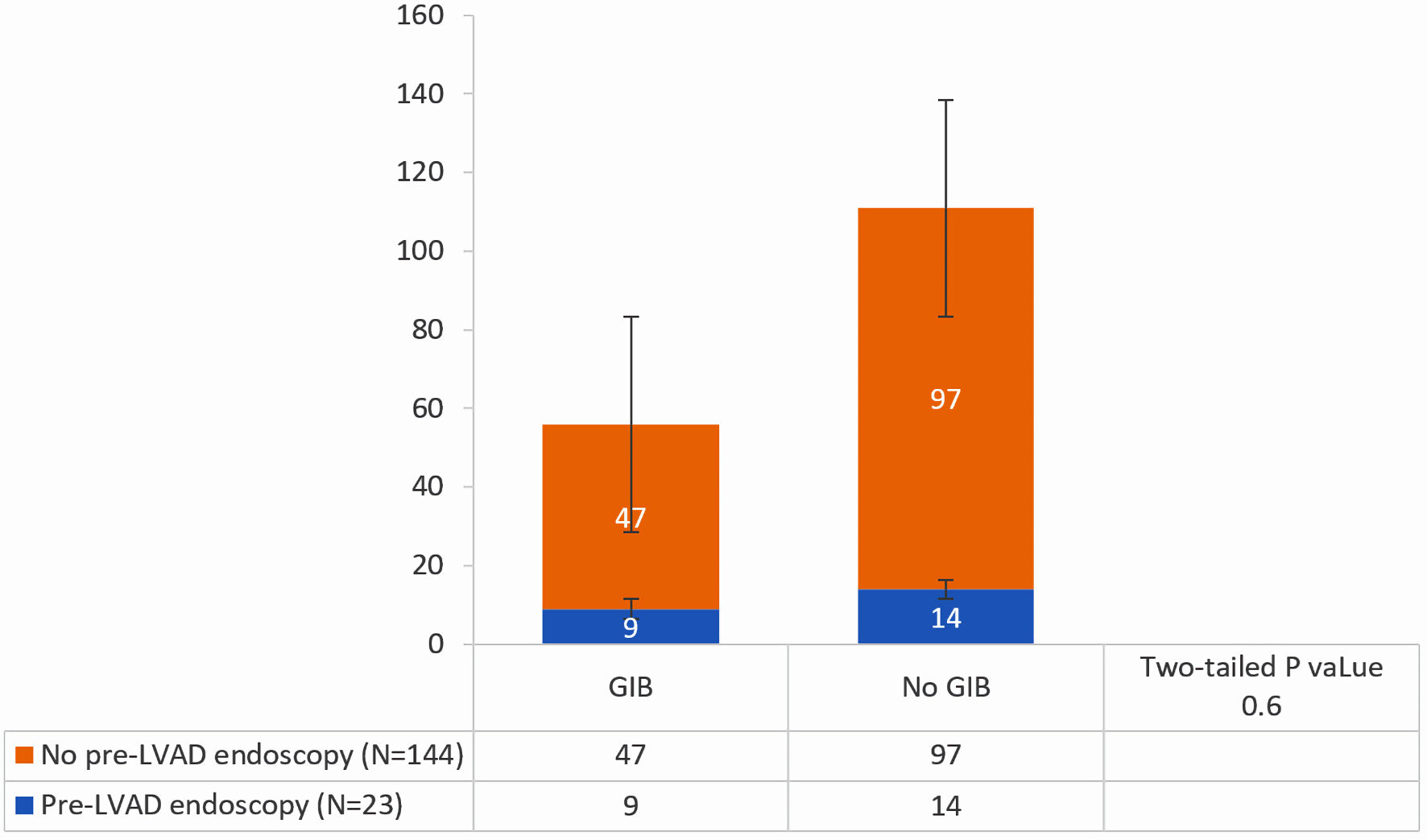
Figure 1. Comparison of the incidence of gastrointestinal bleeding (GIB) between patients who underwent pre-left ventricular assist device (LVAD) endoscopic evaluation and those who did not.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 1, February 2024, pages 1-9
Pre- and Post-Implant Endoscopy in Left Ventricular Assist Device Recipients: A Single-Center Experience
Figures

Tables
| Variable | Cohort (n = 167) |
|---|---|
| GIB: gastrointestinal bleeding; LVAD: left ventricular assist device. | |
| Age at LVAD implantation, years, mean (IQR 25-75%) | 61.5 (17 - 79) |
| Gender | |
| Male | 130 (77.8%) |
| Female | 37 (22.2%) |
| Race | |
| White | 152 (91%) |
| Black | 14 (8.4%) |
| Hispanic | 1 (0.6%) |
| Type of LVAD | |
| HeartWare | 38 (22.8%) |
| HeartMate 2 | 97 (58%) |
| HeartMate 3 | 26 (15.6%) |
| PVAD | 6 (3.6%) |
| Indication for LVAD implantation | |
| Ischemic cardiomyopathy | 91 (55.5%) |
| Non-ischemic cardiomyopathy | 76 (44.5%) |
| Previous history of GIB before LVAD implantation | |
