| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 6, December 2023, pages 281-288
Timing and Outcomes of Percutaneous Endoscopic Gastrostomy After Ischemic Stroke
Shoma Bommenaa, d , Pooja Rangana, Joyce Lee-Iannottib, Wahid Wassefa, Rakesh Nandac
aDepartment of Internal Medicine, University of Arizona College of Medicine-Phoenix, Banner University Medical Center, Phoenix, AZ, USA
bDepartment of Neurology, University of Arizona College of Medicine-Phoenix, Banner University Medical Center, Phoenix, AZ, USA
cDivision of Gastroenterology, University of Arizona College of Medicine-Phoenix, Phoenix VA Health Care System, Phoenix, AZ, USA
dCorresponding Author: Shoma Bommena, Department of Internal Medicine, University of Arizona College of Medicine-Phoenix, Banner University Medical Center, Phoenix, AZ 85006, USA
Manuscript submitted June 22, 2023, accepted October 23, 2023, published online December 9, 2023
Short title: PEG in Stroke
doi: https://doi.org/10.14740/gr1653
| Abstract | ▴Top |
Background: Guidelines recommend using percutaneous endoscopic gastrostomy (PEG) for dysphagia after 2 weeks of stroke onset. We aimed to study the impact of PEG timing on outcomes in patients with ischemic stroke.
Methods: In this retrospective study of patients with ischemic stroke and PEG between 2014 and 2019, early PEG was defined as PEG tube placed within 14 days of stroke and late PEG after 14 days. Outcomes of 30-day mortality, PEG-related complications, and functional swallow recovery were compared between early and late PEG. Logistic regression model assessed factors associated with PEG timing.
Results: The median time of PEG tube placement after stroke was 10.9 days. Of the 161 included patients, 60.9% had early PEG, and its associated patient factors were nursing facility discharge (adjusted odds ratio (OR): 3.4, confidence interval (CI): 1.48 - 7.82) and infection (OR: 0.32, CI: 0.139 - 0.178). Late PEG had 3.27 times greater odds of swallowing recovery, but mortality and complications were not significantly different between early and late PEG.
Conclusions: Skilled nursing facility disposition and lack of infection were predictors of early PEG, constituting the majority of PEG placed for ischemic stroke-related dysphagia. Although better odds of swallowing recovery were seen with late PEG, likely implicating better patient selection, overall, the timing of PEG tube placement did not impact short-term mortality and complications.
Keywords: Percutaneous endoscopic gastrostomy; Ischemic stroke; Dysphagia; Early PEG; Late PEG
| Introduction | ▴Top |
The reported long-term outcomes in patients who receive percutaneous endoscopic gastrostomy (PEG) for stroke-related dysphagia are poor [1, 2], with 2-year mortality as high as 65% and only 14% alive with the regained ability to eat after 2 years of hospitalization. Furthermore, PEG during the index stroke admission is also an independent predictor of 30- and 60-day hospital readmissions [1, 3]. Hence, in patients with dysphagia due to acute stroke, careful patient selection is essential for PEG tube placement, which is, after all, an invasive procedure that is not risk-free [4, 5].
The incidence of dysphagia after acute stroke is greater than 60% [6]. Typically, a nasogastric tube (NGT) or nasoduodenal tube is used initially to administer medications and nutrition, and a PEG is placed later in those with prolonged dysphagia [7]. Regarding timing, the American Stroke Association (ASA) and American Heart Association (AHA) guidelines suggest NGT feeding until 2 - 4 weeks after stroke onset and consider PEG afterward [8]. However, in practice, the PEG tube placement rates in acute ischemic strokes vary widely across US hospitals, and most PEG tube placement happens earlier than the recommended guidelines [9-11]. However, there is a knowledge gap on the factors associated with early vs. late PEG tube placement timing relative to stroke onset and its impact on patient outcomes. The significance of the patient selection criterion is particularly relevant in the widely prevailing heterogeneity in the timing of PEG tube placement in patients with ischemic stroke [12].
