| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 16, Number 5, October 2023, pages 276-279
Primary Squamous Cell Biliary Carcinoma With Liver Metastasis Is Rare but Malicious
Mohamad Khaled Almujarkesha, e , Anirudh R. Damughatlaa, Jasdeep Bathlaa, Kyle Sugga, Dana LaBudab, Samer Alkassisc, Mohammed Najeeb Al Hallaka, d
aDepartment of Internal Medicine, Wayne State University & Detroit Medical Center, Detroit, MI, USA
bSchool of Medicine, Wayne State University, Detroit, MI, USA
cDepartment of Oncology, University of California, Los Angeles, CA, USA
dDepartment of Oncology, Karmanos Cancer Institute, Detroit, MI, USA
eCorresponding Author: Mohamad Khaled Almujarkesh, Department of Internal Medicine, Wayne State University & Detroit Medical Center, Detroit, MI, USA
Manuscript submitted May 29, 2023, accepted September 12, 2023, published online October 21, 2023
Short title: Primary SCBC With Liver Metastasis
doi: https://doi.org/10.14740/gr1637
| Abstract | ▴Top |
Primary squamous cell carcinoma (SCC) of the liver is quite rare, and to our knowledge, very few cases have been reported in the literature. The exact pathogenesis of the disease is unestablished; however, it is mostly reported to be associated with hepatic cyst, Caroli’s disease, hepatolithiasis, hepatic cirrhosis, and hepatic teratoma. We report a case of a 50-year-old woman with no prior medical history initially, who presented with postprandial epigastric and right upper quadrant pain that continued to worsen and was associated with early satiety, nausea, and weight loss of 25 pounds over 2 months, which prompted further evaluation by her primary care physician. Magnetic resonance imaging (MRI) examination a month later revealed a large heterogeneous area measuring 8.5 × 2.4 × 7.4 cm in the inferior right hepatic lobe with heterogeneous enhancement and involvement of the gallbladder, concerning for cholangiocarcinoma. Given radiographic findings, she underwent a computed tomography (CT)-guided core biopsy of the liver, which showed a necrotic malignant tumor favoring adenocarcinoma and was also found to have germline BRCA mutation. A positron emission tomography (PET) scan revealed a large partially necrotic fluorodeoxyglucose (FDG) avid mass, possibly arising from the gallbladder fossa with an invasion of both lobes of the liver and probable involvement of a portion of the ascending colon. There was no gross evidence of distant metastatic disease. The patient underwent staging laparoscopy prior to initiating chemotherapy, and another biopsy was done, which returned in favor of SCC, with immunohistochemical stains being positive for cytokeratin (CK)19, Ber-EP4 (epithelial antigen recognized by Ber-EP4 antibody), and P40 (DeltaNp63); while negative for CK7, CK20, caudal-type homeobox 2 (CDX-2), paired box 8 (PAX-8), and mucicarmine. The patient started platinum-based chemotherapy due to germline BRCA mutation. However, due to complications associated with therapy and the progression of the disease, the patient eventually chose hospice. Primary SSC remains an unexplored aggressive malignancy that carries an overall poor prognosis. Diagnosis can be challenging and requires high clinical suspicion due to the scarcity in specific laboratory workup. Pathological diagnosis remains the gold standard; however, it also carries its own challenges. Treatment is usually case-oriented, and definitive protocols have yet to be established.
Keywords: Primary squamous cell biliary carcinoma; Cholangiocarcinoma; Squamous carcinoma; Cholangiocarcinoma transformation to squamous carcinoma; Adenocarcinoma
| Introduction | ▴Top |
Primary squamous cell biliary carcinoma (SCBC) with liver metastasis is rare and only reported occasionally at irregular intervals. To our knowledge, fewer than 12 cases have been described since Cabot’s first case, which was reported in 1930 [1]. Moreover, only a few of these cases were well documented [2]. Biliary tract cancers increase in incidence with age and are most commonly present in the fifth to seventh decades of life [3]. Although multiple theories have been proposed, the exact pathogenesis of primary SCBC is not yet established. Previous literature considered chronic inflammation of the bile duct, gallstones, congenital cysts, and liver cysts resulting from infections as potential contributing factors [4]. Gallstones as a cause of cholangiocarcinoma is unclear; however, it has been well documented that infectious diseases can cause chronic inflammation of the biliary tract, eventually developing into biliary tract malignancies.
