| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 1, February 2024, pages 1-9
Pre- and Post-Implant Endoscopy in Left Ventricular Assist Device Recipients: A Single-Center Experience
Wael T. Mohameda, Vinay Jahagirdara, Fouad Jabera, e, Mohamed K. Ahmedb, Hassan M. Ghozb, Brett W. Sperryc, Wendell K. Clarkstonb, d
aDepartment of Internal Medicine, University of Missouri-Kansas City, Kansas City, MO, USA
bDivision of Gastroenterology, University of Missouri-Kansas City, Kansas City, MO, USA
cSaint Luke’s Mid America Heart Institute, Kansas City, MO, USA
dDivision of Gastroenterology, Saint Luke’s Hospital, Kansas City, MO, USA
eCorresponding Author: Fouad Jaber, Department of Internal Medicine, University of Missouri-Kansas City, Kansas City, MO, USA
Manuscript submitted July 26, 2023, accepted January 4, 2024, published online February 28, 2024
Short title: Endoscopy in LVAD Patients
doi: https://doi.org/10.14740/gr1661
| Abstract | ▴Top |
Background: Gastrointestinal bleeding (GIB) is common in left ventricular assist devices (LVADs) patients, but the optimal screening approach before LVAD implantation is still unclear. The aim of the study was to describe our experience with pre- and post-LVAD implantation endoscopic screening and subsequent GI bleeding in this cohort.
Methods: A retrospective review was conducted among all patients who underwent LVAD implantation at Saint Luke’s Hospital, between 2010 and 2020. The data were reviewed to determine the yield and safety of endoscopic procedures performed within 1 month before LVAD placement and the incidence of GIB within 1 year after implantation.
Results: A total of 167 LVAD patients met the inclusion criteria, and 23 underwent pre-implantation endoscopic evaluation. Angiodysplasia had a significantly higher odds ratio (OR) of 9.41 (95% confidence interval (CI): 2.01 - 44.09) in post-LVAD endoscopy, while there was no significant difference in bleeding from other sources such as peptic ulcer disease or diverticular bleeding. There was no difference in the incidence of GIB in patients who underwent endoscopic evaluation pre-LVAD compared to post-LVAD GIB (32.6% vs. 39.1%, P = 0.64). Endoscopy was well-tolerated in this cohort, and argon plasma coagulation was the most commonly used intervention to achieve hemostasis.
Conclusions: According to our results, we recommend against routine pre-LVAD endoscopic screening. Instead, we suggest an individualized approach, where decisions are made on a case-by-case basis.
Keywords: Gastrointestinal bleeding; Left ventricular assist device; Endoscopy; Angiodysplasias
| Introduction | ▴Top |
Left ventricular assist devices (LVADs) have emerged as established treatments for patients with advanced heart failure [1]. Originally utilized as a bridge to transplantation (BTT), their usage has evolved to include destination therapy (DT) [1]. Remarkable advancements in LVAD technology have significantly improved the functional status, as well as the quality and duration of life for patients [2]. However, one common complication observed in LVAD patients is gastrointestinal bleeding (GIB), with a meta-analysis reporting a pooled prevalence of 23% (95% confidence interval (CI): 20.5% - 27%) [3]. The etiology of GIB in LVAD patients is multifactorial, with angiodysplasias [4, 5], particularly in the small bowel, being the most common culprit [6]. Similar to Heyde’s syndrome [7, 8], increased circulatory shear forces can trigger the development of bleeding-prone angiodysplasia. In addition, long-term prophylactic anticoagulation and antiplatelet therapy are required in LVAD recipients to prevent pump thrombosis and embolic stroke, which increases the risk of bleeding [9]. Individuals with older age [3, 10], a history of GIB [6, 11], and right ventricular dysfunction [1, 3], are more susceptible to GIB with LVAD.
Before LVAD implantation, patients are often evaluated using endoscopic procedures to rule out cancer, assess anemia, and identify and treat sources of bleeding. However, the optimal screening algorithm prior to LVAD implantation is unclear. Prior studies have provided evidence of the potential advantages of pre-LVAD endoscopy in identifying and managing the cause of GIB and facilitating transplant eligibility [1, 12]. However, contrasting findings have emerged, suggesting that initial endoscopy may not be linked to a decreased risk of recurrent GIB in patients with LVADs [13-16]. Furthermore, 30-60% of patients develop recurrent bleeding independent of prior endoscopic intervention [12, 17]. Dakik et al reported that 64.1% of patients required a second endoscopy for GIB [18].
Considering the scarcity of data regarding the impact of pre-LVAD endoscopic intervention on reducing recurrent bleeding, its efficacy in the LVAD population remains uncertain. Hence, the main objective of our study was to provide a comprehensive analysis of our experience with pre-LVAD implantation endoscopic examination and the subsequent occurrence of GIB in this particular group. Additionally, our secondary objectives encompassed assessing the overall incidence of post-LVAD GIB, analyzing the presentation and sources of bleeding, and evaluating the safety profile associated with pre-LVAD endoscopy.
| Materials and Methods | ▴Top |
Study population
A comprehensive retrospective chart review was conducted, encompassing all patients aged 18 years and above, who underwent LVAD implantation at Saint Luke’s Health System in Kansas City, Missouri, USA, between January 1, 2010, and December 31, 2020. Patients with incomplete medical records, those lost to follow-up, and individuals who passed away within 1 year of LVAD placement due to causes other than GIB were excluded from the study. Approval for the study was obtained from the Institutional Review Board at Saint Luke’s Health System. This study was conducted according to the Helsinki Declaration.
