| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 6, December 2023, pages 307-317
Marital Status Is a Prognostic Factor for Cardiovascular Mortality but Not a Prognostic Factor for Cancer Mortality in Siewert Type II Adenocarcinoma of the Esophagogastric Junction
Zhong Qiang Zhenga, Xuan Zi Sunb, c
aDepartment of General Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
bDepartment of Radiation Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
cCorresponding Author: Xuan Zi Sun, Department of Radiation Oncology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi’an, Shaanxi Province, China
Manuscript submitted August 24, 2023, accepted November 10, 2023, published online December 9, 2023
Short title: Marital Status in Siewert Type II AEG
doi: https://doi.org/10.14740/gr1670
| Abstract | ▴Top |
Background: The impact of marital status on the prognosis of patients with Siewert type II adenocarcinoma of the esophagogatric junction (AEG) remained unclear. This study aimed to investigate the associations of marital status with cancer-specific death risk and cardiovascular death risk in Siewert type II AEG patients.
Methods: Data for Siewert type II AEG patients were obtained from the Surveillance, Epidemiology, and End Results database from 2010 to 2015. A 1:1 propensity score matching (PSM) was applied to reduce inter-group bias between the married and unmarried groups. Kaplan-Meier analysis, a competing risk model and the Fine-Gray multivariable regression model were used to identify the prognostic value of marital status.
Results: In total, 1,623 subjects were included. After PSM, according to Fine-Gray multivariable regression analysis, there was no significant difference in the cumulative cancer-specific death rate between the married and the unmarried groups (hazard ratio (HR): 1.160, 95% confidence interval (CI): 0.994 - 1.354, P = 0.060). Patients in unmarried group had a higher cardiovascular death rate than patients in married group (HR: 3.066, 95% CI: 1.372 - 6.850, P = 0.006).
Conclusions: Our study demonstrates that unmarried Siewert type II AEG patients are associated with higher cardiovascular death risk but not cancer-specific death risk compared with married patients.
Keywords: Adenocarcinoma of the esophagogatric junction; SEER; Marital status; Competing risk model; Cardiovascular mortality
| Introduction | ▴Top |
Gastric cancer is one of the most commonly diagnosed malignant cancers, with one million new cases and 770,000 related deaths each year worldwide [1]. The prevalence of adenocarcinoma of the esophagogastric junction (AEG) has dramatically increased in the past decades, and the prognosis remains dismal [2, 3]. AEG is defined as the tumor that the epicenter of the tumor is within 5 cm of the esophagogastric junction (EGJ) [4]. According to the Siewert classification, AEG is classified into three subgroups: Siewert type I, Siewert type II and Siewert type III [5]. Siewert types I and III AEGs are characterized by a consistent pathology that closely resembles esophageal cancer and gastric cancer, respectively. The treatment strategy for Siewert type II AEG still remains controversial because it is difficult to define its origin. Siewert type II AEG is defined as the tumor with the epicenter located from 1 cm above to 2 cm below the EGJ, and is usually defined as the true tumor of EGJ. Previous studies have mainly focused on cancer-specific death in Siewert type II AEG patients [6]. However, related research for cardiovascular death (CVD), as the major cause of non-cancer-specific mortalities, is relatively scarce in the worldwide. Therefore, further studies are urgently needed to identify the prognostic factors for CVD in Siewert type II AEG patients.
Previous studies largely focused on the relationship between clinicopathological characteristics, anti-tumor therapy status and cardiovascular mortalities (CVMs) in patients with cancer [7-9]. Several studies reported that socioeconomic factors, especially marital status, are prognostic factors for CVD in patients with cancer [8, 10]. However, the prognostic value of marital status for CVM in patients with Siewert type II AEG is rarely reported. The clinical value of marital status for Siewert type II AEG patients remains unknown. Therefore, more real-world studies are needed to confirm the impact of marital status in Siewert type II AEG patients.
| Materials and Methods | ▴Top |
Patient cohort
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) is a cancer database that consists of clinical data of cancer patients from 18 registries and covers approximately 28% of the United States population [11]. The data of Siewert type II AEG patients with primary resection and regional lymph nodes dissection in the SEER database between January 2010 and December 2015 were extracted from SEER*Stat software version 8.3.9 (Information Management Service, Inc., Calverton, MD, USA). Although the information of Siewert AEG classification was not included in the SEER database, Siewert type II patients satisfied a primary site entry of “Cardia, NOS” and a collaborative staging (CS) Schema V0204+entry of “EsophagusGEJunction” were extracted [12, 13]. All procedures were performed in accordance with guidelines in NCI SEER program [14]. The SEER database is a public anonymized database, so ethical approval was not required for this retrospective study. The inclusion criteria included the following: 1) primary Siewert type II AEG (histology codes based on ICD-O-3: 8140-8147, 8160-8162, 8180-8221, 8250-8507, 8514-8551, 8571-8574, 8576, and 8940-8941); 2) aged ≥ 20 years; 3) undergone radical surgery (surgery encode: 30-80) and regional lymph node dissection; 4) without distant metastasis; 5) with follow-up time ≥ 2 months. Patients with missing or unknown clinical records and with multiple primary tumors were excluded in this study. The selection procedure is shown in Figure 1.