Additionally, in the literature, a strong association has been noted to exist between liver fluke infestation and the development of biliary tract malignancies [5]. The majority of gallbladder cancer and cholangiocarcinoma cases are linked to recurring pyogenic cholangitis. Moreover, patients with primary sclerosing cholangitis (PSC) have a lifetime risk of over 30% [6, 7]. Cholangiocarcinoma is a noted complication of malignant strictures of choledochal cyst and choledocholithiasis [8]. Other rare conditions associated with cholangiocarcinoma development include choledochal cysts, Caroli’s disease, exposure to the radiopaque medium thorium dioxide (thorotrast), bile-duct adenoma, and multiple biliary papillomatosis [9]. The direct causality of some of these risk factors has been questioned in previous literature [9]. Many cases of cholangiocarcinoma occur in patients with no risk factors. Adenomas involving the ampulla of Vater are known to be premalignant, especially if they are villous in nature. Adenomas are frequently seen in patients with familial adenomatous polyposis. These individuals have approximately 100 times greater risk of ampullary adenocarcinoma than the average population [10, 11]. Human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) have been associated with malignancies throughout the entire biliary tract [12]. Adenocarcinomas and squamous cell carcinomas tend to metastasize to the liver, as seen in this case, and to regional lymph nodes [13]. SCBC tumors have an extremely poor prognosis, with only a few surviving beyond 12 months despite treatment [14].
| Case Report | ▴Top |
A 50-year-old woman with no prior medical history initially presented with postprandial epigastric and right upper quadrant pain that continued to worsen and was associated with early satiety, nausea, and weight loss of 25 pounds over 2 months, which prompted her primary care physician to get further evaluation. Computed tomography (CT) and magnetic resonance imaging (MRI) scans were done, which revealed a large heterogeneous area measuring 8.5 × 2.4 × 7.4 cm in the inferior right hepatic lobe with heterogeneous enhancement and involvement of the gallbladder concerning cholangiocarcinoma (Fig. 1). Given the concerning radiographic findings, the patient underwent a CT-guided core biopsy of the liver, and the pathology returned as a necrotic malignant tumor, favoring adenocarcinoma, with cytokeratin (CK)7, CK20, and pan keratin returning positive, and negative thyroid transcription factor 1 (TTF-1), and GATA binding protein-3 (GATA-3). The patient was also found to be with germline BRCA mutation. A positron emission tomography (PET) scan was done, which revealed a large partially necrotic fluorodeoxyglucose (FDG) avid mass possibly arising from the gallbladder fossa with an invasion of both lobes of the liver and probable involvement of a portion of the ascending colon. There was no gross evidence of distant metastatic disease. It was decided to initiate neoadjuvant chemotherapy before a staging laparoscopy was done. Biopsy obtained during laparoscopy indicated squamous cell carcinoma, with immunohistochemical stains being positive for CK19, Ber-EP4 (epithelial antigen recognized by Ber-EP4 antibody), and P40 (DeltaNp63) while negative for CK7, CK20, caudal-type homeobox 2 (CDX-2), paired box 8 (PAX-8), and mucicarmine (Figs. 2, 3). Endoscopic retrograde cholangiopancreatography (ERCP) was done to release the obstruction and revealed high-grade biliary stricture of 3 - 4 cm involving the proximal common bile duct and common hepatic duct with significant dilation of intrahepatic ducts and severe narrowing of the duodenum from malignant duodenal wall compression and invasion (Fig. 4). Papillotomy was performed, after which a 4 cm × 60 French Hurricane balloon was used for biliary dilation. An 8 cm × 10 mm fully covered metal stent was deployed successfully throughout the biliary structure, and a significant amount of contrast and biliary material was obtained. The patient was started on platinum-based chemotherapy due to germline BRCA mutation. However, due to complications associated with therapy and the progression of the disease, the patient eventually chose hospice care.