Outcomes and variables
Various variables were gathered to assess the efficacy and safety of endoscopic procedures conducted within 1 month prior to LVAD placement and to determine the incidence of GIB over a 1-year period following implantation.
Regarding the primary aim, the main outcome variable of interest was the occurrence of the first episode of GIB. Independent variables encompassed the presentation of GIB (hematochezia, melena, hematemesis), occurrence of GIB before LVAD placement, and GIB incidence after LVAD implantation.
As for the secondary objectives, independent variables included demographic information, the specific type of LVAD utilized, the purpose of LVAD placement, the method employed for endoscopic intervention, the source and location of bleeding, as well as the number of hospital admissions due to GIB.
Data collection
LVAD recipients were identified via the International Classification of Diseases,10th Revision (ICD-10) code (Z95.811). We obtained baseline data, including demographics, LVAD device parameters, endoscopic results, and bleeding outcomes, through reviewing electronic medical records. Pre-LVAD bleeding was defined as documented overt GIB in the form of melena, hematochezia, or bright red blood per rectum with or without a drop in hemoglobin level, or GIB seen on endoscopy and documented in endoscopy reports by endoscopists within 1 month before LVAD placement. This was identified via records from our hospital system and outside records available to us. Post-LVAD bleeding was defined as documented overt GIB in the form of melena, hematochezia, or bright red blood per rectum with or without a drop in hemoglobin level, or GIB seen on endoscopy and documented in endoscopy reports by endoscopists within 1 year of LVAD placement. The location and treatment of the GIB were recorded as documented by the endoscopist.
Statistical analysis
In the comparative analysis of data between groups, categorical measures were assessed using the Fisher’s exact test, while continuous measures were evaluated using the Student’s t-test. Continuous variables are presented as means ± standard deviations (SDs), and categorical variables are expressed as numbers and proportions. A two-sided P value of < 0.05 was considered statistically significant. For the comparison of pre- and post-endoscopic findings, odds ratios (ORs) and their corresponding 95% CIs were calculated. All statistical analyses were conducted using SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp).