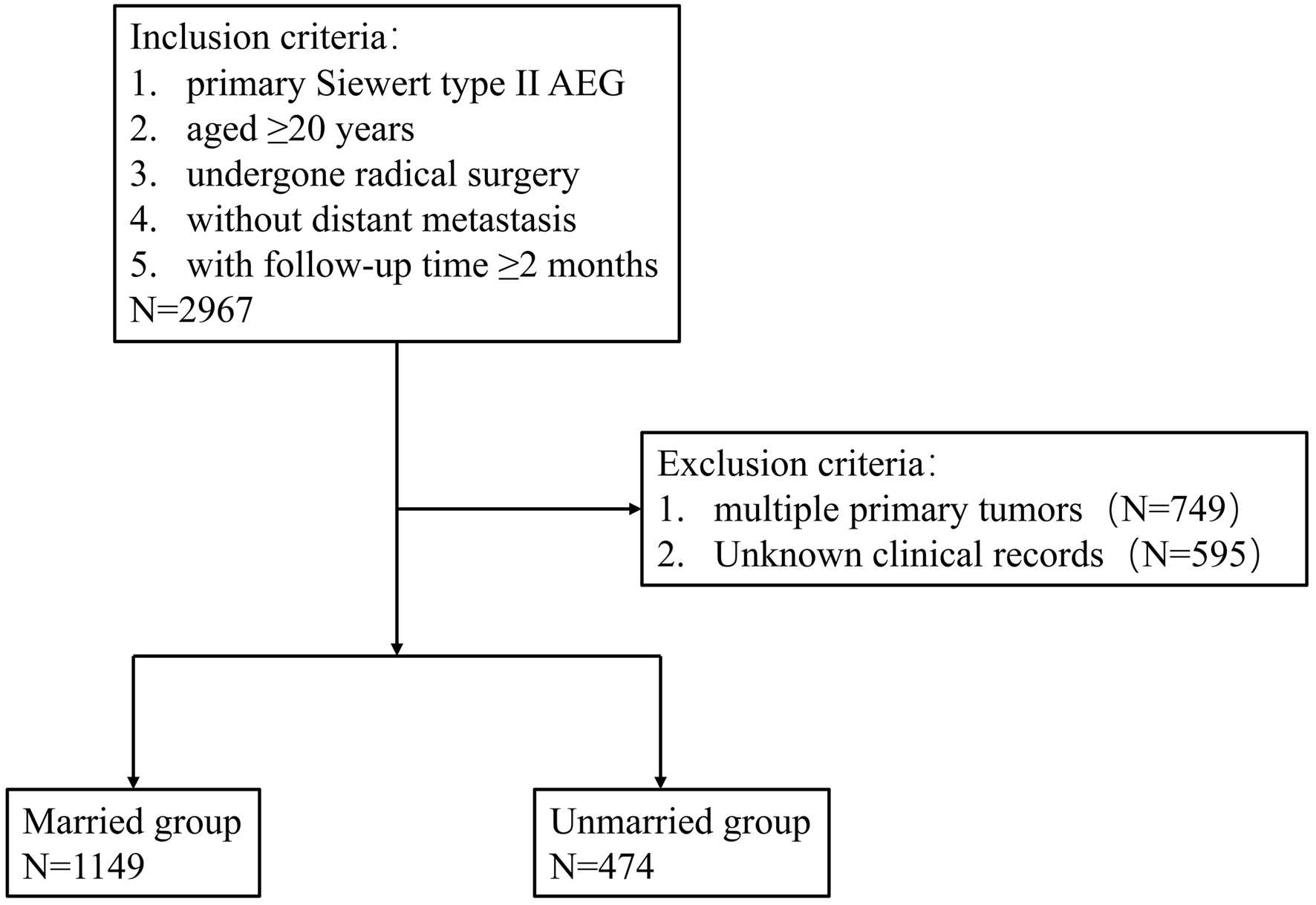 Click for large image | Figure 1. Eligibility, inclusion, and exclusion criteria of the study population. AEG: adenocarcinoma of the esophagogatric junction. |
In our study, the following variables were included: age at diagnosis, sex, race, grade, stage, T status according to American Joint Committee on Cancer (AJCC) seventh edition, N status according to AJCC seventh edition, number of removed lymph nodes (RLN), lymph node metastasis ratio (LNR), tumor size, chemotherapy status, radiotherapy status, marital status, vital status, survival months, and cause of death.
