| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 3, June 2024, pages 109-115
Association of Baseline Hepatitis B Virus DNA and On-Treatment Risk of Cirrhosis and Hepatocellular Carcinoma
Zeyuan Yanga, Ramsey C. Cheunga, b, Janice H. Jouc, d, Joseph K. Lime, Young-Suk Limf, Robert J. Wonga, b, g
aGastroenterology Section, Veterans Affairs Palo Alto Healthcare System, Palo Alto, CA, USA
bDivision of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, CA, USA
cDivision of Gastroenterology and Hepatology, Department of Medicine, Oregon Health & Science University Hospital, Portland, OR, USA
dDivision of Gastroenterology and Hepatology, Department of Medicine, Portland VA Medical Center, Portland, OR, USA
eSection of Digestive Diseases and Yale Liver Center, Yale University School of Medicine, New Haven, CT, USA
fDepartment of Gastroenterology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
gCorresponding Author: Robert J. Wong, Division of Gastroenterology and Hepatology, Veterans Affairs Palo Alto Healthcare System, Stanford University School of Medicine, Palo Alto, CA 94304, USA
Manuscript submitted April 23, 2024, accepted May 16, 2024, published online June 29, 2024
Short title: Baseline HBV DNA and Long-Term Outcomes
doi: https://doi.org/10.14740/gr1735
| Abstract | ▴Top |
Background: Recent studies suggest an inverse relationship between baseline levels of hepatitis B virus (HBV) DNA and on-treatment risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB). However, data are limited to Asian cohorts, and it is unclear if similar associations hold true for non-Asians with CHB. We aimed to evaluate association of baseline HBV DNA with long-term risks of cirrhosis and HCC among a predominantly non-Asian cohort of CHB patients in the USA.
Methods: Using longitudinal data from the national Veterans Affairs database, we evaluated the risk of cirrhosis or HCC among adults with non-cirrhotic CHB who are on continuous antiviral therapy, stratified by moderate levels of baseline HBV DNA (4.00 - 6.99 log10 IU/mL) vs. high levels of baseline HBV DNA (7.00 log10 IU/mL or higher). Propensity score weighting was applied, and competing risks cumulative incidence functions and Cox proportional hazards models were utilized.
Results: Among 1,129 non-cirrhotic CHB patients (41% non-Hispanic White, 36% African American, mean age 57.0 years, 62.2% hepatitis B e antigen (HBeAg) positive), 585 had moderate levels of baseline HBV DNA and 544 had high HBV DNA. After propensity score weighting, no significant difference in risk of cirrhosis was observed between moderate vs. high baseline HBV DNA (4.55 vs. 5.22 per 100 person-years, hazard ratio (HR): 0.87, 95% confidence interval (CI): 0.69 - 1.09, P = 0.22), but risk of HCC was significantly higher in patients with moderate vs. high baseline HBV DNA (0.84 vs. 0.69 per 100 person-years, HR: 1.33, 95% CI: 1.09 - 1.62, P < 0.01).
Conclusions: Among a national cohort of predominantly non-Asian US veterans with non-cirrhotic CHB on antiviral therapy, moderate levels of baseline HBV DNA was associated with higher risk of HCC than high HBV DNA.
Keywords: HBV; Cirrhosis; Hepatocellular carcinoma; Veterans; Antivirals
| Introduction | ▴Top |
Chronic hepatitis B (CHB) remains a major contributor to liver related morbidity and mortality globally [1, 2]. In the USA, recent data estimate that nearly 2.4 million adults are affected by CHB [1]. Delays in diagnosis, timely linkage to care, and appropriate antiviral therapy can lead to liver disease progression to cirrhosis and hepatocellular carcinoma (HCC) [3-7]. While CHB antiviral therapy is associated with significant reductions in long-term risks of HCC, it remains unclear whether baseline factors contribute to differences in HCC risk despite patients being on antiviral therapy [8-10]. Understanding differences in baseline factors and their impact on HCC risk may guide discussions regarding early initiation of antiviral therapy or closer monitoring of high-risk patients while on antiviral therapy.
