| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 3, June 2024, pages 133-145
The Risk of Infection-Caused Mortality in Gastric Adenocarcinoma: A Population-Based Study
Adnan Malika, d, Farman Alib, Muhammad Imran Malikc, Shahbaz Qureshia
aDivision of Gastroenterology, Mountain Vista Medical Center, Mesa, AZ, USA
bCorewell Health Dearborn Hospital, Dearborn, MI, USA
cAiredale General Hospital, Keighley, UK
dCorresponding Author: Adnan Malik, Mountain Vista Medical Center, Mesa, AZ, USA
Manuscript submitted March 19, 2024, accepted May 4, 2024, published online June 29, 2024
Short title: Infection-Caused Mortality in GAC
doi: https://doi.org/10.14740/gr1715
| Abstract | ▴Top |
Background: Gastric adenocarcinoma (GAC) is a deadly tumor. Postoperative complications, including infections, worsen its prognosis and may affect overall survival. Little is known about perioperative complications as well as modifiable and non-modifiable risk factors. Early detection and treatment of these risk factors may affect overall survival and mortality.
Methods: We extracted GAC patient’s data from the Surveillance, Epidemiology, and End Results (SEER) database and analyzed using Pearson’s Chi-square, Cox regression, Kaplan-Meier, and binary regression methods in SPSS.
Results: At the time of analysis, 59,580 GAC patients were identified, of which 854 died of infection. Overall, mean survival in months was better for younger patients, age < 50 years vs. ≥ 50 years (60.45 vs. 56.75), and in females vs. males (65.23 vs. 53.24). The multivariate analysis showed that the risk of infectious mortality was higher in patients with age ≥ 50 years (hazard ratio (HR): 3.137; 95% confidence interval (CI): 2.178 - 4.517), not treated with chemotherapy (HR: 1.669; 95% CI: 1.356 - 2.056), or surgery (HR: 1.412; 95% CI:1.132 - 1.761) and unstaged patients (HR: 1.699; 95% CI: 1.278 - 2.258). In contrast, the mortality risk was lower in females (HR: 0.658; 95% CI: 0.561 - 0.773) and married patients (HR: 0.627; 95% CI: 0.506 - 0.778). The probability of infection was higher in older patients (odds ratio (OR) of 2.094 in ≥ 50 years), other races in comparison to Whites and Blacks (OR: 1.226), lesser curvature, not other specified (NOS) as a primary site (OR: 1.325), and patients not receiving chemotherapy (OR: 1.258).
Conclusion: Older, unmarried males with GAC who are not treated with chemotherapy or surgery are at a higher risk for infection-caused mortality and should be given special attention while receiving treatment.
Keywords: Infections; Gastric adenocarcinoma; Mortality; SEER; Large database; Public health
| Introduction | ▴Top |
Gastric cancer (GC) is overall the fifth most common cancer. It is the third most common cause of mortality among all cancers. In 2018, approximately one million new cases and more than 780,000 deaths occurred due to GC worldwide [1]. In 2019, the estimated number of new GC cases in the United States was 27,510, with 4,340 deaths [2]. Gastric adenocarcinoma (GAC) is the predominant type of GC, accounting for more than 90% of the cases. GAC has two main types: the most common is the intestinal type, and the other is the diffuse type [3]. Other gastric malignancies include lymphoma and leiomyosarcoma [4, 5]. The incidence and prognosis of GC vary according to demographic and other personal factors; for example, it has more incidence in Asian and Black races than in a White race and in males than females. The incidence increases in older age groups. The median age of GC diagnosis is 70 years for males and 74 for females [2]. Risk factors for GC include various dietary, occupational, and personal characteristics. Dietary risk factors include low vitamins A and C, a high-salt diet, and dietary N-nitroso compounds. Occupational factors include exposure to rubber manufacturing, metal processing, and coal. Other risk factors include smoking, blood group A, exposure to radiation, and gastroesophageal reflux disease (GERD). The latter increases the incidence of adenocarcinoma in the distal esophagus and proximal stomach, including the gastroesophageal junction. Infection with Helicobacter pylori is a definite gastric carcinogen [6-8]. A previous study estimated that H. pylori infection accounts for 6.2% of all GCs [9]. Another study found that GAC incidence in persons with H. pylori colonization is about 3% compared with zero in persons without colonization [10].
GAC is more prevalent in developing countries, but its incidence has decreased in developed countries over the past decades. This is primarily due to the control of risk factors, including lifestyle modification, environmental risk factors, and infection treatment with H. pylori [11-14]. However, the incidence of proximal GAC is increasing in developed countries [15, 16].
Localized stage GAC has a cure rate greater than 50%. However, GAC is an aggressive cancer, and only 10-20% of cases are diagnosed in the early stage of the disease in the United States. At the same time, most patients present with a regional or distant spread [17]. Surgery, including resection and adequate lymphadenectomy, is the only curative intervention in cases with localized disease. In patients with apparently localized proximal GAC, the 5-year survival is still low and ranges from 10% to 15% [6, 18]. Even after complete resection, 20-60% of patients experience the disease’s recurrence [19, 20]. Patients with metastatic GAC receive palliative therapy aiming at improving survival via better tolerance of chemotherapy [18].
The advanced tumor stage is the primary predictive index for survival and mortality in GAC due to the lymphatic and blood spread [4]. Male sex is associated with a higher risk of mortality [2]. Also, postoperative complications are associated with a worse prognosis in gastrointestinal and other malignancies [21-24]. Ma et al (2020) stated that postoperative infections independently affect cancer recurrence and patients’ survival after curative GAC resection surgery [25]. These complications include postoperative infections that independently worsen the prognosis of GAC [26, 27]. Studies suggest prolonged inflammations weaken the patients’ immunity and allow the micro-metastasis to regrow [25]. Perioperative treatment aiming to improve immunity limits postoperative immunosuppression and infections and decreases metastasis progression [25, 28].
Risk factors of mortality due to infections in patients with GAC need to be identified and controlled to reduce the morbidity and mortality related to these infectious diseases. In this retrospective study, we aimed to identify the risk factors associated with increased mortality risk due to infectious diseases in patients with GAC.
| Materials and Methods | ▴Top |
Study population
Based on the Surveillance, Epidemiology, and End Results (SEER) database, we conducted this retrospective cohort study in patients with stomach adenocarcinoma. The patient’s data, including the treatment field from 1975 to 2016, were extracted using SEER*Stat version 8.3.8.
Information collection
The dependent variables were executed from the SEER program including: 1) death due to infectious diseases, including pneumonia, influenza, septicemia, tuberculosis, and other infections, i.e., human immunodeficiency virus (HIV); 2) death due to other causes rather than infection; and 3) survivors.