| No | 134 (80.2) |
| Yes | 33 (19.8) |
| GIB within 1 year after LVAD | |
| No | 111 (66.5) |
| Yes | 56 (33.5) |
| Antiplatelets within 1 year after LVAD | |
| None | 3 |
| Single agent (aspirin) | 34 |
| Dual agents (aspirin + clopidogrel) | 1 |
| N/A | 18 |
| Number of hospital admission for GIB within 1 year after LVAD | |
| 1 | 25 |
| 2 | 13 |
| 3 | 0 |
| 4 | 1 |
| 5 | 1 |
| Indication for hospital admission for GIB within 1 year after LVAD | |
| Melena | 32 (80) |
| Hematochezia | 5 (12.5) |
| Combined melena and hematochezia | 2 (5) |
| Hematemesis and melena | 1 (2.5) |
| Pre-LVAD (n = 23) | Post-LVAD (n = 55) | |
|---|---|---|
| EGD: esophagogastroduodenoscopy; LVAD: left ventricular assist device. | ||
| Scope modality | ||
| EGD | 14 (60.9%) | 37 (67.3%) |
| Colonoscopy | 9 (39.1%) | 27 (49.1%) |
| Enteroscopy | 3 (13.0%) | 20 (36.4%) |
| Capsule | 1 (4.3%) | 4 (7.27%) |
| Source of bleeding | ||
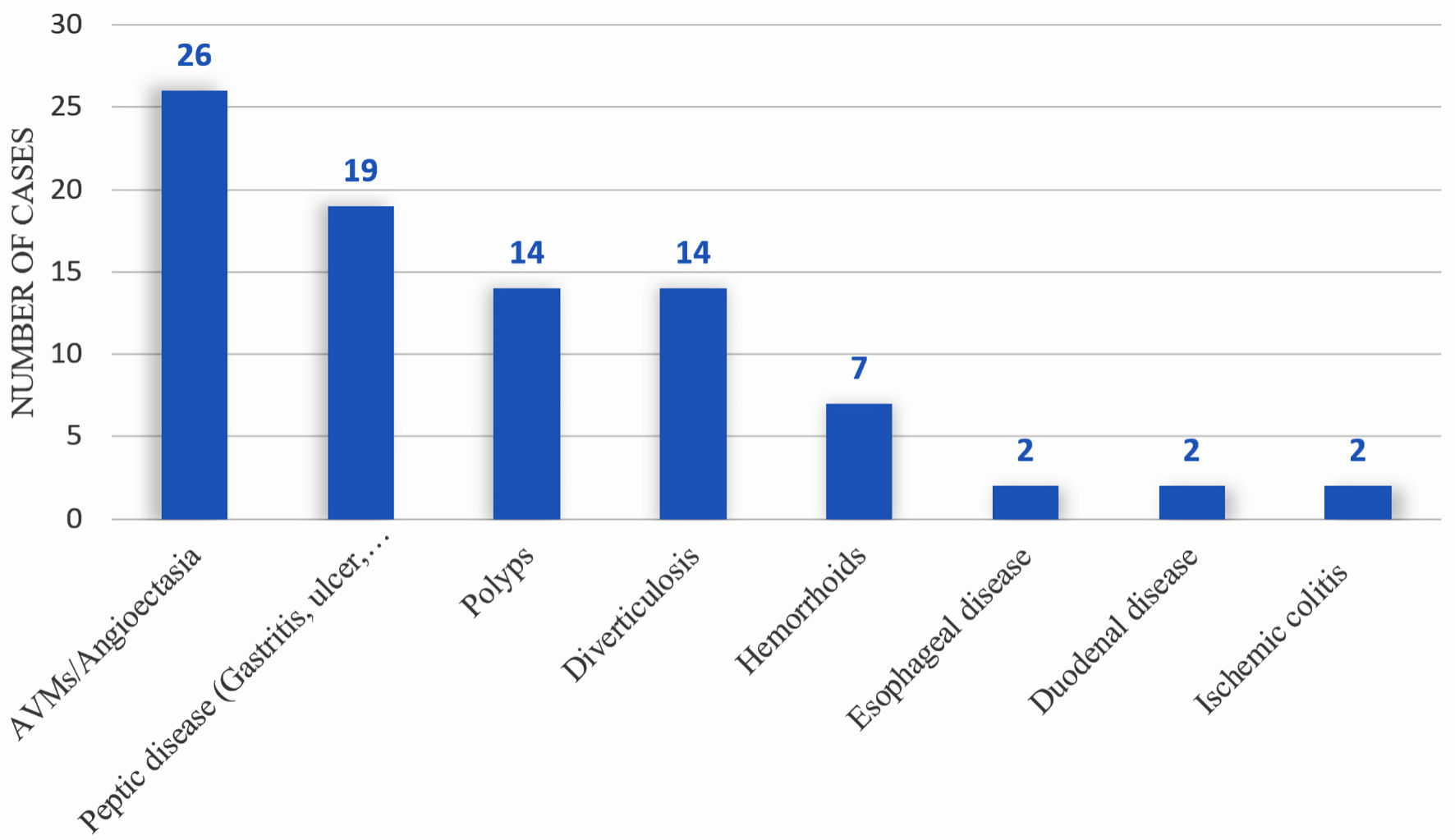
| Peptic ulcer disease | 7 (30.4%) | 19 (34.6%) |
| Angiodysplasia | 2 (8.70%) | 26 (47.3%) |