Recent data suggest that baseline hepatitis B virus (HBV) DNA is associated with long-term on-treatment risks of HCC [11]. Choi et al [11] evaluated 2,073 patients with CHB across three centers in Korea who were maintained on entecavir or tenofovir therapy. The investigators observed an inverse relationship between baseline HBV DNA and long-term risk of HCC. For example, compared to CHB patients with 8.00 log10 IU/mL or higher HBV DNA at baseline, the adjusted hazard ratios (HRs) for HCC risk were 2.48 for patients with HBV DNA levels of 7.00 - 7.99 log10 IU/mL, 3.69 for HBV DNA levels of 6.00 - 6.99 log10 IU/mL, and 6.10 for HBV DNA levels of 5.00 - 5.99 log10 IU/mL [11]. In another study of 4,693 CHB patients on antiviral therapy across multiple centers in Korea, the investigators observed significant differences in risk of HCC by baseline HBV DNA, with the highest risk observed in patients with moderate baseline viral loads (5.00 - 7.99 log10 IU/mL) and the lowest risk observed in patients with high viral loads (8.00 log10 IU/mL or higher) [12].
However, both of these studies were conducted in Asian cohorts from a single country, and it remains unclear if similar patterns would be seen in non-Asian cohorts. Data among predominantly non-Asian populations, and specifically data evaluating both risks of HCC and cirrhosis while on CHB antiviral therapy, are lacking. The current study utilizes a large longitudinal cohort of predominantly non-Asian veterans in the USA with CHB to investigate the association between baseline HBV DNA and long-term risks of cirrhosis and HCC while on antiviral therapy.
| Materials and Methods | ▴Top |
We utilized data from the 2010 - 2022 Veterans Affairs (VA) Corporate Data Warehouse (CDW). The VA CDW is a national longitudinal database of all veterans receiving care at VA healthcare facilities in the USA. The VA CDW allows for longitudinal assessment of patient outcomes, laboratory data, and clinical encounters, and captures important demographic data and comorbidities. The VA health system is the largest integrated health system in the USA, caring for more than 9 million individuals.
Adults with CHB were identified by at least two positive results for hepatitis B surface antigen (HBsAg), HBV DNA, or hepatitis B e antigen (HBeAg) at least 6 months apart, or at least one positive result for HBsAg, HBV DNA, or HBeAg, and one International Classification of Diseases, 9th/10th Revision (ICD-9/10) code for chronic HBV (ICD-9: 070.2x, 070.3x, V02.61; ICD-10: B16.x, B18.0-1, B19.1x). CHB patients were excluded if there was evidence of concurrent human immunodeficiency virus (HIV), hepatitis C, or hepatitis delta infection. The current study focused on differences in on-treatment risks of cirrhosis or HCC by baseline HBV DNA. Each patient with CHB was retrospectively evaluated to determine the first start date of CHB antiviral treatment documented in the medical record, which was set as the index date for assessing outcomes in the follow up period. Comprehensive review of pharmacy data ensured continuous antiviral therapy prescription, and patients were followed until the last date of antiviral therapy prescribed, development of study outcomes (i.e., cirrhosis, HCC), death, or until end of the study period. Patients with cirrhosis or HCC at index date or within 12 months of starting antiviral therapy were excluded to ensure that we are capturing incident events. Cirrhosis and HCC were identified using established definitions based on ICD-9/10 codes [13]. Race/ethnicity in the VA CDW was based on self-reporting and included non-Hispanic White (NHW), Black or African American (AA), Asian or Pacific Islander (API), Hispanic, and American Indian or Alaska Native. Alcohol use was assessed based on documented AUDIT-C scores [14] closest to the time of first start of antiviral therapy and were categorized as: 1) no alcohol use (AUDIT-C = 0); 2) mild levels of alcohol use (AUDIT-C 1 - 2 for women and 1 - 3 for men); and 3) moderate-high levels of alcohol use (AUDIT-C ≥ 3 for women and ≥ 4 for men). Baseline fibrosis-4 (FIB-4) scores at index date were calculated and grouped into three categories based on established criteria: FIB-4 < 1.45, FIB-4 1.45 - 3.25, and FIB-4 > 3.25 [15].