Besides, we gathered the independent variables such as: 1) age at diagnosis; 2) sex; 3) race, including White, Black, and others (American Indian/Alaska Native, Asian or Pacific Islander); 4) marital status, including single, married, separated, divorced, and widowed; 5) primary site in the stomach, including the fundus, body, gastric antrum, pylorus, and not otherwise specified (NOS) stomach, cardia, lesser and greater curvature; 6) stage, including localized, regional, distant, and unstaged; and 7) treatment field, including chemotherapy, surgery, and radiation (beam, other types, and none).
Statistical analysis
We conducted the data analyses using SPSS for Windows version 26.0. The relationships among categorical variables were observed using Pearson’s Chi-square test, and the data were presented as mean and standard deviation (SD) or number and percentage (%). The Cox regression analysis was used in the multivariate analysis to analyze the effect of the variables on the time of death, and the results showed a hazard ratio (HR) and 95% confidence interval (CI). The time to death among the patients was investigated as a univariate analysis by the Kaplan-Meier method, and the data were displayed as mean (months) and 95% CI. The probability of infection was assessed using binary regression analysis, and the results were presented as odds ratio (OR) and 95% CI. The P-value of less than 0.05 means the analysis showed a significant effect.
Ethical approval
Ethical and IRB approval are not applicable.
| Results | ▴Top |
Clinicopathologic characteristics
Table 1 shows the clinicopathologic features of the included patients. Overall, 59,580 patients with stomach adenocarcinoma were in our cohort, with a mean age of 68.49 years. The total survival of patients, infectious mortality, and other mortality were 7,427 (12.46%), 854 (1.44%), and 51,299 (86.10%), respectively.
 Click to view | Table 1. Clinicopathologic Features of Gastric Adenocarcinoma Patients |
Univariate analysis of infectious patients