A total of 1,623 Siewert type II AEG patients were included in the study. Patients were categorized into two groups: married and unmarried (which includes single, divorced, separated, and widowed individuals), based on their marital status. X-tile was used to determine optimal cutoff values for age (56 and 75 years), RLN (10 and 16), LNR (4.2 and 16.7) and tumor size (19 and 44 mm) [15]. “No/unknown” radiotherapy records were considered as no radiotherapy. “No/unknown” chemotherapy records were considered as no chemotherapy. CVD was defined as death due to cardiovascular disease as follows according to International Classification of Diseases-Tenth Revision: heart disease (I00-I09, I11, I13, I20-I51), hypertension without heart disease (I10, I12), cerebrovascular disease (I60-I69), atherosclerosis (I70), aortic aneurysm and dissection (I71) and other diseases of arteries, arterioles and capillaries (I72-I78).
The final follow-up was performed in November 2021, and the median follow-up was 37 months (ranging from 2 to 107 months). The follow-up period was defined as the time from the data of first diagnosis with AEG to the date of last follow-up or death.
Statistical analysis
In the present study, we used Kaplan-Meier analysis, a competing risk model and the Fine-Gray multivariable regression model to identify the prognostic value of marital status in Siewert type II AEG patients based on the SEER database. Additionally, we also used Kaplan-Meier analysis, a competing risk model and the Fine-Gray multivariable regression model to further explore the association between marital status and CVD risk.
The statistical analysis in this study was performed in IBM SPSS 24.0 software or R software (Version 4.1.3). To balance the bias between the married and unmarried subgroups, a 1:1 propensity score matching (PSM) was used to match one married patient with one unmarried patient by variables included in baseline [16]. Descriptive statistics and frequency tables were used to summarize the data. Categorical data were evaluated with the Chi-square test. The Kaplan-Meier survival curve analysis was applied to show the difference in overall survival (OS), AGE-specific survival and cardiovascular-specific survival between the married group and unmarried group, while the log-rank test was performed to estimate differences among groups. Competing risk model analysis and Gray’s test were used to identify statistical differences between AGE-specific death and non-AGE-specific death or cardiovascular disease-specific death and non-cardiovascular disease-specific death due to marital status by using the R package cmprsk. The Fine-Gray multivariable regression model was used to examine associations between factors and AEG cancer-specific death or cardiovascular disease-specific death. The difference was considered statistically significant for a two-sided P value < 0.05.
| Results | ▴Top |
Baseline characteristics
A total of 1,623 patients with Siewert type II AEG were included in this study before PSM. There were significant differences in age (P = 0.025), sex (P < 0.001) and race (P < 0.001) between married and unmarried groups. After PSM, 948 AEG patients were eventually included. There were no statistically differences between the two groups on the mentioned factors, except sex (P = 0.04), race (P < 0.001) and chemotherapy status (P = 0.04). The key information of the variables is shown in Table 1.
 Click to view | Table 1. Baseline Clinical Characteristics of Patients With Siewert Type II AEG |
Kaplan-Meier survival analysis
OS, cancer-specific survival and cardiovascular disease-specific survival in Siewert type II AEG patients were explored using Kaplan-Meier analysis. Among all 1,623 patients included in this study, 937 (57.73%) died. Compared with patients in the married group, patients in the unmarried group had worse OS, cancer-specific survival and cardiovascular disease-specific survival both before and after PSM (Fig. 2).