We aimed to assess the impact of the timing of PEG tube placement relative to stroke onset on outcomes in patients with dysphagia from an acute ischemic stroke. The primary outcome was 30-day mortality. Secondary outcomes were PEG-related complications and functional swallowing recovery. We also aimed to assess factors associated with the timing of PEG tube placement in patients with acute ischemic stroke.
| Materials and Methods | ▴Top |
We conducted a retrospective single-center cohort study of all adult patients admitted for an acute ischemic stroke who received a PEG between 2014 and 2019. The study population was limited to ischemic strokes without including hemorrhagic stroke, as the neurological outcomes of hemorrhagic stroke are very different compared to ischemic stroke [13]. Inclusion criteria were patients with acute ischemic stroke with a PEG placed for dysphagia during the same hospital admission. In our study, early PEG was defined as PEG placed within 14 days of ischemic stroke onset, and late PEG was defined as PEG placed at or 14 days after the ischemic stroke. We used 14 days from stroke onset to classify the timing of PEG, based on the ASA guidelines that recommend choosing NGT for 2 - 3 weeks after stroke. Additionally, this definition was used in a previous study that evaluated the trends of PEG tube timing in stroke patients [3, 8]. We compared outcomes between the patients who received early and late PEG. The primary outcome of our study was 30-day mortality. Secondary outcomes included PEG-related complications and functional swallowing recovery. The complications related to PEG included minor (tube dislodgement, PEG wound leakage, and wound infection) and major complications (peritonitis, gastric peroration, and significant bleeding). Functional swallowing recovery was defined as the ability to independently consume a pre-stroke oral diet and have PEG removed at follow-up visits. Partial recovery was defined as oral intake along with supplemental PEG tube feeding.
International Classification of Disease, Ninth Revision, and Clinical Modification (ICD-9-CM) codes of 434.xx and International Classification of Disease, Tenth Revision, and Clinical Modification (ICD-10-CM) codes of I63.xx were used to identify all cases of ischemic stroke in the study period. In addition, the PEG procedure during the hospital stay was identified by the procedure order (PEG tube placement, AN plc G tube Perc) and the ICD-10-CM of Z 93.1. In addition, medical records were reviewed, and the presence of ischemic stroke was verified based on documentation in the clinical progress notes and findings on diffusion-weighted imaging (DWI) on the brain magnetic resonance imaging (MRI). Among the confirmed patients with ischemic stroke, only those PEG placed due to dysphagia after the index stroke in the same admission were included (Fig. 1), and the rest were excluded.