| Polyps | 3 (13.0%) | 14 (25.5%) |
| Hemorrhoids | 3 (13.0%) | 7 (12.7%) |
| Diverticulosis | 5 (21.7%) | 14 (25.5%) |
| Esophageal disease | 2 (8.7%) | 2 (3.64%) |
| Duodenal disease | 4 (17.4%) | 2 (3.64%) |
| Ischemic colitis | 0 (0.0%) | 2 (3.64%) |
| Location of lesion | ||
| No source identified | 5 (21.7%) | 9 (16.3%) |
| Stomach | 3 (13.0%) | 27 (49.1%) |
| Small intestine | 3 (13.0%) | 25 (45.5%) |
| Large intestine | 3 (13.0%) | 30 (54.5%) |
| Ano-rectal | 3 (13.0%) | 8 (14.5%) |
| GIB | No GIB | P value | |
|---|---|---|---|
| GIB: gastrointestinal bleeding; LVAD: left ventricular assist device. | |||
| No pre-LVAD endoscopy (n = 144) | 32.6% (n = 47) | 67.3% (n = 97) | |
| Pre-LVAD endoscopy (n = 23) | 39.1% (n = 9) | 60.9% (n = 14) | 0.6 |
| Endoscopic findings | OR | 95% CI |
|---|---|---|
| LVAD: left ventricular assist device; CI: confidence interval. | ||
| Peptic ulcer disease | 1.21 | 0.42 - 3.44 |
| Angiodysplasia | 9.41 | 2.01 - 44.09 |
| Polyps | 2.28 | 0.59 - 8.84 |
| Hemorrhoids | 0.97 | 0.23 - 4.14 |
| Diverticulosis | 1.23 | 0.38 - 3.93 |