Our initial cohort included 2,510 patients with CHB. From this cohort, we specifically focused on patients with baseline HBV DNA ≥ 4 log10 IU/mL, and categorized patients into moderate levels of baseline HBV DNA (4.00 - 6.99 log10 IU/mL) and high levels of baseline HBV DNA (7.00 log10 IU/mL or higher). Propensity score weighting was applied to these two groups, which was subsequently used to analyze cirrhosis and HCC outcomes. Baseline demographics and disease characteristics are presented as frequencies and proportions for categorical variables and mean and standard deviation or median and interquartile range (IQR) for continuous variables. The standard mean difference was utilized to compare differences in patient’s characteristics before and after propensity score weighting. Incidence of cirrhosis or HCC was presented as incidence per 100 person-years. Comparisons of univariate unadjusted outcomes were compared between groups using Chi-square testing as well as the Z statistic using standard equations. Overall incidence of cirrhosis or HCC was also evaluated using the Nelson-Aalen methods for estimating cumulative hazards rate, and competing risks methods were applied with death as a censoring event. The log-rank testing was used to compare cumulative incidence curves between groups. Statistical analyses were performed using SQL and SAS® Studio 3.6 on SAS® 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as a two-tailed P value < 0.05. This study was approved by the Stanford University Institutional Review Board and the VA Palo Alto Healthcare System Research and Development Committee. Waiver of informed consent was granted by the aforementioned institutional review board.
| Results | ▴Top |
A total of 585 CHB patients were in the moderate HBV DNA level group, and 544 patients were in the high HBV DNA level group (Table 1). Median follow-up time was 57 months (IQR: 29 - 106). The majority of patients were men and were on entecavir antiviral therapy. Among patients with moderate HBV DNA, 41.3% were HBeAg positive, whereas 85.0% were HBeAg positive in the high HBV DNA group. Race/ethnicity distribution was similar between both groups, with the majority being non-Asian (mostly NHW and Black/AA). Patients in the high HBV DNA were slightly older than those in the moderate HBV DNA group (58.1 vs. 55.9 years). Table 1 also describes alcohol use and tobacco use between the two groups. When evaluating by baseline FIB-4 scores, 21.1% of patients with moderate HBV DNA had FIB-4 > 3.25, compared to 27.7% of patients in the high HBV DNA group (Table 1).
 Click to view | Table 1. Characteristics of the Study Cohort |
The incidence of cirrhosis was 4.15 per 100,000 person-years (95% confidence interval (CI): 3.49 - 4.93) in the moderate HBV DNA group vs. 4.84 per 100,000 person-years (95% CI: 4.08 - 5.74) in the high HBV DNA group (Table 2). After propensity score weighting, no significant difference in the incidence of cirrhosis was observed between the two groups (4.55 per 100,000 person-years in the moderate HBV DNA vs. 5.22 per 100,000 person-years in the high HBV DNA) (Table 2, Fig. 1). On Cox regression analyses, when compared to CHB patients with high baseline HBV DNA, no significant difference in risk of cirrhosis was observed in the moderate HBV DNA group both before propensity score weighting (HR: 0.87, 95% CI: 0.69 - 1.11, P = 0.28) and after propensity score weighting (HR: 0.87, 95% CI: 0.69 - 1.09, P = 0.22) (Table 2).