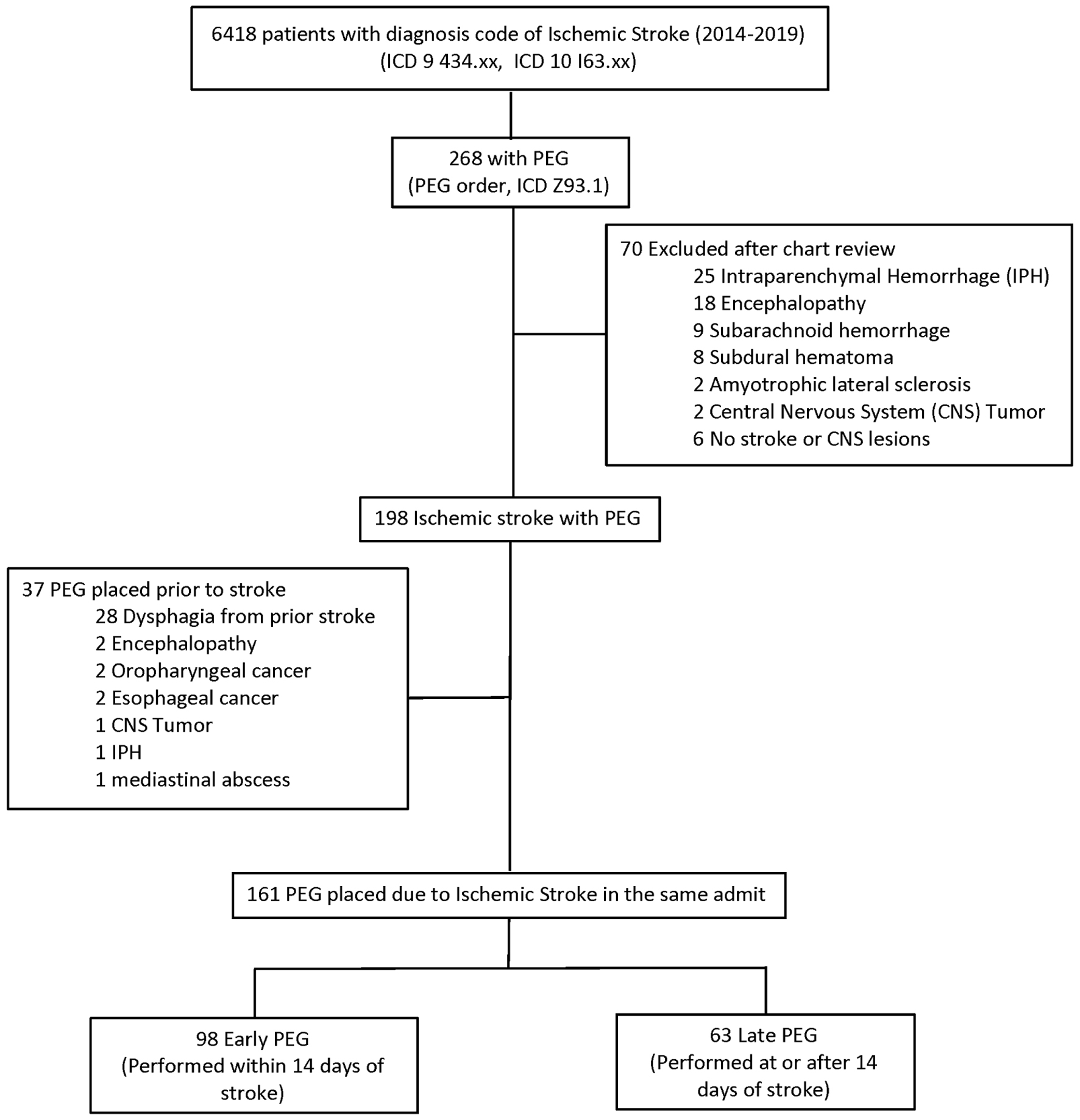 Click for large image | Figure 1. Distribution of the cohort. |
Patient demographics, comorbidities, stroke-related vascular risk factors, and characteristics were abstracted from medical records. For comorbidity assessment, we utilized the Charlson Comorbidity Index (CCI), an index associated with outcomes and mortality in patients with ischemic stroke [14]. Based on diabetes mellitus, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, malignancies, peripheral vascular disease, and dementia, the CCI score was calculated to categorize them into three groups: mild (CCI score 1 - 2), moderate (CCI score 3 - 4), and severe (CCI score > 5). In addition, mechanical ventilation, tracheostomy status, and infections, including sepsis, pneumonia, urinary tract infections, and complications after PEG tube placement, were collected. The mean follow-up period of our study was 5.4 months. The study was conducted in accordance with the approval of the institutional review board in compliance with all the applicable institutional ethical guidelines for the care, welfare and use of animals.
Data analysis
The data were analyzed to estimate 30-day mortality (primary outcome), PEG-related complications, and functional swallow recovery after PEG removal (secondary outcomes). Data were described using descriptive statistics, and non-parametric methods were employed when data were not normally distributed. Continuous data were compared between groups with two-group t-tests or Wilcoxon rank-sum (Mann-Whitney) test as appropriate. The Chi-square test compared categorical data. Logistic regression was used to study the effect of PEG timing on dichotomous outcomes of 30-day mortality, PEG-related complications, and swallowing recovery. Clinical factors associated with early compared to late PEG tube placement were identified by univariable analysis with an alpha of 0.2 cut-offs, followed by multivariable logistic regression analysis. A backward stepwise selection method was implemented for variable selection, and statistical significance was defined as an alpha of 0.05, with two-sided alternative hypotheses. Data were analyzed using STATA® Version 17 (StataCorp, College Station, TX).
| Results | ▴Top |
Distribution and demographics
A total of 6,418 ischemic stroke patients were identified based on ICD codes (Fig. 1). Of these, 268 (4.2%) were identified as having PEG. Of the 268 patients, 70 were excluded after chart review as they did not have an ischemic stroke. Of the 198 patients with confirmed ischemic stroke and PEG, 37 were excluded as they had PEG placed before the ischemic stroke for dysphagia unrelated to the stroke. The final cohort comprised 161 patients with ischemic stroke and gastrostomy tubes placed after the index stroke.