| Results | ▴Top |
A total of 205 patients who underwent LVAD placement at our institution were identified. Of these, 167 met the inclusion criteria, 23 of the 167 patients had pre-LVAD endoscopy within 1 month of LVAD, while 144 did not undergo endoscopy (Fig. 1). The median age at the time of implantation was 61.5 years, 77.8% were males, 91% were white, and 55.5% underwent LVAD implantation for end-stage ischemic cardiomyopathy (Table 1). Type of LVAD included HeartWare: 38 (22.8%), HeartMate 2: 97 (58%), HeartMate 3: 26 (15.6%), and percutaneous ventricular assist device (PVAD): six (3.6%). Thirty-three patients (19.8%) had a documented history of GIB prior to LVAD placement (Table 1).
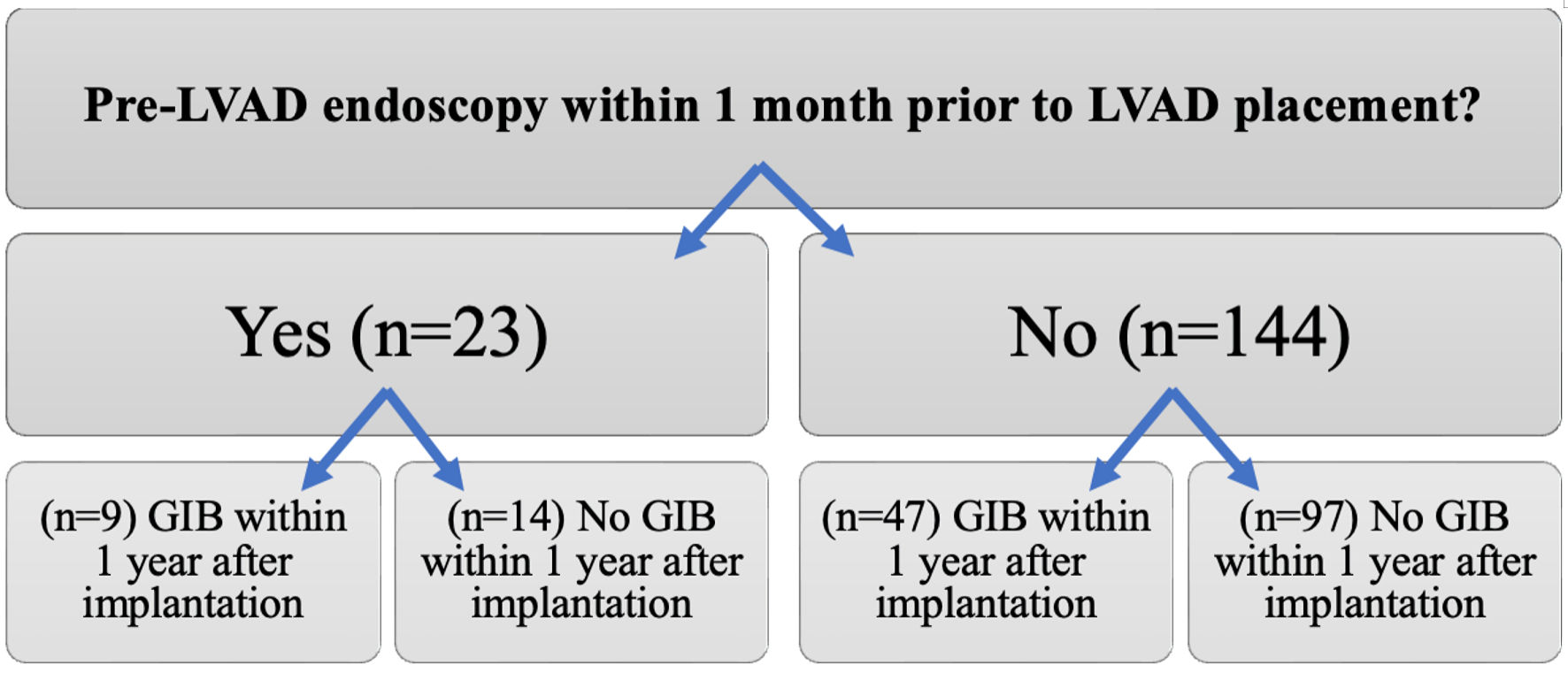 Click for large image | Figure 1. Comparison of the incidence of gastrointestinal bleeding (GIB) between patients who underwent pre-left ventricular assist device (LVAD) endoscopic evaluation and those who did not. |
 Click to view | Table 1. Demographics and Patient Characteristics of Study Population |
Endoscopic modalities for the 23 pre-LVAD endoscopies included esophagogastroduodenoscopy (EGD) (n = 14), colonoscopy (n = 9), enteroscopy (n = 3), and capsule endoscopy (n = 1) (Table 2). Peptic ulcer disease and diverticulosis were the most common findings. Nine of these patients (39.1%) developed GIB within 1 year after implantation compared to 32.6% (47/144) patients who did not undergo pre-LVAD endoscopy (P = 0.64) (Table 3, Figs. 1, 2). Of those who underwent pre-LVAD endoscopy, two (8.7%) patients underwent therapeutic endoscopic interventions. The first patient received argon plasma coagulation (APC) thermal therapy and endoclip placement, while the other patient received APC thermal therapy and epinephrine injection. Therapeutic measures were successful in 100% of patients, as documented on endoscopy reports.
 Click to view | Table 2. Comparison of Endoscopic Modality and Findings in Two Groups |