 Click to view | Table 2. Incidence and Risk of Cirrhosis Before and After Propensity Score Weighing by Baseline Levels of HBV DNA |
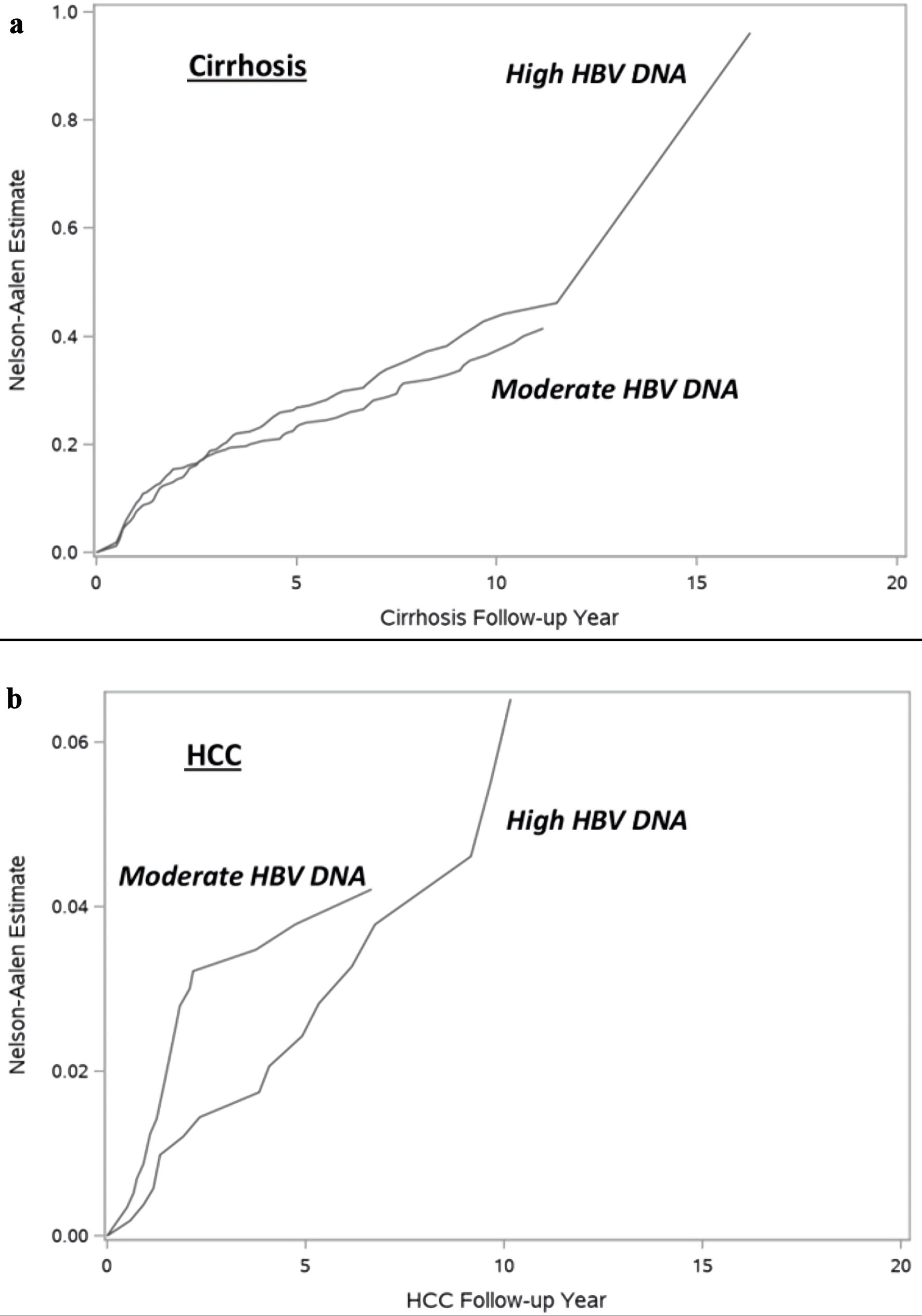 Click for large image | Figure 1. Figure 1. Incidence of (a) cirrhosis and (b) hepatocellular carcinoma by baseline HBV DNA. HBV: hepatitis B virus; HCC: hepatocellular carcinoma. |
The incidence of HCC was 0.55 per 100,000 person-years (95% CI: 0.35 - 0.85) in the moderate HBV DNA group vs. 0.50 per 100,000 person-years (95% CI: 0.31 - 0.82) in the high HBV DNA group (Table 3). After propensity score weighting, the incidence of HCC was 0.84 per 100,000 person-years in the moderate HBV DNA and 0.69 per 100,000 person-years in the high HBV DNA) (Table 3, Fig. 1). On Cox regression analyses, when compared to CHB patients with high baseline HBV DNA, no significant difference in risk of HCC was observed in the moderate HBV DNA group before propensity score weighting (HR: 1.12, 95% CI: 0.58 - 2.17, P = 0.73). However, after propensity score weighting, patients with moderate HBV DNA had significantly higher risk of HCC compared to patients with high HBV DNA (HR: 1.33, 95% CI: 1.09 - 1.62, P < 0.01) (Table 3).