The incidence of gastrostomy tubes in patients with ischemic stroke was 2.54%. Overall, the median time of PEG tube placement after ischemic stroke was 10.9 days (interquartile range (IQR): 7.6 - 17.9). Of them, 60.9% (n = 98) had an early PEG with a median duration of 8.4 days (IQR: 6.2 - 11.8) (Fig. 2). The clinical characteristics of the cohort and a comparison between the two groups of early and late PEG are shown in Table 1. The two groups had no significant difference in patient demographics, clinical features, comorbidities, and stroke severity based on National Institute of Health Stroke Scale (NIHSS) scores (16 vs. 17; P = 0.968). However, early PEG was associated with a significantly shorter length of stay (mean 15.8 vs. 20.11; P = 0.029).
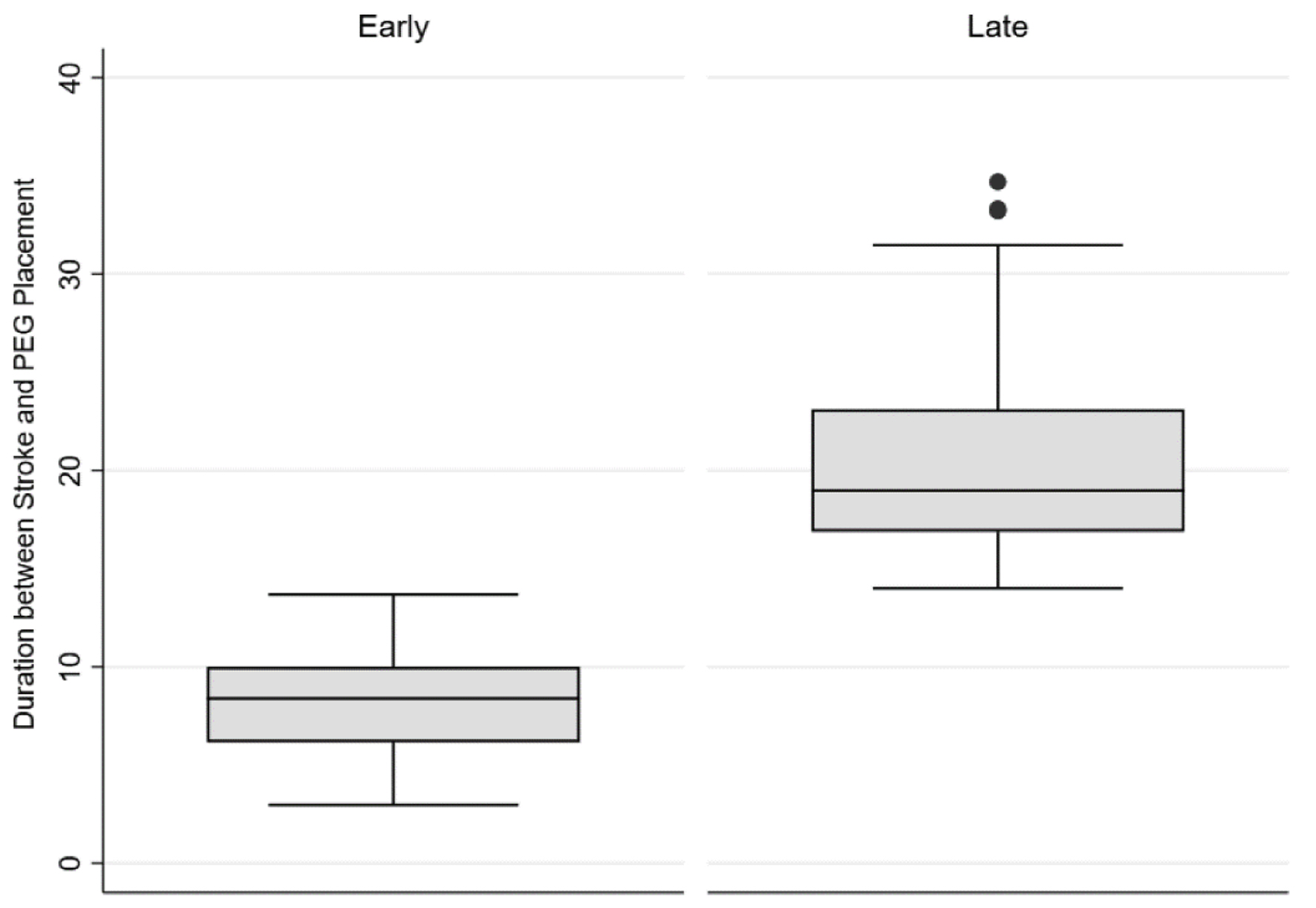 Click for large image | Figure 2. Distribution of median time from stroke to PEG in early vs. late PEG. PEG: percutaneous endoscopic gastrostomy. |
 Click to view | Table 1. Patient Demographics and Comorbidities in the Early and Late PEG Groups |
The association of the timing of PEG with primary and secondary outcomes
The overall 30-day mortality of the cohort was 6.8% (n = 11). The 30-day mortality was not significantly different between early and late PEG (7.14% vs. 6.35%, P = 0.558) (Table 2). The functional swallow recovery rate in the cohort was 26.7% (n = 43), and it was significantly higher in the late PEG compared to early PEG at a mean follow-up of 5.4 months (36.51% vs. 20.41%, respectively; P = 0.024). In the multiple logistic regression model, those patients with late PEG had 3.27 times greater odds of swallowing recovery and PEG removal after adjusting for covariates, including age, gender, race comorbidities, and stroke severity (adjusted OR: 3.27; 95% CI: 1.22 - 8.74; P = 0.02). A low NIHSS score was the other factor that predicted swallowing recovery with PEG removal in this model (Table 3).
 Click to view | Table 2. Primary and Secondary Outcomes in the Early and Late PEG Groups |
 Click to view | Table 3. Univariable and Multivariable Logistic Regression Model for PEG Removal and Swallowing Recovery |
There were no significant differences in overall PEG-associated complications between late PEG and early PEG (3.2% vs. 9.2%; P = 0.204). Our study had two major PEG-related complications (1.24%): one case of PEG malposition in the transverse colon and one case of gastric wall necrosis at the gastrostomy tube site that required partial gastric resection. The minor PEG-related complications included four dislodged gastrostomies, one G-tube leakage, one G-tube clog, and one G-tube malposition, most requiring PEG tube replacement. In addition, 75.8% of patients in the cohort were on uninterrupted antiplatelet (100 aspirin + 9 dual antiplatelet therapy with aspirin and clopidogrel) or anticoagulation therapy (n = 13 including intravenous heparin drip held 6 h prior to procedure; enoxaparin and apixaban with morning of procedure dose held). No direct PEG procedure-related gastrointestinal bleeding was observed in our cohort.