 Click to view | Table 3. Incidence of GIB in Those With Pre-LVAD Endoscopic Evaluation Compared to Those Without Endoscopic Evaluation |
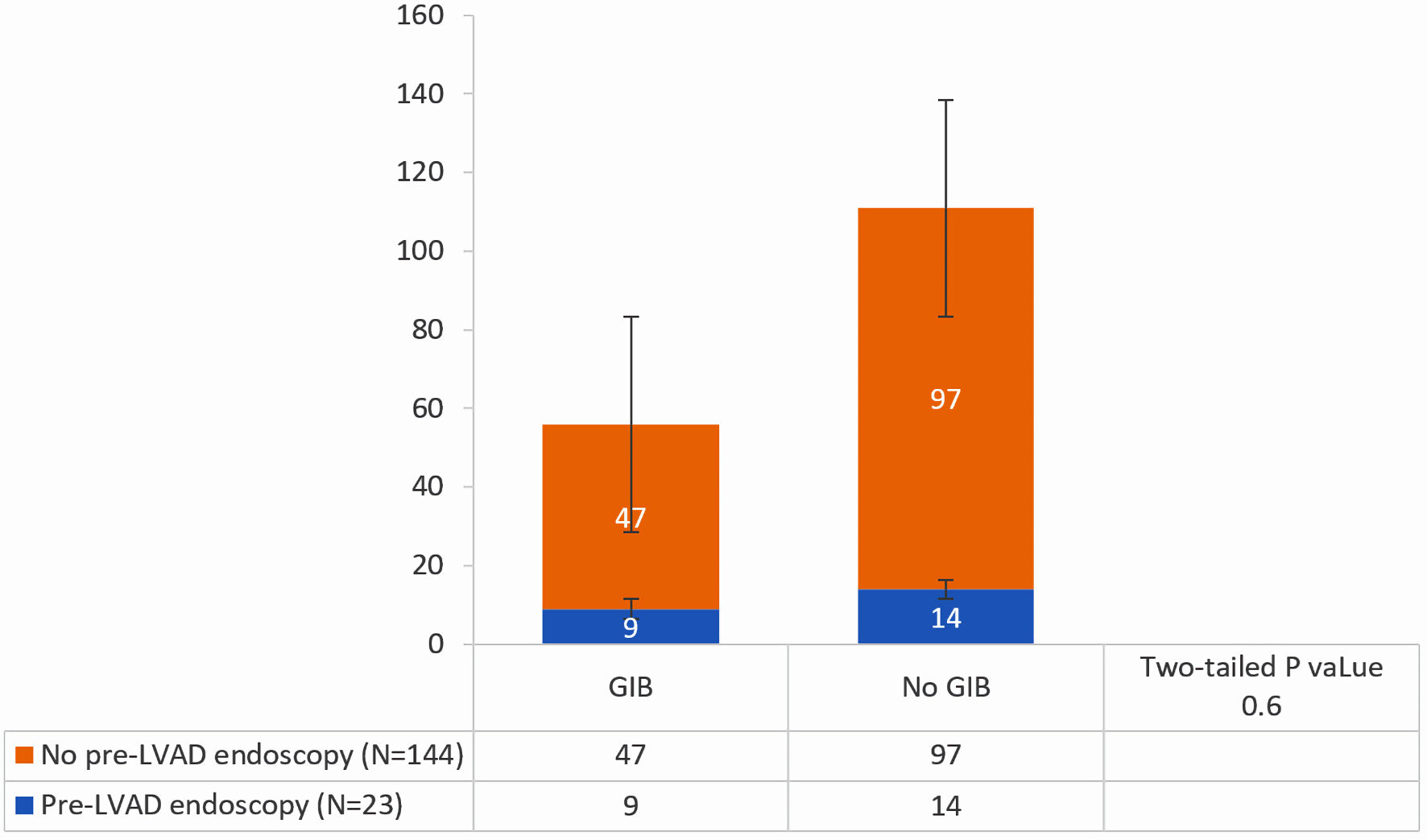 Click for large image | Figure 2. Incidence of GIB in those who did undergo pre-LVAD endoscopic evaluation compared to those who did not. GIB: gastrointestinal bleeding; LVAD: left ventricular assist device. |
We compared the OR for the endoscopic findings at post-LVAD endoscopy (55 patients who underwent post-LVAD endoscopy for GIB) versus pre-LVAD endoscopy (23 patients who underwent pre-LVAD endoscopic screening). Within 1 year of LVAD implantation, 56 patients (33.5%) experienced GIB, and 55 underwent endoscopic evaluation (Table 2, Fig. 1). The most frequent cause of bleeding was angiodysplasias (n = 26, 47.3%), followed by peptic ulcer disease 19 (34.6%), then polyps and diverticulosis (seven each, 25.5%) (Fig. 3). We compared the OR for the endoscopic findings at post-LVAD endoscopy (55 patients who underwent post-LVAD endoscopy for GIB) versus pre-LVAD endoscopy (23 patients who underwent pre-LVAD endoscopic screening) (Table 4). The only significant endoscopic finding was angiodysplasia (OR = 9.41, 95% CI = 2.01 - 44.09) (Table 4). Of those who underwent endoscopy, 33 (58.9%) patients were treated. All treated patients received APC thermal therapy, 21 (63.6%) had concomitant endoclip placement, and five had injections (epinephrine, sclerosants, or cyanoacrylate glues) (15.1%) (Fig. 4). Therapeutic measures were successful in 100% of patients, as documented on endoscopy reports.
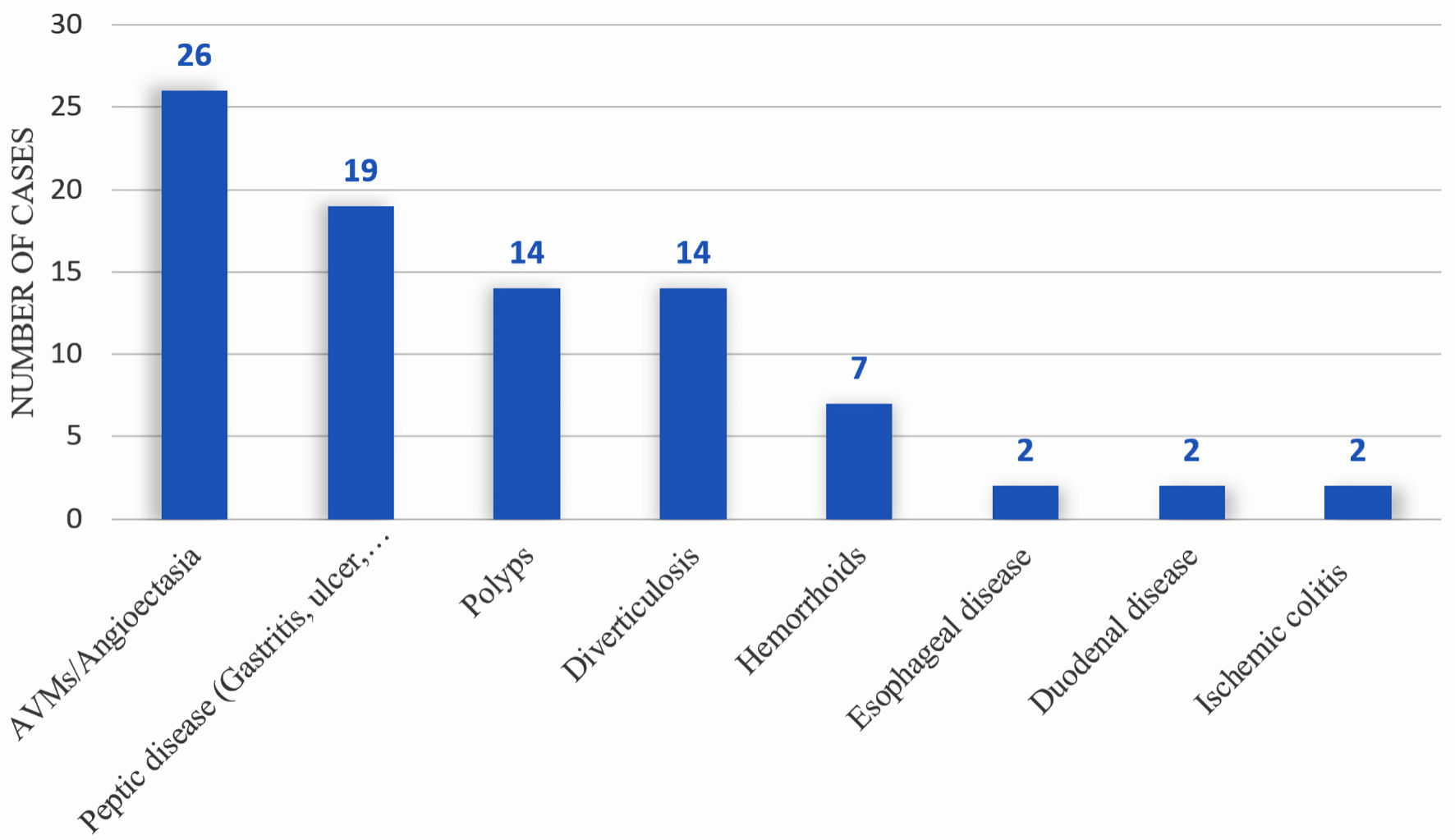 Click for large image | Figure 3. Diagnostic yield of endoscopy in identifying source of bleeding within 1 year post-LVAD placement. LVAD: left ventricular assist device. |
 Click to view | Table 4. Odds Ratio (OR) for Post-LVAD Endoscopic Findings Compared to Pre-LVAD Findings |
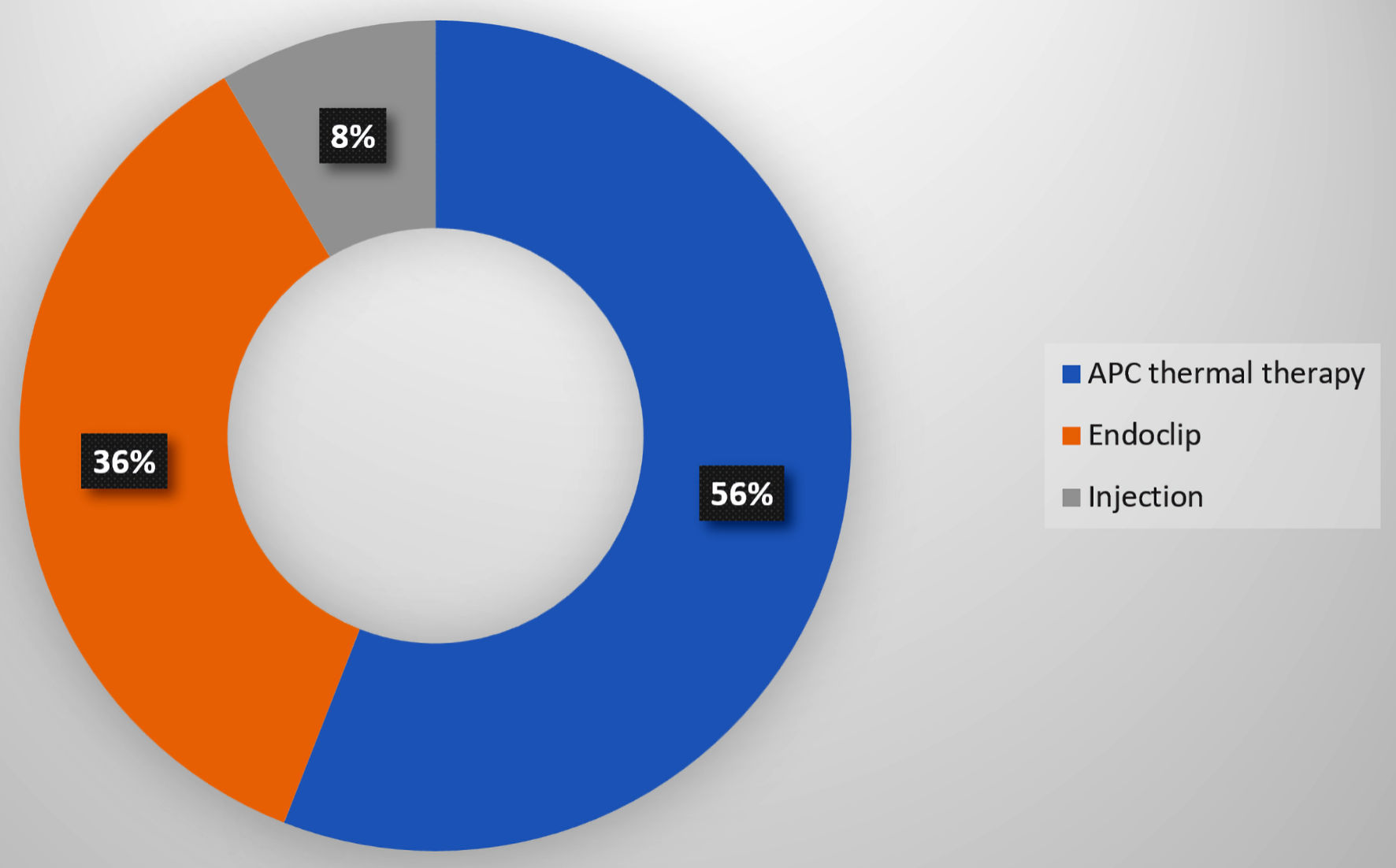 Click for large image | Figure 4. Therapeutic yield of endoscopy within 1year post-LVAD placement. LVAD: left ventricular assist device; APC: argon plasma coagulation. |
| Discussion | ▴Top |