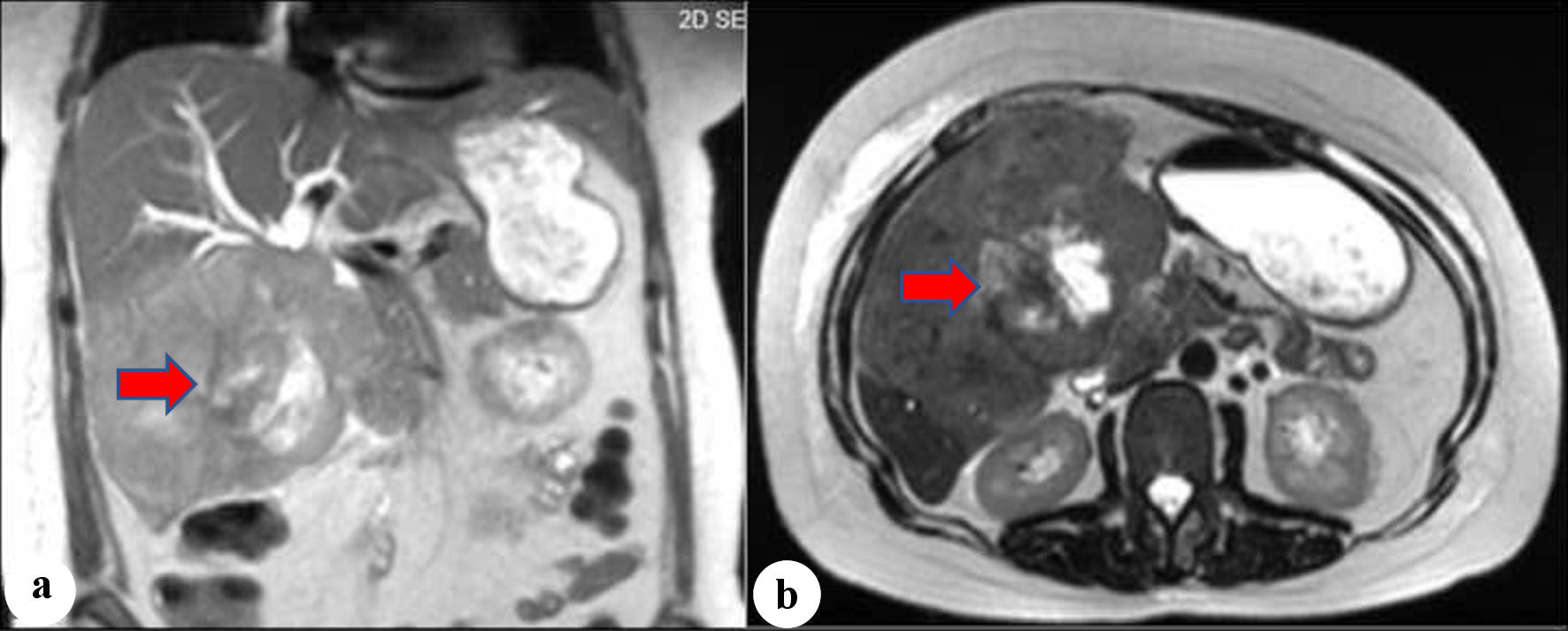 Click for large image | Figure 1. (a, b) Magnetic resonance imaging (MRI). The arrows show heterogeneous mass with a large necrotic center involving the right and left hepatic lobes, common hepatic duct, and proximal common bile duct. |
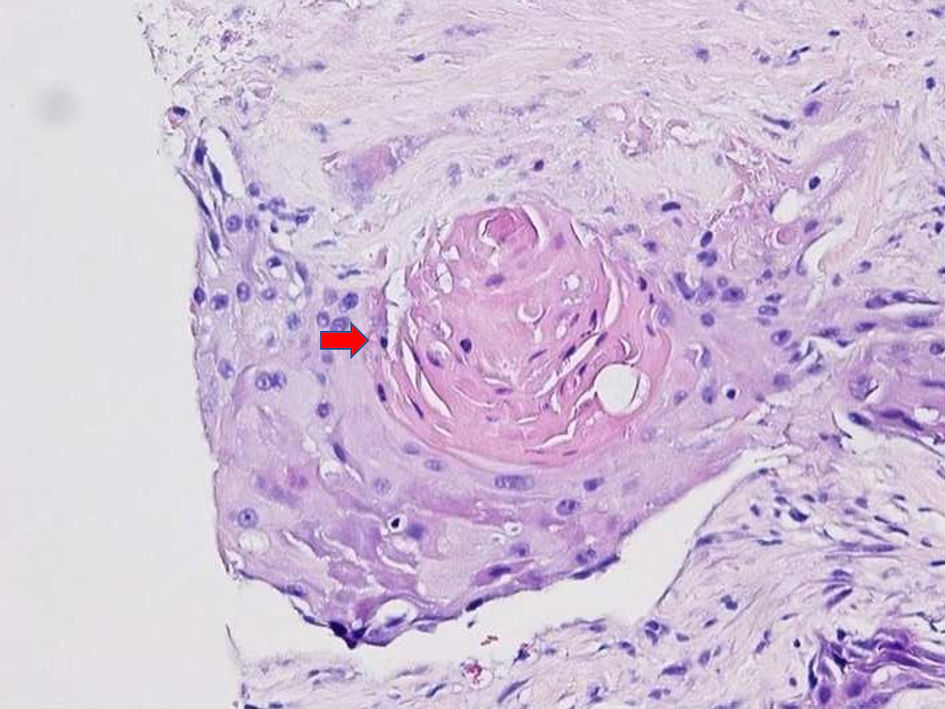 Click for large image | Figure 2. H&E staining images at × 20 showing squamous cell carcinoma, and the arrow shows focal keratinization. H&E: hematoxylin and eosin. |