Patient factors associated with the timing of PEG tube placement
In order to evaluate the factors associated with the timing of PEG relative to stroke onset, we performed a univariate followed by multivariable regression analysis (Table 4). After adjusting for age, sex, comorbidities, intubation, tracheostomy status, and length of stay, those discharged to a skilled nursing facility after an ischemic stroke had 3.4 times higher odds of receiving an early PEG tube placement (adjusted odds ratio (OR): 3.4, confidence interval (CI): 1.48 - 7.82, P = 0.004) than those discharged to home or acute rehabilitation. In addition, those with infection during the ischemic stroke-related hospital stay (including sepsis from any cause, pneumonia, and urinary tract infection) had lower odds of having an early PEG (adjusted OR: 0.32, CI: 0.139 - 0.178, P = 0.007).
 Click to view | Table 4. Univariable and Multivariable Logistic Regression Model Assessing the Association Between Early PEG and Patient Factors |
| Discussion | ▴Top |
The incidence of PEG tube placement for dysphagia after an acute ischemic stroke was 2.54% in our cohort. The majority (60.9%, n = 63) had an early PEG within 2 weeks of acute ischemic stroke. Disposition to a skilled nursing facility from the stroke hospital admission was associated with 3.4 times higher odds of receiving an early PEG. In addition, sepsis and infection during the stroke hospital admission were associated with 0.32 times lower odds of early PEG. Although late PEG had higher odds of functional swallowing recovery and PEG removal at follow-up, we did not find significant differences in the 30-day mortality and PEG-related complications between early and late PEG groups. Our results suggest that patients who received PEG after 14 days were adequately prepared for PEG construction
A PEG tube was placed at a median of 10.9 days after the stroke. The distribution of early vs. late PEG in our study is aligned with the previously reported discrepancy between guidelines and practice trends in PEG tube placement across the USA. However, our late PEG rate of 39% is higher than that reported in previous studies. For example, a state inpatient database study on PEG after an acute stroke had a 14% prevalence of late PEG [9], and another study utilized National Inpatient Sample (NIS) data to study PEG timing in patients with ischemic stroke, where greater than 50% PEG happened within a week of admission [10]. Differences in dysphagia assessments of speech pathologists [15], physicians overestimating PEG benefits, and lack of awareness of late PEG benefits among providers are some of the speculated factors associated with varied PEG practices of nationwide hospitals after stroke [10, 11, 16].