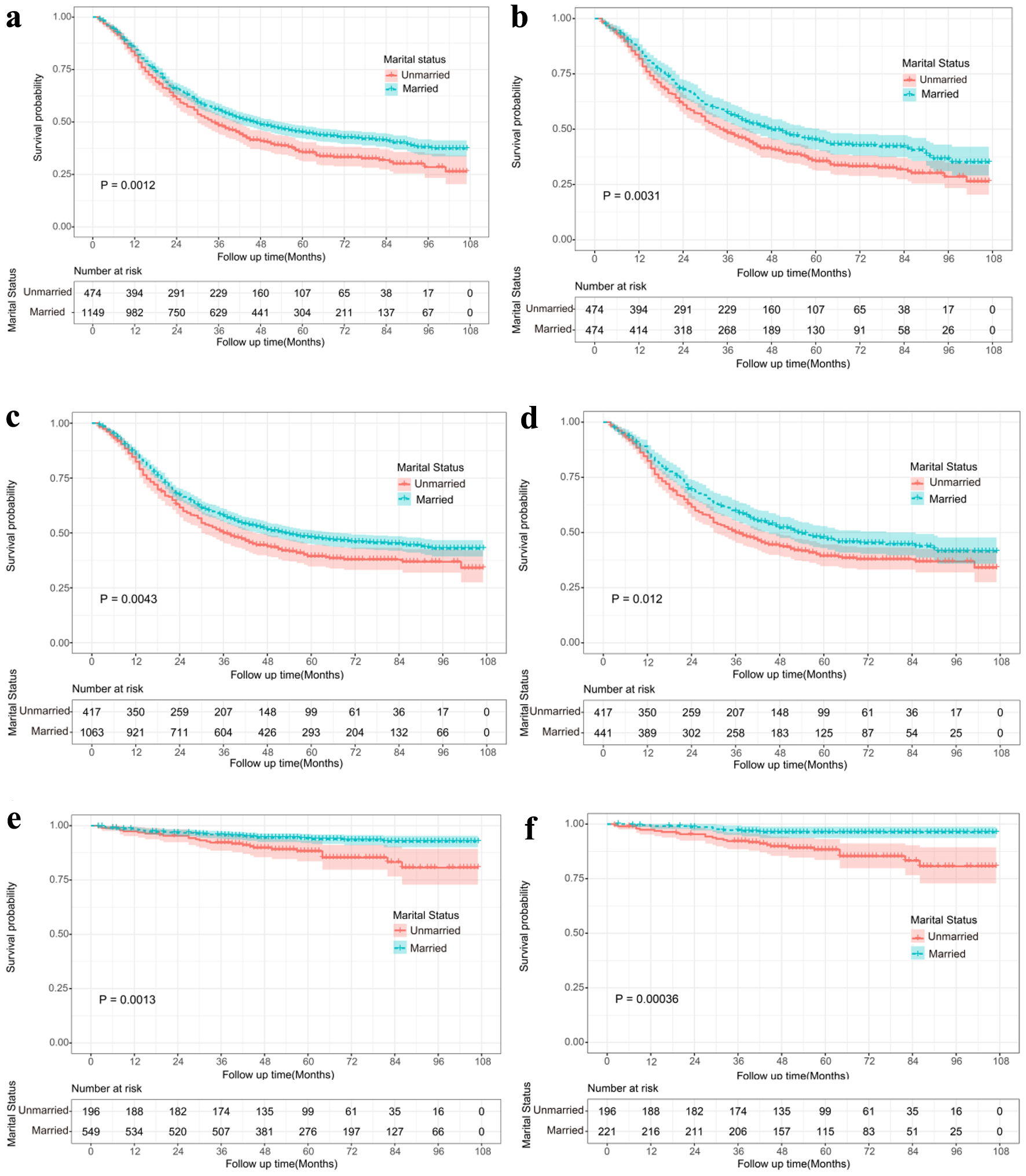 Click for large image | Figure 2. Kaplan-Meier survival analysis for married and unmarried Siewert type II AEG patients. Overall survival curves for the married and unmarried group before (a) and after PSM (b). Cancer-specific survival curves for the married and unmarried group before (c) and after PSM (d). Cardiovascular specific survival curves for the married and unmarried group before (e) and after PSM (f). AEG: adenocarcinoma of the esophagogatric junction; PSM: propensity score matching. |
Association of marital status with cancer death risk in patients with Siewert type II AEG
In this study, a total of 937 patients died before PSM, of which 84.74% (794/937) were cancer-specific death. According to a competing risk model, there was no significant difference in the cumulative cancer-specific death rate between the married and the unmarried groups (Gray’s test, P = 0.08) (Fig. 3). To further investigate the independent prognostic factors of cancer-specific death, the Fine-Gray multivariable regression model was established (Table 2). We noted no statistically significant difference for marital status between groups (hazard ratio (HR): 1.160, 95% confidence interval (CI): 0.994 - 1.354, P = 0.060). Additionally, compared with the corresponding subgroups, patients with age 56 - 75 years, grade G3, higher T status, higher N status, and lower RLN tended to have significantly higher cancer-specific death (P < 0.05).