GIB is a significant concern after LVAD placement and is a leading cause of readmission in this patient population [19]. Our study findings indicate that pre-LVAD endoscopic evaluation performed within 1 month before implantation did not demonstrate a reduction in the incidence of post-LVAD bleeding. The primary source of bleeding in this cohort was identified as angiodysplasias located in the proximal upper gastrointestinal (GI) tract. Diagnostic and therapeutic endoscopy was well-tolerated in this cohort, with APC being the most commonly used intervention.
Recent literature has suggested that 32.9-36.2% of LVAD recipients have GIB after device placement, which aligns with our findings. The higher incidence of GIB in recent studies [12, 20] compared to earlier ones [4, 6, 16, 21] may be attributed to various factors, including the introduction of continuous flow devices and the increased use of LVADs for extended periods as DT. The overall higher risk of GIB in the LVAD population is partly due to the use of antiplatelets/anticoagulants to minimize the high risk of hypercoagulability and thrombus formation in this group [3]. Our findings also support this, as nearly two-thirds of patients who experienced post-LVAD GIB were on anticoagulants or antiplatelets. Consistent with other studies [1, 3], angiodysplasias of the proximal upper GI tract were the predominant etiology of post-LVAD bleeding. Among patients who underwent endoscopy for GIB, the OR for angiodysplasia was higher in post-LVAD endoscopy compared to pre-LVAD (OR = 9.41, 95% CI = 2.01 - 44.09). One possible explanation is that the celiac trunk, which supplies the foregut, lies in close proximity to LVAD and is subjected to greater stress than the distal vasculature, which supplies the lower GI tract [22]. It has been demonstrated that patients with congestive heart failure have inherently weaker blood vessels than healthy individuals [23], making them more prone to develop angiodysplasias when exposed to higher pressures after LVAD implantation. However, it is paramount to note that other sources of bleeding in this study were still fairly common in patients with LVADs, including peptic disease, polyps, and diverticulosis. Nevertheless, the OR for these bleeding sources after LVAD compared to pre-LVAD was not statistically significant.