Higher mean survival month of GAC patients was noted in younger individuals (< 50 years: 60.45 vs. ≥ 50 years: 56.75), female gender vs. males (65.23 vs. 53.24), and other races vs. Whites and Blacks (78.91 vs. 54.01 and 47.21, respectively). Overall, the mean survival in months based on marital status was 33.28 for singles, 62.79 for married, 96.66 for separated, 50.37 for divorced, and 54.18 for widowed subjects. The mean survival months in descending order for the primary site was 77.88 in lesser curvature, NOS vs. 74.63 for pylorus vs. 62.49 for gastric antrum vs. 55.88 for fundus vs. 55.32 for body vs. 53.48 for greater curvature, NOS vs. 52.29 for stomach, NOS and 42.39 for cardia, NOS. Localized stage patients have a higher mean survival month of 76.28 vs. 59.83 for regional vs. 23.90 for unstaged and 15.13 for distant spread stage. The overall survival month was higher, i.e., 68.25 in patients with surgery vs. 14.01 without surgery. Also, the overall survival month was 61.60 for patients receiving chemotherapy vs. 42.23 for patients not receiving/unknown chemotherapy. The overall survival month was 59.47 for patients treated with another type of radiation vs. 45.31 for patients not treated with radiation, and 44 for patients treated by beam radiation. Details of the univariate analysis are shown in Table 2.
 Click to view | Table 2. Univariate Analysis Using Kaplan-Meier Test |
Multivariate analysis of infectious patients
Figures 1-9 show the survival curves of each variable for infection-caused mortality. The multivariate analysis showed that the increased risk of infection-related mortality in patients with age ≥ 50 years (HR: 3.137; 95% CI: 2.178 - 4.517), patients not treated with chemotherapy (HR: 1.669; 95% CI: 1.356 - 2.056), or surgery (HR: 1.412; 95% CI: 1.132 - 1.761) and unstaged patients (HR: 1.699; 95% CI: 1.278 - 2.258). In contrast, a reduced risk of infection-related mortality was noted in females (HR: 0.658; 95% CI: 0.561 - 0.773) and married patients (HR: 0.627; 95% CI: 0.506 - 0.778). Table 3 shows details of the multivariate analysis.