In our study, discharge to a skilled nursing facility was associated with early PEG tube placement. Previous studies have speculated that policies of skilled nursing homes and their preferences for accepting PEG due to potential NGT dislodgement might contribute to early PEG tube placement [17]. Consistent with this association are findings of a nationwide survey study of speech pathologists that system pressures for early discharge influenced 35% of their recommendations for PEG tube placement [15]. Whether the disposition to skilled nursing influenced the decision for early PEG tube placement within 2 weeks of acute stroke and whether this practice, to facilitate early discharge to a skilled nursing facility, might over-select patients for the PEG procedure remains to be investigated further. Incorporating a multidisciplinary approach with the palliative care team while focusing on patient care goals in PEG tube placement, using a nasal bridle in patients who temporarily need NGT to recover swallowing or wish not to get a PEG due to their poor prognosis, and improving education among providers and the nursing homes are some of the proposed solutions [18, 19]. The validated objective tools such as the validated Sheffield Gastrostomy Scoring System (SGSS) and predictive swallowing score to predict swallowing recovery in those with dysphagia from ischemic strokes are important clinical tools that can help clinicians and family members in identifying those who would benefit from a feeding tube [20-22]. In addition, in our study, we found that those with infections including pneumonia, urinary tract infections, and sepsis during the stroke hospitalization had lower odds of receiving an early PEG, likely reflecting that these patients were sicker from non-stroke-related issues that delayed the PEG tube placement decision. In comparision, a previous study that looked at factors associated with PEG timing found older age and large stroke volume to be associated with early PEG, placed within a week of stroke [10].
Although the 30-day mortality rate between early and late PEG in our study was not significantly different, the findings of more significant swallowing recovery followed by PEG removal support the guideline recommendations of PEG timing in stroke. In order to manage dysphagia after stroke in the first 2 - 3 weeks, the guidelines recommend placing an NGT [8], which helps appropriate patient selection for PEG in stroke by limiting its use to those with persistent dysphagia. A possible explanation for our findings of greater odds of swallowing recovery in the late PEG group may be attributed to better patient selection based on the independent association of swallowing recovery with NIHSS score. In addition, the effect of intense speech therapy services that this group had more access to, as those with late PEG were frequently discharged to an acute rehabilitation facility, might have played a role. Finally, dependency on PEG caused by its early placement might negatively impact swallowing recovery. Similar to our study, the authors of the FOOD randomized controlled trial found that more patients in the early PEG group remained PEG dependent at follow-up, in contrast with the NGT group, in which only 30% of patients randomized to NGT feeding ended up receiving PEG later [23]. With no difference in the two groups, the rate of post-PEG complications was 6.8%, which is similar to the reported rate in the literature [4]. Interestingly, our cohort did not have any direct PEG-related bleeding complications, despite 75.8% of patients being on peri-procedural antiplatelet or anticoagulation therapy. This is consistent with findings of recent studies, including a meta-analysis of 12 studies that included 8,471 patients, where antiplatelet therapy did not increase the risk of bleeding after PEG [24].
Here we review the literature on the outcomes of PEG after an ischemic stroke based on the timing of its placement. The PEG vs. NGT arm trial of multicenter randomized controlled FOOD trial studied the effect of the route of the enteral tube and timing on outcomes and found that early PEG in a week of stroke was associated with an increased borderline significance of the absolute risk of death [23]. A recent large observational study of patients that included both ischemic stroke and intracerebral hemorrhage found that gastrostomy and jejunostomy tubes placed within week 1 of stroke had significantly greater 30-day mortality than those placed after 5 weeks. However, a severe disability with a modified Rankin Scale (MRS) of 4 - 5 at discharge was also noted in these late tube placement survivors. Unlike our study, patients who died after early PEG more commonly had dementia, advanced age, and greater stroke severity [25]. Another observational study of 154 patients did not show any difference in 30-day mortality similar to our results, although the authors considered early PEG as those placed within 7 days of stroke [26]. On the contrary, a systemic review that evaluated the effectiveness and safety of PEG as opposed to NGT in adults with dysphagia, not limited to stroke, found that PEG had lower chances of intervention failure. There was no significant difference in mortality between the two groups. The evidence, however, was of low quality [27].