 Click to view | Table 3. Incidence and Risk of Hepatocellular Carcinoma Before and After Propensity Score Weighing by Baseline Levels of HBV DNA |
| Discussion | ▴Top |
In a national population-based analysis of US veterans with CHB, we observed that patients with moderate levels of baseline HBV DNA had a significantly higher risk of HCC compared to those with high levels of baseline HBV DNA, but no difference in risk of cirrhosis was observed. Our findings confirm what has been recently observed in predominantly Asian populations with CHB. For example, in a previous study of 2,073 HBeAg-positive CHB patients across three centers in Korea who were maintained on CHB antiviral therapy (entecavir or tenofovir), an inverse relationship between baseline HBV DNA and long-term risk of HCC was reported [11]. Compared to CHB patients with 8.00 log10 IU/mL or higher HBV DNA, the adjusted HRs for HCC risk were 2.48 for patients with HBV DNA levels of 7.00 - 7.99 log10 IU/mL, 3.69 for HBV DNA levels of 6.00 - 6.99 log10 IU/mL, and 6.10 for HBV DNA levels of 5.00 - 5.99 log10 IU/mL [11]. In a more recent multicenter study of 4,693 CHB in Korea maintained on CHB antiviral therapy, the highest risk of HCC was observed in patients with moderate baseline viral loads (5.00 - 7.99 log10 IU/mL), and the lowest risk observed in patients with high viral loads (8.00 log10 IU/mL or higher) [12]. Our current study observed similar findings but is unique in demonstrating similar patterns in the association of baseline HBV DNA and risk of HCC in a predominantly non-Asian population with CHB. This is particularly important given the overall paucity of CHB data in non-Asian cohorts, and these observations support the suggestion for earlier initiation of CHB antiviral therapy tailored to HBV viral load to reduce long-term risks of HCC even among non-Asian populations. However, it is important to interpret the findings of our study in the context that the overall distribution of HBV DNA levels was lower than in previous Korean cohorts. Hence the thresholds we used for defining moderate and high levels of baseline HBV DNA were slightly different from previous studies. Nevertheless, the overall similar trends observed in the relationship between baseline HBV DNA and risk of HCC demonstrate consistent findings.
In addition, our study also evaluated incidence of cirrhosis as an outcome, which has not been extensively evaluated in prior studies. While we did not observe significant differences in risk of cirrhosis between those with moderate vs. high levels of baseline HBV DNA, the data seem to suggest a trend towards higher risk of cirrhosis in those with high HBV DNA vs. moderate HBV DNA. While cirrhosis certainly modulates the risk of long-term HCC, it is also important to emphasize that nearly a quarter of patients with CHB-related HCC do not have evidence of cirrhosis [16-18]. This emphasizes the point that the HBV itself is carcinogenic and even in the setting of non-cirrhosis, can induce mutations contributing to the higher risk of developing HCC. While the exact reasons for this inverse relationship between baseline HBV DNA and HCC and cirrhosis risk are not clear, several hypotheses have been discussed. Prior studies have suggested that high HBV DNA is usually associated with HBeAg positive stage of disease, whereas patients with a lower HBV DNA may reflect HBeAg negative patients, who have longer duration of infection. Studies have suggested that the low level persistent immune-mediated killing of HBV-infected hepatocytes, by the infiltration of cytotoxic T lymphocytes, may contribute to adaptive responses in the liver along the development of HBV-resistant hepatocytes. While this leads to overall declines in HBV DNA, this may represent progressive injury of hepatocytes through attempted immune mediated viral clearance, clonal hepatocyte repopulation, and a subsequent increase in the risk of HCC. Along the same lines, those with lower baseline HBV DNA likely reflect longer duration of potential HBV DNA integration into the host’s genome, and hence greater risk of HCC.