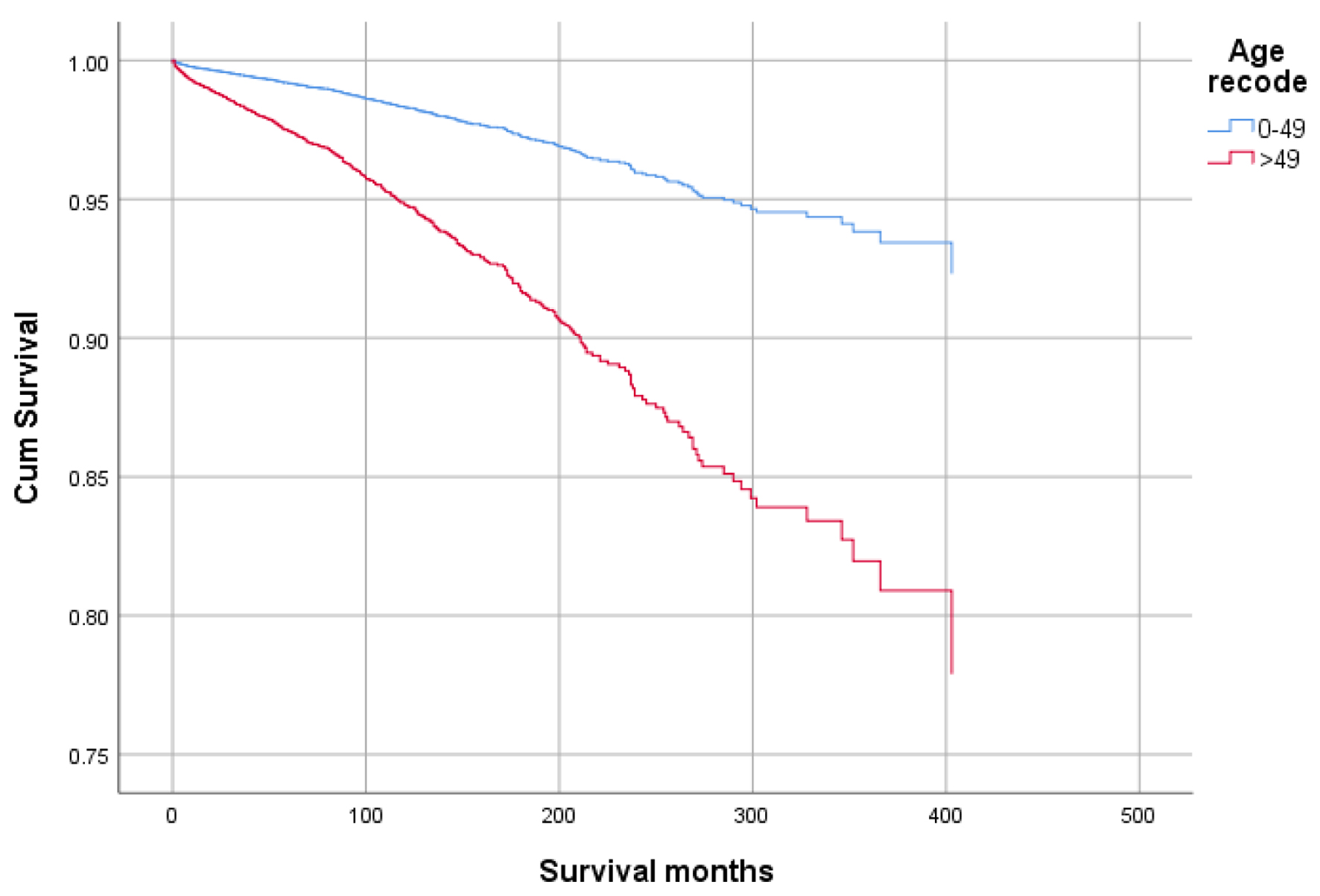 Click for large image | Figure 1. Survival curve for infection-caused mortality with age. |
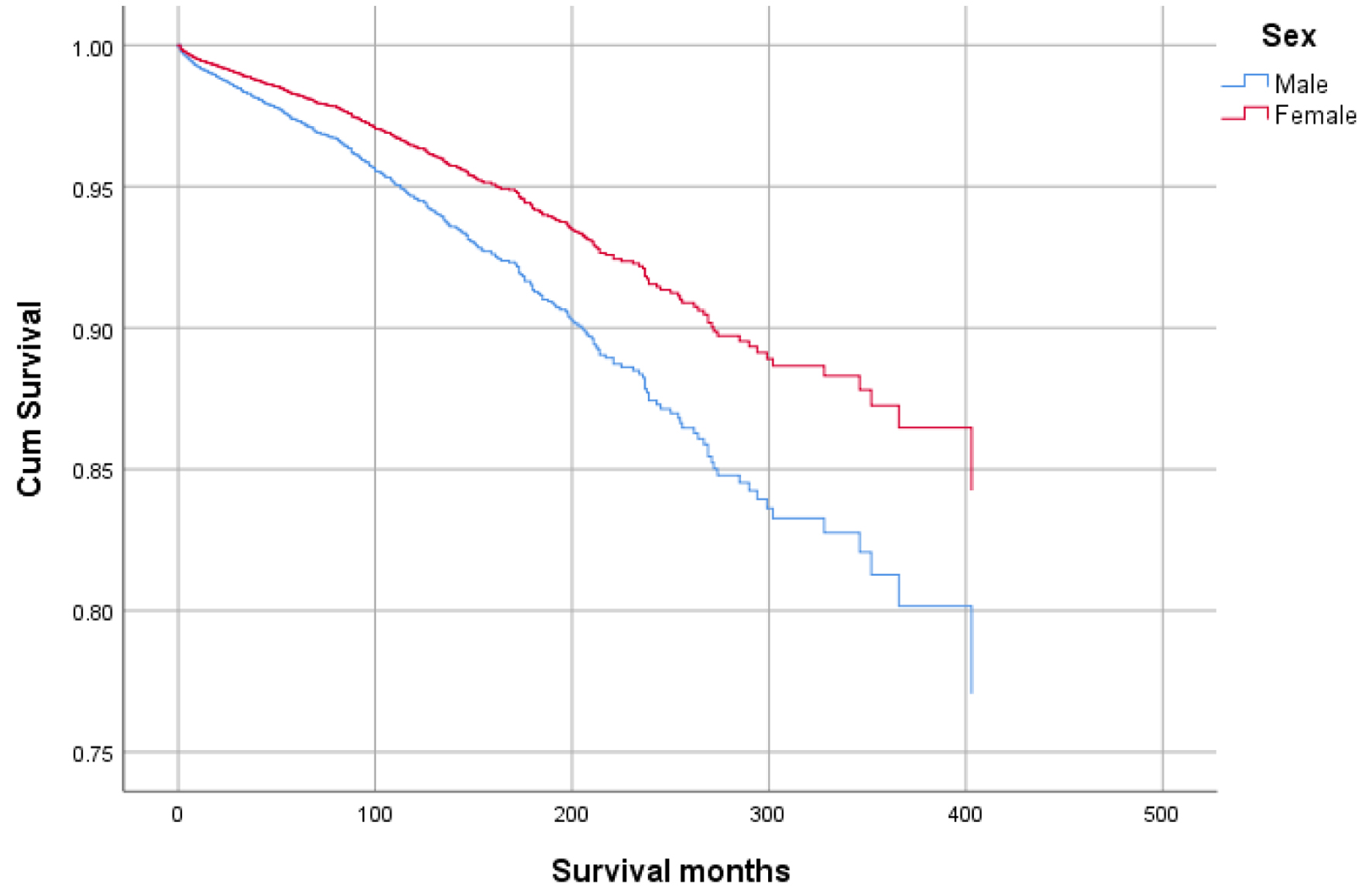 Click for large image | Figure 2. Survival curve for infection-caused mortality with sex. |
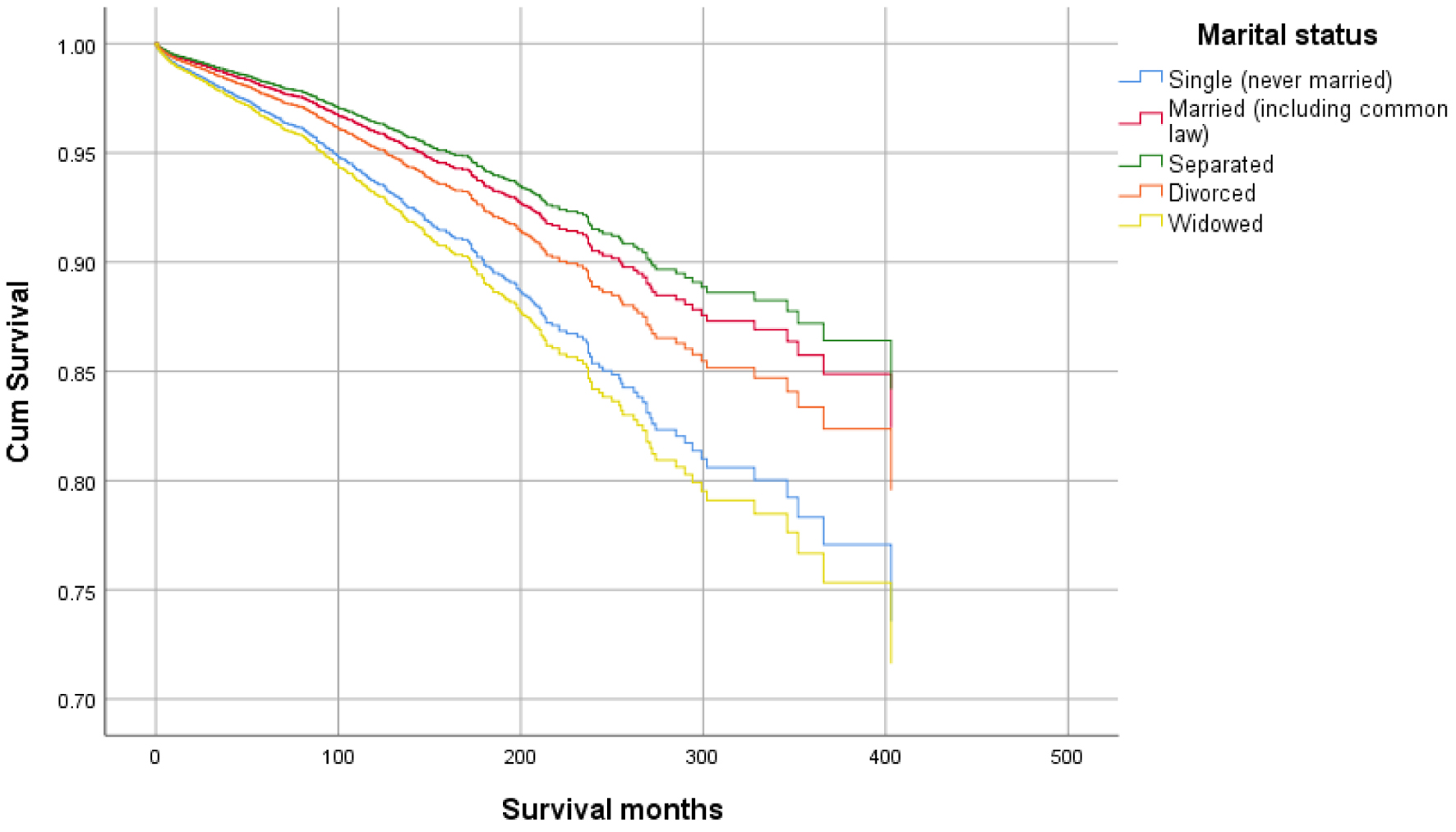 Click for large image | Figure 3. Survival curve for infection-caused mortality with marital status. |
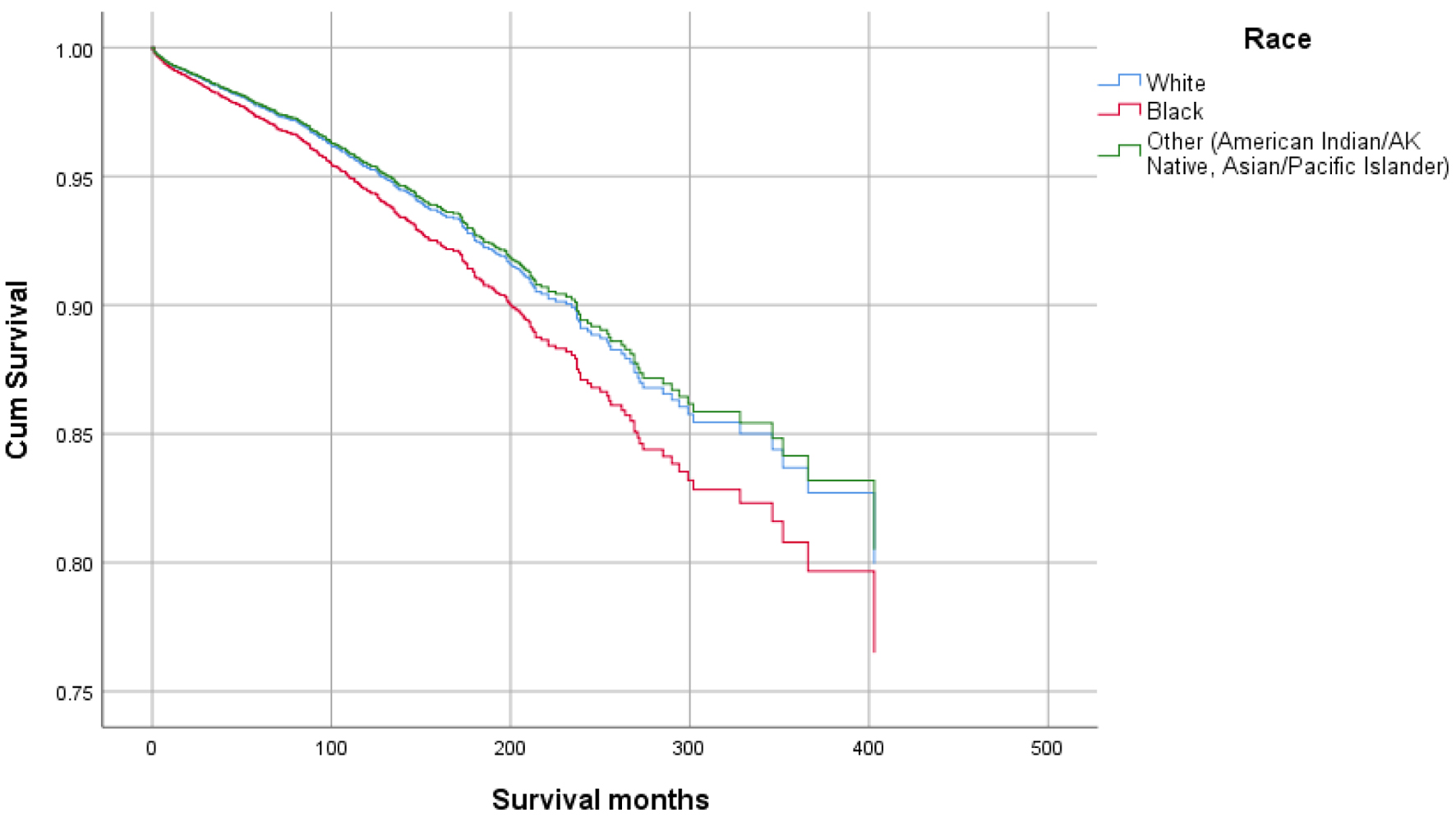 Click for large image | Figure 4. Survival curve for infection-caused mortality with race. |
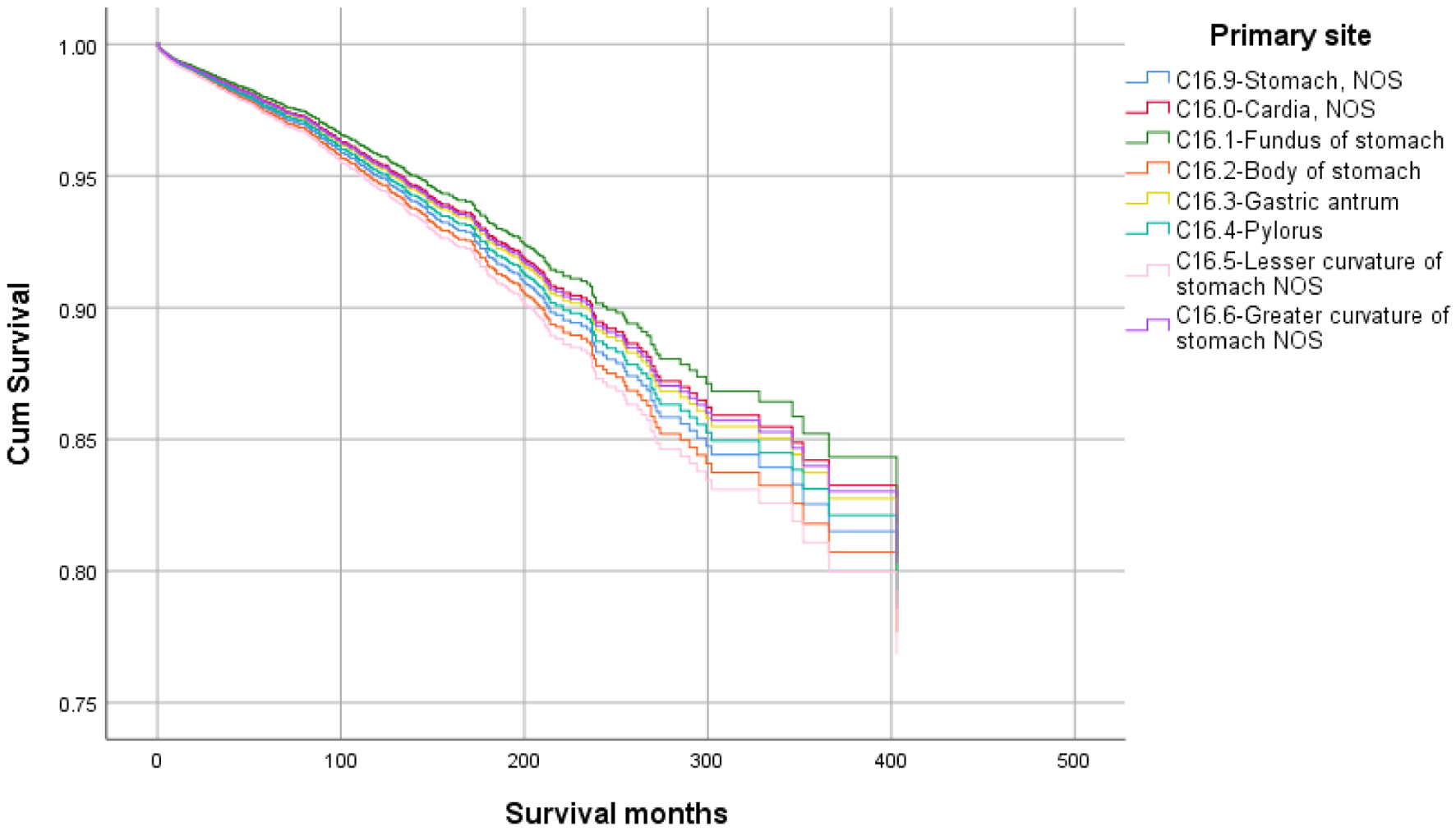 Click for large image | Figure 5. Survival curve for infection-caused mortality with primary site. |
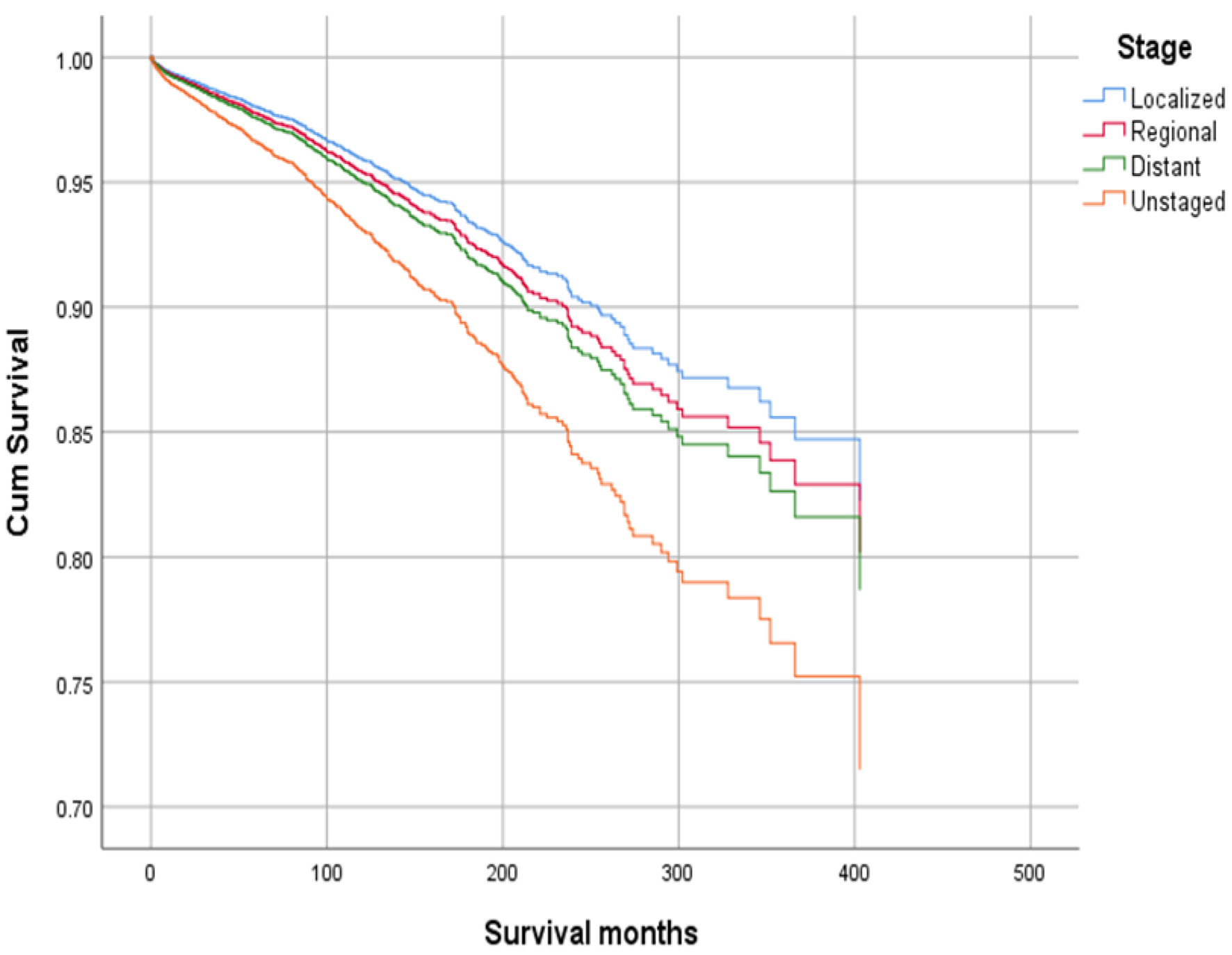 Click for large image | Figure 6. Survival curve for infection-caused mortality with stage. |
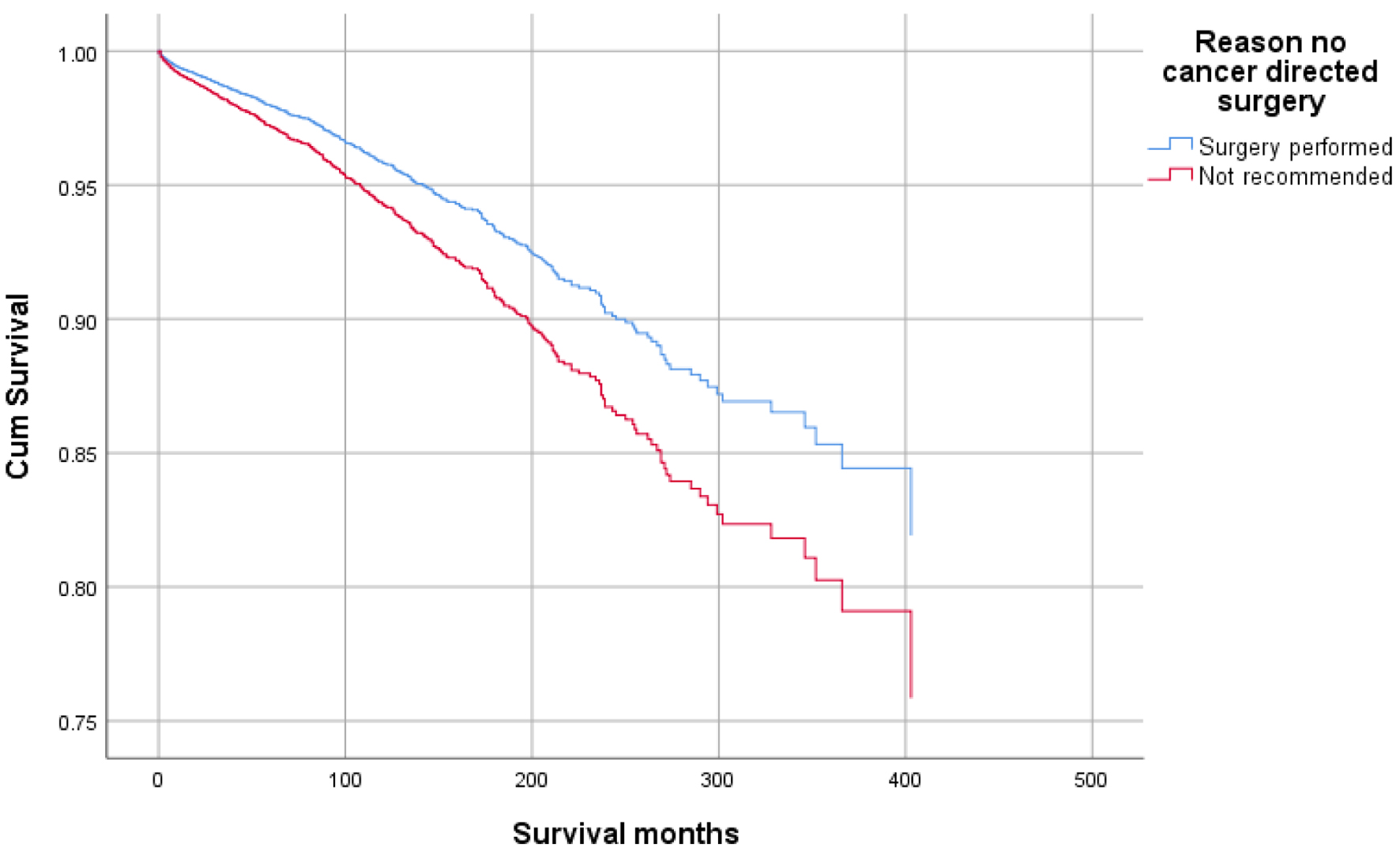 Click for large image | Figure 7. Survival curve for infection-caused mortality with surgery. |
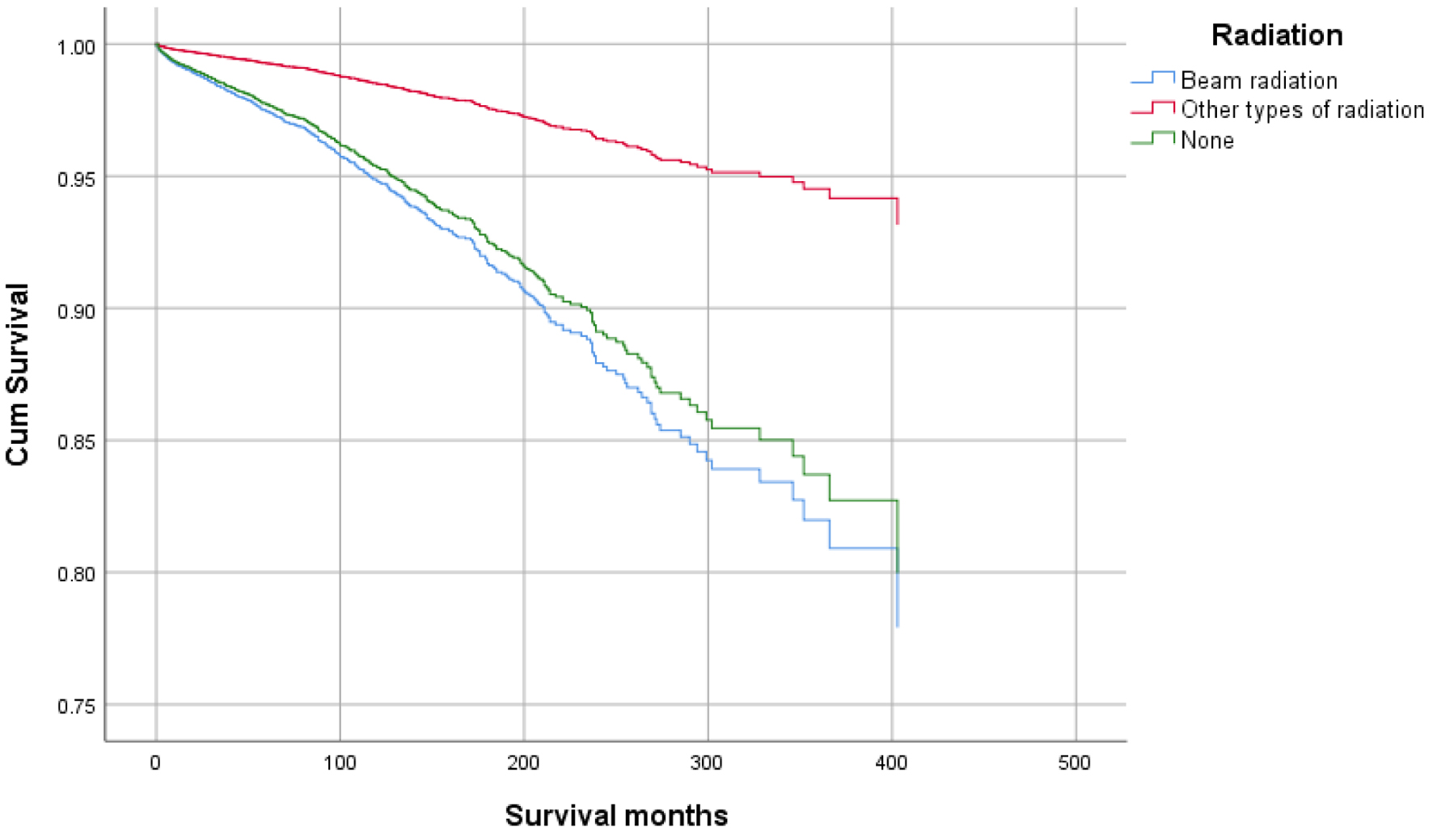 Click for large image | Figure 8. Survival curve for infection-caused mortality with radiation. |