A history of GIB may also be a confounding factor as it increases the risk of recurrent bleeding in this population [2]. Morgan et al [11] reported that a history of GIB prior to LVAD implantation was the only independent predictor of GIB and was the only significant variable between patients with and without GIB after implantation (21.1% vs. 10.4%, P = 0.016). In our study, 37.5% of patients who developed GIB were readmitted for GIB, with four out of five presenting with melena. This finding is consistent with the recent results published by Palchaudhuri et al [20], where 55.5% of those who developed index GIB had a recurrence episode, and around 10% of LVAD patients had ≥ 3 admission encounters for GIB. The higher number of recurrent bleeding episodes in Palchaudhuri et al [20] study may be due to the longer follow-up of patients (median follow-up of 601 days) compared to our study, where we followed these patients for only 1 year after LVAD placement. Rebleeding may reflect the natural history of angiodysplastic bleeding, especially when patients are taking anticoagulants. Additionally, in our study, patients with normal pre-LVAD endoscopy with no previous history of GIB still developed post-LVAD GIB. This is due to the development of angiodysplasia after LVAD implantation, as we mentioned earlier, along with the use of anticoagulants and antiplatelet therapy. Furthermore, patients with a history of pre-LVAD GIB and successful pre-LVAD therapeutic interventions continued to develop post-LVAD GIB from other sources, such as proximal small bowel angiodysplasia.
Our study showed no significant difference in the incidence of post-LVAD GIB between patients who underwent pre-LVAD upper endoscopy and those who did not. Specifically, 39% of patients who had pre-LVAD endoscopy developed GIB within 1 year of implantation, while 32% of those without prior endoscopy experienced GIB (P value = 0.64). This result is consistent with other studies showing that endoscopic evaluation does not effectively reduce the risk of GIB recurrence in LVAD patients [13-16, 19, 20]. The pathophysiology, prevalence, and distribution of angioectatic lesions throughout the GI tract may account for why endoscopic interventions have limited efficacy in reducing the risk of GIB recurrence in this population [20].