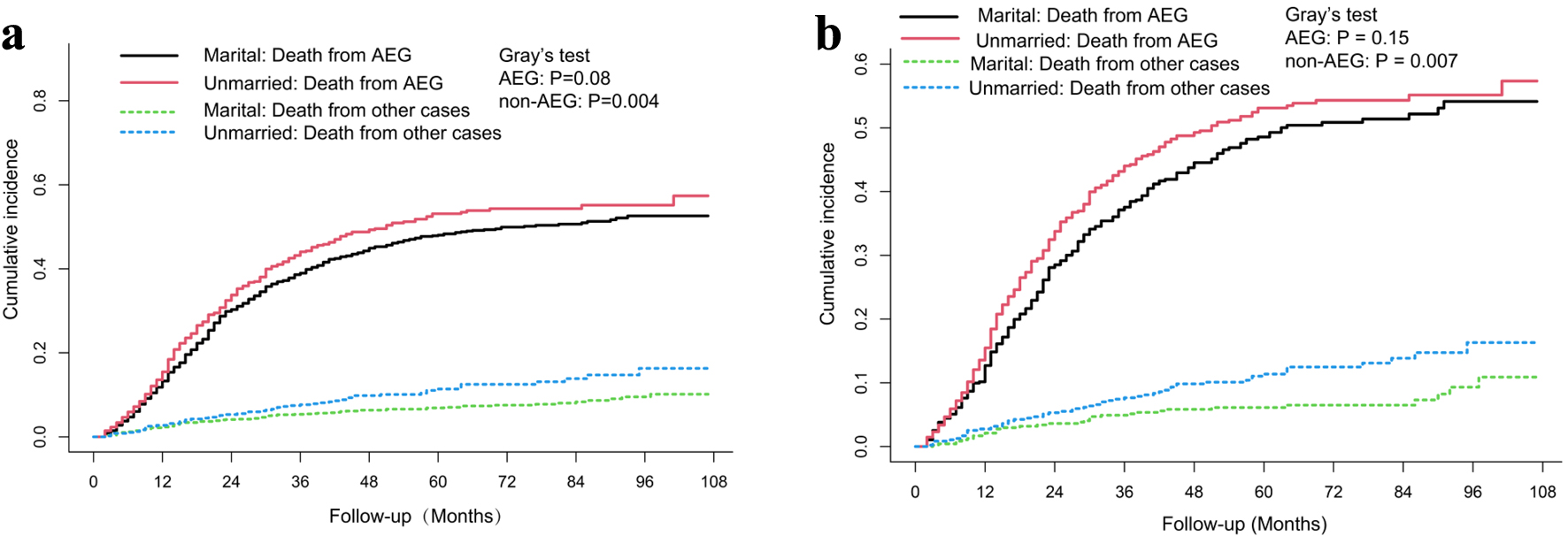 Click for large image | Figure 3. Cumulative incidence of cancer specific death and non-cancer specific death in married and unmarried Siewert type II AEG patients before (a) and after PSM (b). AEG: adenocarcinoma of the esophagogatric junction; PSM: propensity score matching. |
 Click to view | Table 2. Multivariable Competing Risk Analysis for Cancer-Specific Death in Patients With Siewert Type II AEG |
A total of 565 patients died after PSM, of which 84.07% (475/565) were cancer-specific death. A similar phenomenon was observed. There was still no significant difference in the cumulative cancer-specific death rate between the married and the unmarried groups (Gray’s test, P = 0.15) (Fig. 3), and there was no statistically significant difference for marital status between groups (HR: 1.179, 95% CI: 0.975 - 1.426, P = 0.089) (Table 2). Compared with the corresponding subgroups after PSM, patients with grade G3, higher T status, higher N status, lower RLN and larger tumor size tended to have significantly higher cancer-specific death (P < 0.05) (Table 2).
Association of marital status with CVD risk in patients with Siewert type II AEG
To further investigate the association of marital status with CVD risk, a competing risk model and the Fine-Gray multivariable regression were used. As shown in Figure 4, patients in the unmarried group had a higher cumulative cardiovascular disease-specific death rate than patients in married group both before and after PSM. According to the Fine-Gray multivariable regression analysis, unmarried status was related to increased CVD risk before PSM (HR: 1.860, 95% CI: 1.096 - 3.160, P = 0.021), and the adjusted HR further increased after PSM (HR: 3.066, 95% CI: 1.372 - 6.850, P = 0.006) (Table 3).