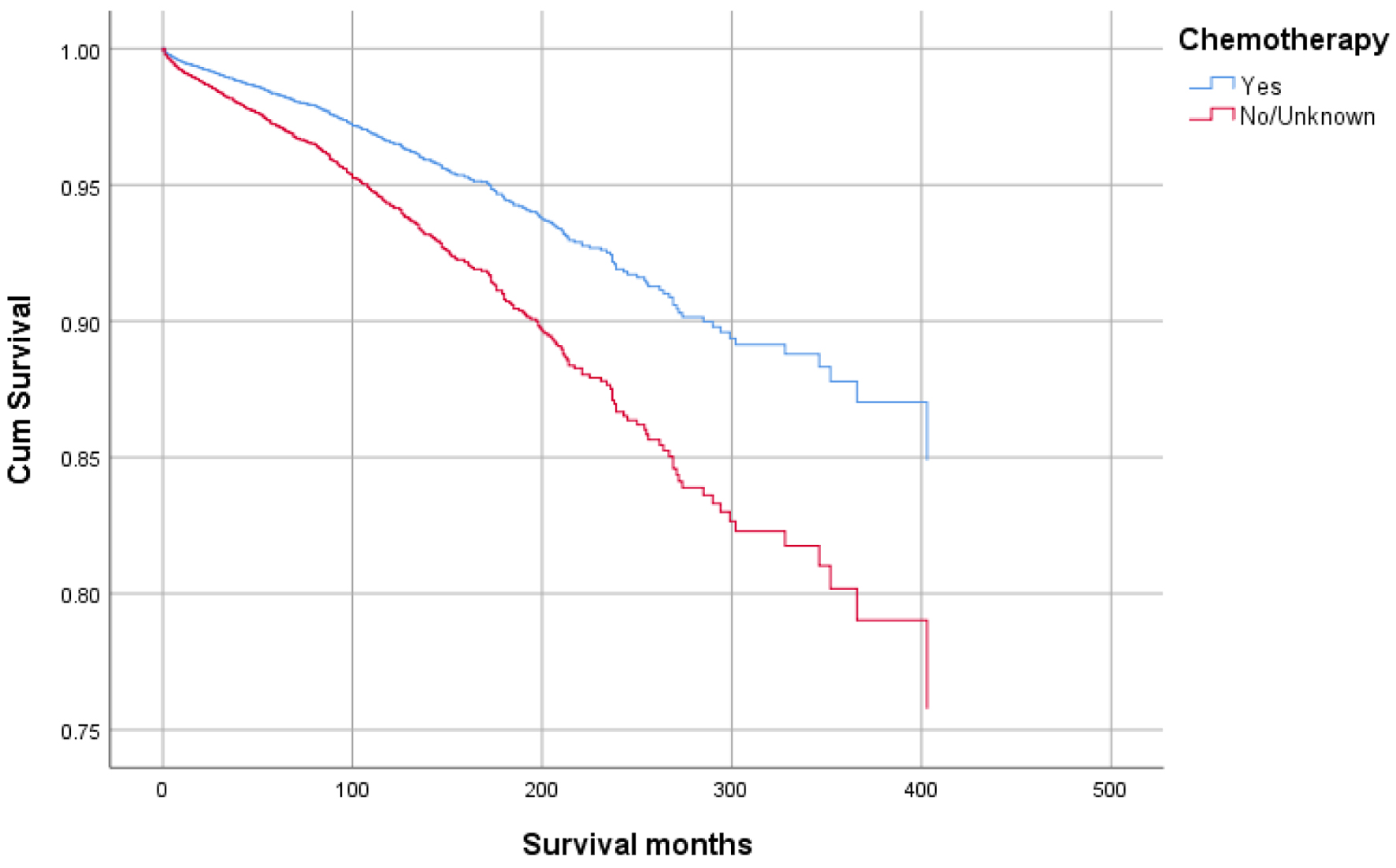 Click for large image | Figure 9. Survival curve for infection-caused mortality with chemotherapy. |
 Click to view | Table 3. Multivariate Analysis Using Cox Regression Test |
Binary regression for infection
The probability of infection in adenocarcinoma patients increased in patients with age ≥ 50 years (OR: 2.094; 95% CI: 1.453 - 3.018), other races vs. White and Black races (OR: 1.226; 95% CI: 1.006 - 1.494), lesser curvature, NOS as a primary site (OR: 1.325; 95% CI: 1.02 - 1.721) and patients without chemotherapy (OR: 1.258; 95% CI: 1.029 - 1.538). The probability of infection decreased in females (OR: 0.789; 95% CI: 0.671 - 0.928), married patients (OR: 0.715; 95% CI: 0.574 - 0.89), patients not treated by surgery (OR: 0.549; 95% CI: 0.447 - 0.673), regional (OR: 0.561; 95% CI: 0.478 - 0.66) and distant stage (OR: 0.354; 95% CI: 0.284 - 0.442). Table 4 shows details of the binary regression analysis.