Our study has several limitations. First, it is a retrospective study. Second, there might be residual confounding even after matching factors such as stroke severity, medical comorbidities, and age that could have affected our study results. Third, we acknowledge a loss to follow up with both groups in evaluating and comparing swallowing recovery between early and late PEG. However, this was non-differential as the baseline characteristics between the two groups were not statistically different. Lastly, we could not capture and adjust for the mRS in all the patients. Despite these weaknesses, we believe our study findings are significant in the context of varied PEG practices and will aid provider in decision-making on the placement of PEG in acute ischemic stroke.
In conclusion, consistent with current PEG practice patterns in the USA, most PEG tubes were placed within 2 weeks of stroke. Disposition to a skilled nursing facility and lack of infection during hospitalization were significant predictors of early PEG tube placement. Despite greater odds of swallowing recovery with less PEG dependency at follow-up in the late PEG group, no differences in 30-day mortality and post-PEG complications were seen between early and late PEG groups. Our results suggest that patients who received a late PEG after 14 days were adequately prepared for PEG construction. In the current era of widely varied PEG timing practices across the USA, with the potential influence of the health care system, education on the timing of PEG among providers and facilities might standardize the management of acute stroke-related dysphagia.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Given the retrospective nature of the study, informed consent was waived.
Author Contributions
Shoma Bommena: conceptualization; investigation; methodology; validation; visualization; writing - initial draft and revisions. Pooja Rangan: methodology; statistical analysis. Joyce Lee-Iannotti: methodology; review and editing. Wahid Wassef: methodology; review and editing. Rakesh Nanda: conceptualization, methodology; review, and editing.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AHA: American Heart Association; ASA: American Stroke Association; CCI: Charlson Comorbidity Index; DWI: diffusion-weighted imaging; ICD: International Classification of Diseases; MBS: modified barium swallow; MRI: magnetic resonance imaging; NGT: nasogastric tube; NIHSS: National Institute of Health Stroke Scale; PEG: percutaneous endoscopic gastrostomy
| References | ▴Top |
- Meisel K, Arnold RM, Stijacic Cenzer I, Boscardin J, Smith AK. Survival, functional status, and eating ability after percutaneous endoscopic gastrostomy tube placement for acute stroke. J Am Geriatr Soc. 2017;65(8):1848-1852.
doi pubmed - Sutcliffe L, Flynn D, Price CI. Percutaneous endoscopic gastrostomy and mortality after stroke in England from 2007 to 2018: a retrospective cohort study. Stroke. 2020;51(12):3658-3663.
doi pubmed - Wilmskoetter J, Simpson KN, Bonilha HS. Hospital readmissions of stroke patients with percutaneous endoscopic gastrostomy feeding tubes. J Stroke Cerebrovasc Dis. 2016;25(10):2535-2542.
doi pubmed pmc - Stein DJ, Moore MB, Hoffman G, Feuerstein JD. Improving all-cause inpatient mortality after percutaneous endoscopic gastrostomy. Dig Dis Sci. 2021;66(5):1593-1599.
doi pubmed - Arora G, Rockey D, Gupta S. High In-hospital mortality after percutaneous endoscopic gastrostomy: results of a nationwide population-based study. Clin Gastroenterol Hepatol. 2013;11(11):1437-1444.e1433.
doi pubmed - Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
doi pubmed - O'Mahony D, McIntyre AS. Artificial feeding for elderly patients after stroke. Age Ageing. 1995;24(6):533-535.
doi pubmed - Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
doi pubmed - Wilmskoetter J, Simpson AN, Simpson KN, Bonilha HS. Practice patterns of percutaneous endoscopic gastrostomy tube placement in acute stroke: are the guidelines achievable? J Stroke Cerebrovasc Dis. 2016;25(11):2694-2700.
