olien CN. Mass media flow and differential growth in knowledge. Public Opin Q. 1970;34(2):159-170.
- Du P, Wang X, Kong L, Riley T, 3rd, Jung J. Changing urban-rural disparities in the utilization of direct-acting antiviral agents for hepatitis C in U.S. Medicare patients, 2014-2017. Am J Prev Med. 2021;60(2):285-293.
doi pubmed pmc - Wong RJ, Jayasekera C, Jones P, Kanwal F, Singal AG, Ahmed A, Taglienti R, et al. An open-access, interactive decision-support tool to facilitate guideline-driven care for hepatocellular carcinoma. Gastroenterology Res. 2022;15(6):297-307.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