 Click to view | Table 4. The Probability of Infection Using Binary Regression Test |
| Discussion | ▴Top |
This study analyzed 59,580 patients with GAC registered in the SEER database. The factors associated with higher mortality rates due to infections in patients with GAC included older age at diagnosis (age > 50 years), male sex, no or unknown history of chemotherapeutic treatment, patients without surgery, and unstaged disease.
In contrast, younger age at diagnosis, female sex, and married status appear to have a protective effect with reduced mortality. Race, other marital statuses (single, separated, divorced, and widowed), disease’s primary site, radiation therapy, and regional and distant spread had no significant effect on mortality.
The binary regression analysis showed that patients with older age at diagnosis, male sex, and those with no or unknown history of exposure to chemotherapy had a higher risk of infection.
However, females and married status were associated with lower infection rates. Also, patients not recommended for surgery and patients with regional and distant disease stages had a lower risk of infection. Race (White and Black), other marital statuses (single, separated, divorced, and widowed), other primary cancer sites, radiation exposure, and unstaged have no significant risk for infection. Races other than Black and White, i.e., Asians and other races, had a higher risk for infection but not for death due to infection. Prior studies have reported that Asians have better survival after diagnosis of GAC compared to Whites, which may be related to earlier diagnosis [29-31].
Prior studies [29, 31] have reported better survival in patients with surgical intervention for GAC due to operability feasibility in early-stage cancers. Married status in patients with GC is associated with lower mortality, as reported in prior studies [32]. It may be indirectly related to lower psychosocial burden and better social support available for these patients, as reported in other studies [33, 34].
Cancer patients are more vulnerable to hospitalization with severe sepsis [35]. Previous studies have reported that severe sepsis has a higher risk of mortality in cancer patients, especially those with a distant spread, compared to concomitant chronic medical conditions, i.e., liver disease, renal failure, obstructive pulmonary diseases, and diabetes [35, 36]. There is a need to evaluate the risk factors for infection in GAC and the risk of increased mortality, which may help choose appropriate therapy for such patients and help improve patient outcomes.
Conclusion
Among patients with GAC, those with older age, unmarried status, male gender, unstaged cancer, history of chemotherapy, and not recommended for surgery have a higher risk of mortality due to infections.
Strengths and limitations
This study is based on large database registered in the SEER database and follow-up information. Death-related information of the study population is well documented in the SEER database. We have considered a wide variety of infections, including tuberculosis, pneumonia, influenza, septicemia, and other infections, including HIV infection, not only sepsis. However, the limitations of this study persist. There is no record of genetic and personal factors that may have affected the liability to infection and mortality due to infectious diseases. Data regarding tumor differentiation and histological subtypes of GAC were not included in analysis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Adnan Malik: supervision, date curation, data analysis, writing and editing the manuscript. Farman Ali: writing and editing the manuscript. Muhammad Imran Malik: data curation, writing and editing the manuscript. Shahbaz Qureshi: writing and editing the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
doi pubmed - Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a Histo-Clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49.
doi pubmed - Milano AF. 20-year comparative survival and mortality of cancer of the stomach by age, sex, race, stage, grade, cohort entry time-period, disease duration & selected ICD-O-3 oncologic phenotypes: a systematic review of 157,258 cases for diagnosis years 1973-2014: (SEER*Stat 8.3.4). J Insur Med. 2019;48(1):5-23.
doi pubmed - Wroblewski LE, Peek RM, Jr. Helicobacter pylori, cancer, and the gastric microbiota. Adv Exp Med Biol. 2016;908:393-408.
doi pubmed - Recio-Boiles A, Babiker HM. Gastric cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing. LLC.; 2020.
- Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50(8):1498-1509.
doi pubmed - Zhang Z, Xu G, Ma M, Yang J, Liu X. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology. 2013;145(1):113-120.e113.
doi pubmed - Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136(2):487-490.
doi pubmed - Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784-789.
doi pubmed - Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333(1):32-41.
doi pubmed - Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
doi pubmed - Song H, Ekheden IG, Zheng Z, Ericsson J, Nyren O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867.
doi pubmed pmc - Blot WJ, Devesa SS, Kneller RW, Fraumeni JF, Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287-1289.
pubmed - Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104(2):510-513.
doi pubmed - Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012.
doi pubmed pmc - Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
doi pubmed - Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87(3):353-357.
doi pubmed - Rivera F, Vega-Villegas ME, Lopez-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33(4):315-324.
doi pubmed - Kano K, Aoyama T, Yoshikawa T, Maezawa Y, Nakajima T, Hayashi T, Yamada T, et al. The negative survival impact of infectious complications after surgery is canceled out by the response of neoadjuvant chemotherapy in patients with esophageal cancer. Ann Surg Oncol. 2018;25(7):2034-2043.
doi pubmed - Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwe H, Decker G, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250(5):798-807.
doi pubmed - McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92(9):1150-1154.
doi pubmed - Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240(2):255-259.
doi pubmed pmc - Maezawa Y, Aoyama T, Ju M, Komori K, Kano K, Sawazaki S, Numata M, et al. The impact of severe infectious complications on long-term prognosis for gastric cancer. Anticancer Res. 2020;40(7):4067-4074.
doi pubmed - Hayashi T, Yoshikawa T, Aoyama T, Hasegawa S, Yamada T, Tsuchida K, Fujikawa H, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer. 2015;18(2):368-374.
doi pubmed - Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, Aikou S, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21(3):891-898.
doi pubmed - Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253(4):798-810.
doi pubmed - Le A, Berger D, Lau M, El-Serag HB. Secular trends in the use, quality, and outcomes of gastrectomy for noncardia gastric cancer in the United States. Ann Surg Oncol. 2007;14(9):2519-2527.
doi pubmed - Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22(9):2965-2971.
doi pubmed - Zhang X, Yang J, Huang Q, Lyu J. Prognostic factors in patients with gastric adenocarcinoma using competing-risk analysis: a study of cases in the SEER database. Scand J Gastroenterol. 2019;54(8):1015-1021.
doi pubmed - Qiu M, Yang D, Xu R. Impact of marital status on survival of gastric adenocarcinoma patients: Results from the Surveillance Epidemiology and End Results (SEER) Database. Sci Rep. 2016;6:21098.
doi pubmed pmc - Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, Baider L. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 2010;21(4):877-883.
doi pubmed - Goldzweig G, Hubert A, Walach N, Brenner B, Perry S, Andritsch E, Baider L. Gender and psychological distress among middle- and older-aged colorectal cancer patients and their spouses: an unexpected outcome. Crit Rev Oncol Hematol. 2009;70(1):71-82.
doi pubmed - Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8(5):R291-298.
doi pubmed pmc - Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