doi pubmed pmc - George BP, Kelly AG, Albert GP, Hwang DY, Holloway RG. Timing of percutaneous endoscopic gastrostomy for acute ischemic stroke: an observational study from the US nationwide inpatient sample. Stroke. 2017;48(2):420-427.
doi pubmed - George BP, Kelly AG, Schneider EB, Holloway RG. Current practices in feeding tube placement for US acute ischemic stroke inpatients. Neurology. 2014;83(10):874-882.
doi pubmed pmc - Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895.
doi pubmed - Chiu D, Peterson L, Elkind MSV, Rosand J, Gerber LM, Silverstein MD, Glycine Antagonist in Neuroprotection Americas Trial I. Comparison of outcomes after intracerebral hemorrhage and ischemic stroke. J Stroke Cerebrovasc Dis. 2010;19(3):225-229.
doi pubmed - Jimenez Caballero PE, Lopez Espuela F, Portilla Cuenca JC, Ramirez Moreno JM, Pedrera Zamorano JD, Casado Naranjo I. Charlson comorbidity index in ischemic stroke and intracerebral hemorrhage as predictor of mortality and functional outcome after 6 months. J Stroke Cerebrovasc Dis. 2013;22(7):e214-218.
doi pubmed - Chen BJ, Suolang D, Frost N, Faigle R. Practice patterns and attitudes among speech-language pathologists treating stroke patients with dysphagia: a nationwide survey. Dysphagia. 2022;37(6):1715-1722.
doi pubmed - Teno JM, Mitchell SL, Kuo SK, Gozalo PL, Rhodes RL, Lima JC, Mor V. Decision-making and outcomes of feeding tube insertion: a five-state study. J Am Geriatr Soc. 2011;59(5):881-886.
doi pubmed pmc - Moran C, O'Mahony S. When is feeding via a percutaneous endoscopic gastrostomy indicated? Curr Opin Gastroenterol. 2015;31(2):137-142.
doi pubmed - Anderson MR, O'Connor M, Mayer P, O'Mahony D, Woodward J, Kane K. The nasal loop provides an alternative to percutaneous endoscopic gastrostomy in high-risk dysphagic stroke patients. Clin Nutr. 2004;23(4):501-506.
doi pubmed - Seder CW, Stockdale W, Hale L, Janczyk RJ. Nasal bridling decreases feeding tube dislodgment and may increase caloric intake in the surgical intensive care unit: a randomized, controlled trial. Crit Care Med. 2010;38(3):797-801.
doi pubmed - Kurien M, Leeds JS, Delegge MH, Robson HE, Grant J, Lee FK, McAlindon ME, et al. Mortality among patients who receive or defer gastrostomies. Clin Gastroenterol Hepatol. 2013;11(11):1445-1450.
doi pubmed - Wilcox CM, McClave SA. To PEG or not to PEG. Clin Gastroenterol Hepatol. 2013;11(11):1451-1452.
doi pubmed - Galovic M, Stauber AJ, Leisi N, Krammer W, Brugger F, Vehoff J, Balcerak P, et al. Development and validation of a prognostic model of swallowing recovery and enteral tube feeding after ischemic stroke. JAMA Neurol. 2019;76(5):561-570.
doi pubmed pmc - Dennis MS, Lewis SC, Warlow C, Collaboration FT. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365(9461):764-772.
doi pubmed - Gangwani MK, Aziz M, Aziz A, Priyanka F, Patel A, Ghaffar U, Weissman S, et al. Dual antiplatelet therapy does not increase bleeding risk in percutaneous gastrostomy tube placement: network meta-analysis. Dig Dis Sci. 2023;68(5):1966-1974.
doi pubmed - Joundi RA, Saposnik G, Martino R, Fang J, Kapral MK. Timing of direct enteral tube placement and outcomes after acute stroke. J Stroke Cerebrovasc Dis. 2019;28(12):104401.
doi pubmed - Reddy KM, Lee P, Gor PJ, Cheesman A, Al-Hammadi N, Westrich DJ, Taylor J. Timing of percutaneous endoscopic gastrostomy tube placement in post-stroke patients does not impact mortality, complications, or outcomes. World J Gastrointest Pharmacol Ther. 2022;13(5):77-87.
doi pubmed pmc - Gomes CA, Jr., Andriolo RB, Bennett C, Lustosa SA, Matos D, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2015;2015(5):CD008096.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