The strengths of this study include the utilization of a large, established, longitudinal cohort, which allows for accurate assessment of long-term CHB outcomes. In particular, there are generally limited data on CHB epidemiology among non-Asian populations, especially as it relates to evaluating baseline factors contributing to long-term risk of cirrhosis and HCC while maintained on CHB antiviral therapy. Thus, our cohort of predominantly non-Asians adds important data to the CHB literature. Furthermore, we applied propensity score weighting to balance potential differences in baseline factors that may confound the assessment of outcomes. However, certain limitations should be acknowledged. The mode of HBV transmission and hence the duration of CHB infection, is likely to be different between Asian and non-Asian cohorts. The majority of veterans with CHB likely acquired CHB infection during adulthood rather than vertical transmission, and hence the overall risk of HCC due to less duration of chronic infection may be lower in veterans with adult acquired CHB. Our cohort was predominantly men, and thus may have limited generalizability to women and non-veterans. However, this limitation should be balanced with the fact that veterans receive most, if not all, of their healthcare within VA health systems. Given the integrated nature of the national VA healthcare system, accurate assessment of long-term health outcomes is ensured. Along similar lines, our sample size had limited statistical power to perform sub-group analyses by patient characteristics. For example, when attempting to stratify the cohort by race/ethnicity, the sample size of some groups, especially in the high baseline HBV DNA group, became much smaller and limited the power to be able to evaluate differences in HCC or cirrhosis outcomes. While we utilized established definitions and previously validated algorithms to identify out study population and to define our study outcomes, there is a possibility of misclassification bias, although we would not necessarily expect any bias to be differential in nature. For example, there may be some misclassifications present such that patients with FIB-4 > 3.25 may have undiagnosed cirrhosis. However, we used established definitions and algorithms that have been previously used with the current dataset to accurately identify cirrhosis and thus we believe the misclassification was likely minimal. As previously noted, while we attempted to adjust for important baseline confounders using propensity score weighting, we acknowledge that unmeasured confounders may have affected the study outcomes as with all observational studies. Finally, one common limitation of observational studies is lost to follow-up or challenges in ascertaining outcome assessment for patients that utilize different healthcare systems. However, we believe the integrated nature of the VA health system is a strength in this regard, given that most veterans will continue longitudinal care within VA healthcare systems.
In conclusion, among a national longitudinal cohort of predominantly non-Asian veterans in the USA, with non-cirrhotic CHB on antiviral therapy, we observed that patients with baseline moderate levels of HBV DNA had significantly higher risk of HCC compared to patients with high levels of baseline HBV DNA. We did not observe differences in risk of cirrhosis by baseline HBV DNA. These data validate previous Asian studies showing the inverse relations of HBV DNA and risk of HCC in a predominantly non-Asian Western CHB population. Our findings taken together with recent data from Asian cohorts suggest that earlier initiation of CHB antiviral therapy targeting HBV DNA may further help reduce long-term risks of HCC.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by an investigator-initiated research study grant from Gilead Sciences.
Conflict of Interest
ZY and RC report no disclosures. JHJ has received grants from Gilead. JKL has received grants from Gilead, Intercept, Inventiva, Novo Nordisk, Pfizer, and Viking. YSL is an advisory board member of Gilead Sciences and receives investigator-initiated research funding from Gilead Sciences. RJW has received funding (to his institution) from Gilead Sciences, Exact Sciences, Theratechnologies, Durect Corporation and has served as a consultant for Gilead Sciences (without compensation).