A previous study by Taylor et al [12] reported a low rate of procedure-related adverse events (2.8%), such as bleeding, infection, and perforation, after performing 533 endoscopic procedures for 297 GIB events in 345 LVAD patients. In our study, upper endoscopy successfully located and treated the source of bleeding with no reported adverse events. This highlights that endoscopy is generally safe and well-tolerated in LVAD population. In our study, we achieved hemostasis in 100% of the cases when the bleeding source was identified. APC thermal therapy was used in all pre- and post-LVAD patients who received therapeutic endoscopic interventions. Other endoscopic treatment modalities included endoclip placement, epinephrine injection, or a combination.
Hirose et al [24] found that the cost of treating GIB in LVAD patients is high, averaging $9,112 ± 520 per episode. This high cost highlights the need for clear guidelines on the use of pre-LVAD implantation endoscopy to optimize healthcare resources. There have been limited proposals to address the challenges associated with low-yield procedures. Axelrad et al [25] have suggested an endoscopic algorithm that involves using push enteroscopy specifically for overt GIB, instead of performing EGD or colonoscopy. They also proposed conservative management for cases of occult bleeding without undergoing endoscopic evaluation. Another suggestion put forth by Palchaudhuri et al [20] is the development of a prognostic risk score to aid in triaging LVAD patients with GIB to different care pathways. More importantly, managing GIB in LVAD patients requires a comprehensive and multidisciplinary approach. It should start with a thorough evaluation in the emergency department, including prompt assessment, hemodynamic resuscitation, and a detailed history and physical examination. The assessment of LVAD function by the cardiology team and consultation with the gastroenterology team should begin in the emergency department. Collaboration between cardiologists and gastroenterologists is essential for optimal patient care, including managing antiplatelet and anticoagulant regimens, providing endoscopic guidance, and adjusting LVAD pump speed [1, 26].
The study has several limitations, primarily due to its retrospective and observational design, which may have restricted the ability to fully demonstrate the impact of factors such as changes in anticoagulation therapy and acid suppression therapy. Additionally, the decision-making process for screening by individual cardiologists and gastroenterologists may not have been fully captured in the chart review. Another limitation is the study’s reliance on data from a single academic center, which may limit its generalizability to other settings. Furthermore, the sample size may not have been large enough to identify additional predictors of GIB. Several factors might influence the outcomes in our study, including the patient’s age, presence of comorbidities, use of anticoagulation, risks of GIB, and prior history of GIB. However, a strength of the study is the close monitoring of LVAD patients at the facility, which may have reduced the likelihood of patients seeking care for GIB episodes elsewhere.
In conclusion, our study shows that angiodysplasia of the proximal upper GI tract is the most frequent cause of post-LVAD GIB. Also, our analysis indicates that pre-LVAD endoscopic evaluation conducted within 1 month prior to implantation does not appear to reduce the incidence of post-LVAD bleeding. The decision for pre-LVAD endoscopic screening should be individualized on a case-by-case basis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All other authors have no conflict of interest to disclose.
Informed Consent
Not applicable.
Author Contributions
Wael T. Mohamed: project administration, conceptualization, data curation, writing - original draft and approval of final draft. Vinay Jahagirdar: data curation, visualization, writing - original draft, and approval of final draft. Fouad Jaber: data curation, data analysis, visualization, writing - original draft, and approval of final draft. Mohamed K. Ahmed: data analysis, visualization, manuscript editing and reviewing, and approval of final draft. Hassan M. Ghoz: conceptualization, supervision, manuscript editing and reviewing, and approval of final draft. Brett W. Sperry: conceptualization, supervision, manuscript editing and reviewing, and approval of final draft. Wendell K. Clarkston: conceptualization, supervision, manuscript editing and reviewing, and approval of final draft.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
APC: argon plasma coagulation; GIB: gastrointestinal bleeding; LVAD: left ventricular assist device; BTT: bridge to transplantation; DT: destination therapy; EGD: esophagogastroduodenoscopy; GI: gastrointestinal
| References | ▴Top |
- Jabbar HR, Abbas A, Ahmed M, Klodell CT, Jr., Chang M, Dai Y, Draganov PV. The Incidence, Predictors and Outcomes of Gastrointestinal Bleeding in Patients with Left Ventricular Assist Device (LVAD). Dig Dis Sci. 2015;60(12):3697-3706.
doi pubmed - Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896.
doi pubmed - Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80(3):435-446.e431.
doi pubmed - Demirozu ZT, Radovancevic R, Hochman LF, Gregoric ID, Letsou GV, Kar B, Bogaev RC, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30(8):849-853.
doi pubmed - Goldstein DJ, Aaronson KD, Tatooles AJ, Silvestry SC, Jeevanandam V, Gordon R, Hathaway DR, et al. Gastrointestinal bleeding in recipients of the HeartWare Ventricular Assist System. JACC Heart Fail. 2015;3(4):303-313.
doi pubmed - Aggarwal A, Pant R, Kumar S, Sharma P, Gallagher C, Tatooles AJ, Pappas PS, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93(5):1534-1540.
doi pubmed - Letsou GV, Shah N, Gregoric ID, Myers TJ, Delgado R, Frazier OH. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial-flow left ventricular assist device. J Heart Lung Transplant. 2005;24(1):105-109.