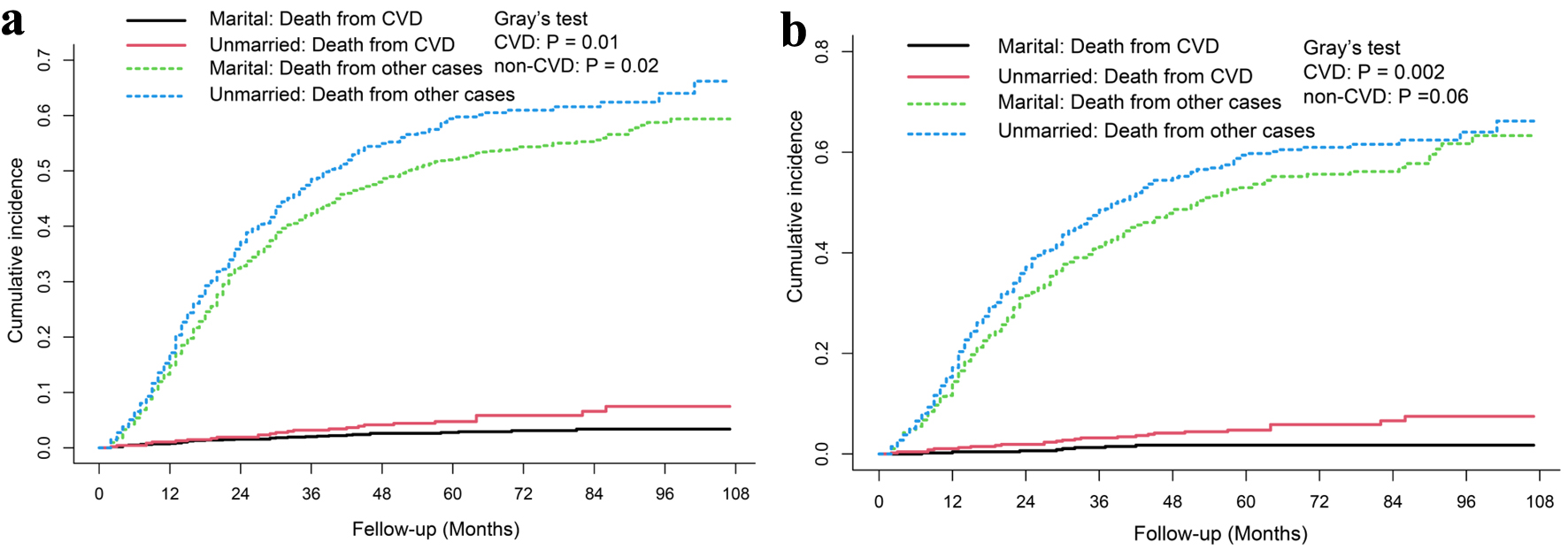 Click for large image | Figure 4. Cumulative incidence of cardiovascular death and non-cardiovascular specific death in married and unmarried Siewert type II AEG patients before (a) and after PSM (b). AEG: adenocarcinoma of the esophagogatric junction; PSM: propensity score matching. |
 Click to view | Table 3. Multivariable Competing Risk Analysis for Cardiovascular Death in Patients With Siewert Type II AEG |
| Discussion | ▴Top |
In the present study, based on the analysis of a cohort of 1,623 patients with Siewert type II AEG in the SEER database from January 2010 to December 2015, we investigated the relationship between survival prognosis and marital status for Siewert type II AEG patients who underwent radical surgery. We confirmed that married patients had better OS and cardiovascular disease-specific survival than unmarried patients, and there was no statistically significant difference for cancer-specific survival between two groups, by using Kaplan-Meier survival analysis, a competing risk model, the Fine-Gray multivariable regression and PSM method.
In current study, based on the Fine-Gray multivariable analysis and PSM method, we have shown that histological grade, T stage, RLN, LNR and tumor size could be prognostic factors for Siewert type II AEG patients. The impacts of histological grade and tumor size were widely reported to predict the prognosis of AEG [6, 17]. In our study, patients with higher grade and larger tumor size tended to have a worse prognosis, which was consist with previous studies. Zhu et al reported that N stage and RLN are both prognostic factors for Siewert type II AEG [3, 18]. Guo et al demonstrated that RLN and LNR are related to prognosis of Siewert type II AEG patients without radiation therapy, and LNR is also a prediction factor for Siewert type II AEG patients with radiation therapy [6]. They also reported that N stage is not a prognostic factor for Siewert type II AEG patients. The differences between studies could be attribute to different study population, different groupings and different PSM methods. In our study, N stage, RLN and LNR were included in our multivariable regression model, and the results demonstrated that RLN and LNR rather than N stage were related to the prognosis of patients with Siewert type II AEG.
Marital status, one of the social and psychological factors, has been widely reported to be related with the prognosis of patients with cancer [19-21]. The association of marital status with prognosis of patient with Siewert type II AEG was less frequently reported. Wang et al found that unmarried status was a negative prognostic factor for both OS (HR: 1.20, 95% CI: 1.10 - 1.31, P < 0.001) and cancer-specific survival (HR: 1.29, 95% CI: 1.12 - 1.48, P < 0.001) in Siewert type II AEG patients by using Cox regression analyses [22]. In this study, we demonstrated that there was no statistically difference in the cancer-specific death rate between the married and unmarried groups using competing risk regression analyses. The discrepancies between the two studies could be attributed to the fact that the features included in two studies and the analysis methods used in two studies were different. Therefore, additional studies are needed to verify the impact of marital status on cancer-specific death in Siewert type II AEG patients.