Informed Consent
This study was approved by the Stanford University Institutional Review Board and the VA Palo Alto Healthcare System Research and Development Committee. Waiver of informed consent was granted by the aforementioned institutional review board.
Author Contributions
Study concept and design: all authors. Acquisition of data: ZY and RJW. Analysis and interpretation of the data: all authors. Statistical analyses: ZY and RJW. Drafting of the manuscript: ZY and RJW. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: RJW. All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AA: African American; API: Asian or Pacific Islander; CDW: Corporate Data Warehouse; CHB: chronic hepatitis B; FIB-4: fibrosis-4; HBeAg: hepatitis B e antigen; HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; NHW: non-Hispanic White; VA: Veterans Affairs
| References | ▴Top |
- Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, Kim WR, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74(2):607-626.
doi pubmed pmc - Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383-403.
doi pubmed - Wong RJ, Jain MK, Therapondos G, Niu B, Kshirsagar O, Thamer M. Low rates of hepatitis B virus treatment among treatment-eligible patients in safety-net health systems. J Clin Gastroenterol. 2022;56(4):360-368.
doi pubmed - Ogawa E, Yeo YH, Dang N, Le MH, Jeong D, Tran S, Henry L, et al. Diagnosis rates of chronic hepatitis B in privately insured patients in the United States. JAMA Netw Open. 2020;3(4):e201844.
doi pubmed pmc - Tang E, Torres S, Liu B, Baden R, Bhuket T, Wong RJ. High prevalence of cirrhosis at initial presentation among safety-net adults with chronic hepatitis B virus infection. J Clin Exp Hepatol. 2018;8(3):235-240.
doi pubmed pmc - Tang E, Liu B, Bhuket T, Wong RJ. Low rates of linkage and retention into care among patients with chronic HBV infection. Clin Gastroenterol Hepatol. 2019;17(9):1909-1911.
doi pubmed - Hu DJ, Xing J, Tohme RA, Liao Y, Pollack H, Ward JW, Holmberg SD. Hepatitis B testing and access to care among racial and ethnic minorities in selected communities across the United States, 2009-2010. Hepatology. 2013;58(3):856-862.
doi pubmed - Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468-475.
doi pubmed - Wong RJ, Jain MK, Therapondos G, Niu B, Kshirsagar O, Thamer M. Antiviral therapy reduces risk of cirrhosis in noncirrhotic HBV patients among 4 urban safety-net health systems. Am J Gastroenterol. 2021;116(7):1465-1475.
doi pubmed - Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58(5):1537-1547.
doi pubmed - Choi WM, Kim GA, Choi J, Han S, Lim YS. Increasing on-treatment hepatocellular carcinoma risk with decreasing baseline viral load in HBeAg-positive chronic hepatitis B. J Clin Invest. 2022;132(10):e154833.
doi pubmed pmc - Choi WM, Kim GA, Choi J, Choi GH, Lee YB, Sinn DH, Lim YS. Non-linear association of baseline viral load with on-treatment hepatocellular carcinoma risk in chronic hepatitis B. Gut. 2024;73(4):649-658.
doi pubmed - Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155(6):1828-1837.e1822.
doi pubmed pmc - Brummer J, Bloomfield K, Karriker-Jaffe KJ, Pedersen MM, Hesse M. Using the alcohol use disorders identification test to predict hospital admission for alcohol-related conditions in the Danish general population: a record-linkage study. Addiction. 2023;118(1):86-94.
doi pubmed pmc - Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61(1):292-302.
doi pubmed - Yen YH, Cheng YF, Wang JH, Lin CC, Wang CC. Characteristics and etiologies of hepatocellular carcinoma in patients without cirrhosis: When East meets West. PLoS One. 2021;16(1):e0244939.
doi pubmed pmc - Kalayci C, Johnson PJ, Davies SE, Williams R. Hepatitis B virus related hepatocellular carcinoma in the non-cirrhotic liver. J Hepatol. 1991;12(1):54-59.
doi pubmed - Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956-967.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