doi pubmed - Pate GE, Chandavimol M, Naiman SC, Webb JG. Heyde's syndrome: a review. J Heart Valve Dis. 2004;13(5):701-712.
pubmed - Baumann Kreuziger LM. Management of anticoagulation and antiplatelet therapy in patients with left ventricular assist devices. J Thromb Thrombolysis. 2015;39(3):337-344.
doi pubmed - Shrode CW, Draper KV, Huang RJ, Kennedy JL, Godsey AC, Morrison CC, Shami VM, et al. Significantly higher rates of gastrointestinal bleeding and thromboembolic events with left ventricular assist devices. Clin Gastroenterol Hepatol. 2014;12(9):1461-1467.
doi pubmed - Morgan JA, Paone G, Nemeh HW, Henry SE, Patel R, Vavra J, Williams CT, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012;31(7):715-718.
doi pubmed - Taylor C, Bittner K, Bartell N, Aranez J, Alexis JD, Carlson B, Chen L, et al. Outcomes of gastrointestinal bleeding in patients with left ventricular assist devices: a tertiary care experience. Endosc Int Open. 2020;8(3):E301-E309.
doi pubmed pmc - Stern B, Maheshwari P, Gorrepati VS, Bethards D, Chintanaboina J, Boehmer J, Clarke K. Initial endoscopic intervention is not associated with reduced risk of recurrent gastrointestinal bleeding in left ventricular assist device patients. Ann Gastroenterol. 2021;34(5):660-668.
doi pubmed pmc - Truss WD, Weber F, Pamboukian SV, Tripathi A, Peter S. Early implementation of video capsule enteroscopy in patients with left ventricular assist devices and obscure gastrointestinal bleeding. ASAIO J. 2016;62(1):40-45.
doi pubmed - Meyer MM, Young SD, Sun B, Azzouz M, Firstenberg MS. Endoscopic evaluation and management of gastrointestinal bleeding in patients with ventricular assist devices. Gastroenterol Res Pract. 2012;2012:630483.
doi pubmed pmc - Sarosiek K, Bogar L, Conn MI, O'Hare B, Hirose H, Cavarocchi NC. An old problem with a new therapy: gastrointestinal bleeding in ventricular assist device patients and deep overtube-assisted enteroscopy. ASAIO J. 2013;59(4):384-389.
doi pubmed - Tabibian JH, Rhoades DP, Forde KA, McLean RC, Chandrasekhara V. Timing of gastrointestinal bleeding after implantation of left ventricular assist devices associates with anatomic location, presentation, and management. Clin Gastroenterol Hepatol. 2019;17(3):448-454.
doi pubmed - Dakik HK, McGhan AA, Chiu ST, Patel CB, Milano CA, Rogers JG, Chow SC, et al. The Diagnostic Yield of Repeated Endoscopic Evaluation in Patients with Gastrointestinal Bleeding and Left Ventricular Assist Devices. Dig Dis Sci. 2016;61(6):1603-1610.
doi pubmed - Elmunzer BJ, Padhya KT, Lewis JJ, Rangnekar AS, Saini SD, Eswaran SL, Scheiman JM, et al. Endoscopic findings and clinical outcomes in ventricular assist device recipients with gastrointestinal bleeding. Dig Dis Sci. 2011;56(11):3241-3246.
doi pubmed pmc - Palchaudhuri S, Dhawan I, Parsikia A, Birati EY, Wald J, Siddique SM, Fisher LR. Does endoscopic intervention prevent subsequent gastrointestinal bleeding in patients with left ventricular assist devices? A retrospective study. World J Gastroenterol. 2021;27(25):3877-3887.
doi pubmed pmc - Balcioglu O, Engin C, Yagdi T, Nalbantgil S, Baysal B, Erkul S, Engin Y, et al. Effect of aortic valve movements on gastrointestinal bleeding that occured in continuous flow left ventricular assist device patients. Transplant Proc. 2013;45(3):1020-1021.
doi pubmed - Scardulla F, Pasta S, D'Acquisto L, Sciacca S, Agnese V, Vergara C, Quarteroni A, et al. Shear stress alterations in the celiac trunk of patients with a continuous-flow left ventricular assist device as shown by in-silico and in-vitro flow analyses. J Heart Lung Transplant. 2017;36(8):906-913.
doi pubmed - Chong AY, Blann AD, Patel J, Freestone B, Hughes E, Lip GY. Endothelial dysfunction and damage in congestive heart failure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 2004;110(13):1794-1798.
doi pubmed - Hirose H, Sarosiek K, Cavarocchi NC. Ad hoc cost analysis of the new gastrointestinal bleeding algorithm in patients with ventricular assist device. ASAIO J. 2014;60(3):351-352.
doi pubmed - Axelrad JE, Pinsino A, Trinh PN, Thanataveerat A, Brooks C, Demmer RT, Effner L, et al. Limited usefulness of endoscopic evaluation in patients with continuous-flow left ventricular assist devices and gastrointestinal bleeding. J Heart Lung Transplant. 2018;37(6):723-732.
doi pubmed - Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125(24):3038-3047.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