CVD, as the leading cause of non-cancer-specific mortalities, has become increasingly appreciated in patients with cancer recent years. As the increasing life span and expectancy, CVD increases among the patients with cancer [23, 24]. Previous studies showed that patients with cancer had a higher CVD rate than patients without cancer [25]. Marital status, as a physiological factor, exhibits a strong relationship to cardiovascular disease [26]. However, the effect of marital status in CVD has not previously been reported in patients with Siewert type II AEG. In our study, we found that patients in unmarried group had a higher CVD rate than patients in married group in Siewert type II AEG patients. To the best of our knowledge, this is the first study of the impact of marital status in cardiovascular prognosis of Siewert type II AEG patients. The cardiovascular benefit of marriage may be associated with social and emotional support. Firstly, supervision and mentally encouragement from spouses promote healthy lifestyle changes. Married patients with cancer are more likely to have a healthier lifestyle than unmarried patients [27, 28]. Secondly, spousal support and supervision from spouses may promote early diagnosis and early treatment for cardiovascular disease for patients with cancer [10, 29]. Thirdly, the cancer diagnosis elicited a range of psychological stress levels, including cancer-related distress, depression, and anxiety, which were identified as risk factors that heighten the likelihood of cardiovascular mortality among individuals with cancer [30].
Inevitably, there are also some notable limitations that need to be addressed in this study. Firstly, unregistered confounding factors and selection bias cannot be avoided due to the retrospective study design. Secondly, we cannot rule out the possibility that risk estimates might be influenced by potential confounders, such as family history and other comorbidities. Thirdly, due to the small sample size, we did not explore the impact of different unmarried status such as divorced, separated, widowed in cardiovascular prognosis of patients with Siewert type II AEG. Finally, we were unable to avoid the possibility that our results might exclude the influence of surgical status because of the focus on surgical cases.
Conclusions
Socioeconomic factors especially marital status were found to be associated with an increased risk of CVM in patients with cancer. However, the clinical value of marital status for Siewert type II AEG patients remains unknown. This study presented a competing risk analysis in a large, population-based, real-world cohort, to further explore the association between marital status and CVD risk. Our study demonstrates that unmarried Siewert type II AEG patients are associated with higher CVD risk but not with cancer-specific death risk compared with married patients. Our results suggest that more attention needs to be offered to unmarried patients with Siewert type II AEG especially for their cardiovascular disease.
Acknowledgments
None to declare.
Financial Disclosure
This study was not funded by public or private institutions or individuals.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was waived.
Author Contributions
ZQZ drafted the manuscript, analyzed data, generated the figure and performed the background research. XZS edited the manuscript. All authors contributed to the article and approved the submitted version.
Data Availability
The data supporting the findings of this study can be obtained in the SEER program.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Amini N, Spolverato G, Kim Y, Squires MH, Poultsides GA, Fields R, Schmidt C, et al. Clinicopathological features and prognosis of gastric cardia adenocarcinoma: a multi-institutional US study. J Surg Oncol. 2015;111(3):285-292.
doi pubmed - Zhu K, Xu Y, Fu J, Mohamud FA, Duan Z, Tan S, Zhao Z, et al. Proximal gastrectomy versus total gastrectomy for siewert type II adenocarcinoma of the esophagogastric junction: a comprehensive analysis of data from the SEER registry. Dis Markers. 2019;2019:9637972.
doi pubmed pmc - Feng H, Zheng J, Zheng C, Deng Z, Liao Q, Wang J, Li Y. The probability of Lymph node metastasis with a tumor size larger than and smaller than 4 cm is different in stages T1-T3 of Siewert type II adenocarcinoma of esophagogastric junction: A Population-Based Study. J Cancer. 2021;12(22):6873-6882.
doi pubmed pmc - Rudiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353-361.
doi pubmed pmc - Guo Z, Wang N, Liu F, Zhao Q. Prognostic nomogram for Siewert type II adenocarcinoma of the esophagogastric junction patients with and without neoadjuvant radiotherapy: a retrospective cohort study. Am J Transl Res. 2022;14(1):135-149.
pubmed pmc - Liu E, Guan X, Wei R, Jiang Z, Liu Z, Wang G, Chen Y, et al. Association between radiotherapy and death from cardiovascular disease among patients with cancer: a large population-based cohort study. J Am Heart Assoc. 2022;11(6):e023802.
doi pubmed pmc - Zhao Y, Qin F, Ji Q, Xia W, He B. Primary site as a novel prognostic factor for cardiovascular mortality post-radiotherapy in limited-stage small cell lung cancer: A large population-based study. Front Cardiovasc Med. 2022;9:922811.
doi pubmed pmc - Guan T, Zhang H, Yang J, Lin W, Wang K, Su M, Peng W, et al. Increased risk of cardiovascular death in breast cancer patients without chemotherapy or (and) radiotherapy: a large population-based study. Front Oncol. 2020;10:619622.
doi pubmed pmc - Guan T, Wang Y, Li F, Chen D, Wei Q, Wang K, Zhang H, et al. Association of marital status with cardiovascular outcome in patients with breast cancer. J Thorac Dis. 2022;14(4):841-850.
doi pubmed pmc - Wingo PA, Jamison PM, Hiatt RA, Weir HK, Gargiullo PM, Hutton M, Lee NC, et al. Building the infrastructure for nationwide cancer surveillance and control—a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) Program (United States). Cancer Causes Control. 2003;14(2):175-193.
doi pubmed - Miccio JA, Oladeru OT, Yang J, Xue Y, Choi M, Zhang Y, Yoon H, et al. Neoadjuvant vs. adjuvant treatment of Siewert type II gastroesophageal junction cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. J Gastrointest Oncol. 2016;7(3):403-410.
doi pubmed pmc - Zhou Y, Tian M, Gungor C, Wang D. Neoadjuvant radiotherapy for locoregional Siewert type II gastroesophageal junction adenocarcinoma: A propensity scores matching analysis. PLoS One. 2021;16(5):e0251555.
doi pubmed pmc - https://seer.cancer.gov/
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252-7259.
doi pubmed - Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242-1258.
doi pubmed pmc - Chen J, Xia YJ, Liu TY, Lai YH, Yu JS, Zhang TH, Ooi S, et al. Development and validation of a survival nomogram for patients with Siewert type II/III adenocarcinoma of the esophagogastric junction based on real-world data. BMC Cancer. 2021;21(1):532.
doi pubmed pmc - Chen L, Tang K, Wang S, Chen D, Ding K. Predictors of lymph node metastasis in siewert type II T1 adenocarcinoma of the esophagogastric junction: a population-based study. Cancer Control. 2021;28:10732748211026668.
doi pubmed pmc - Zhang S, Yang Z, Qiu P, Li J, Zhou C. Research on the role of marriage status among women underwent breast reconstruction following mastectomy: a competing risk analysis model based on the SEER database, 1998-2015. Frontiers in Surgery. 2021;8:803223.
- Salmon C, Song L, Muir K, Collaborators U, Pashayan N, Dunning AM, Batra J, et al. Marital status and prostate cancer incidence: a pooled analysis of 12 case-control studies from the PRACTICAL consortium. Eur J Epidemiol. 2021;36(9):913-925.
doi pubmed - Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: An analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 2018;54:119-124.
doi pubmed - Wang S, Chen L, Chen D, Chao J, Shao Y, Tang K, Chen W. Effect of marital status on the survival of patients with adenocarcinoma of the esophagogastric junction: a population-based, propensity-matched study. Cancer Control. 2021;28:10732748211066309.
doi pubmed pmc - Kolodziejczyk C, Jakobsen M, Sall Jensen M, Poulsen PB, Khan H, Kumler T, Andersson M. Mortality from cardiovascular disease in women with breast cancer - a nationwide registry study. Acta Oncol. 2021;60(10):1257-1263.
doi pubmed - Ell P, Martin JM, Cehic DA, Ngo DTM, Sverdlov AL. Cardiotoxicity of radiation therapy: mechanisms, management, and mitigation. Curr Treat Options Oncol. 2021;22(8):70.
doi pubmed - Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70(8):926-938.
doi pubmed pmc - Dhindsa DS, Khambhati J, Schultz WM, Tahhan AS, Quyyumi AA. Marital status and outcomes in patients with cardiovascular disease. Trends Cardiovasc Med. 2020;30(4):215-220.
doi pubmed - Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595.
doi pubmed pmc - Kilpi F, Konttinen H, Silventoinen K, Martikainen P. Living arrangements as determinants of myocardial infarction incidence and survival: A prospective register study of over 300,000 Finnish men and women. Social Science & Medicine (1982). 2015;133:93-100.
- Wong CW, Kwok CS, Narain A, Gulati M, Mihalidou AS, Wu P, Alasnag M, et al. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart. 2018;104(23):1937-1948.
doi pubmed - Bucciarelli V, Caterino AL, Bianco F, Caputi CG, Salerni S, Sciomer S, Maffei S, et al. Depression and cardiovascular disease: The deep blue sea of women's heart. Trends Cardiovasc Med. 2020;30(3):170-176.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