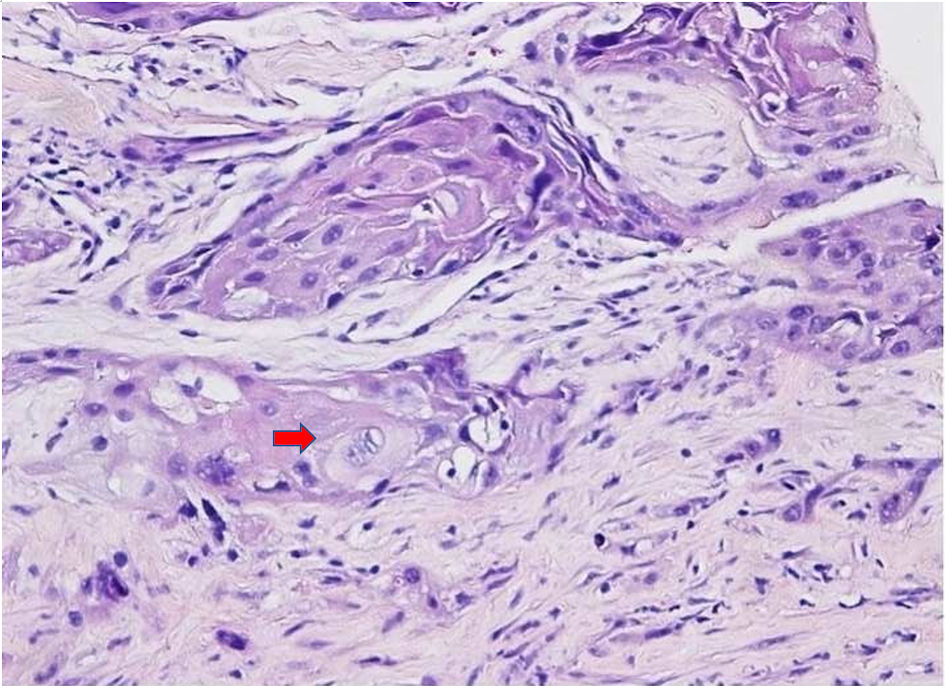 Click for large image | Figure 3. H&E staining images (× 20) showing squamous cell carcinoma with necrosis. The arrow shows typical squamous cell carcinoma cells which are large with abundant eosinophilic cytoplasm with often vesicular, nuclei. No normal hepatic tissue is present in the biopsy. H&E: hematoxylin and eosin. |
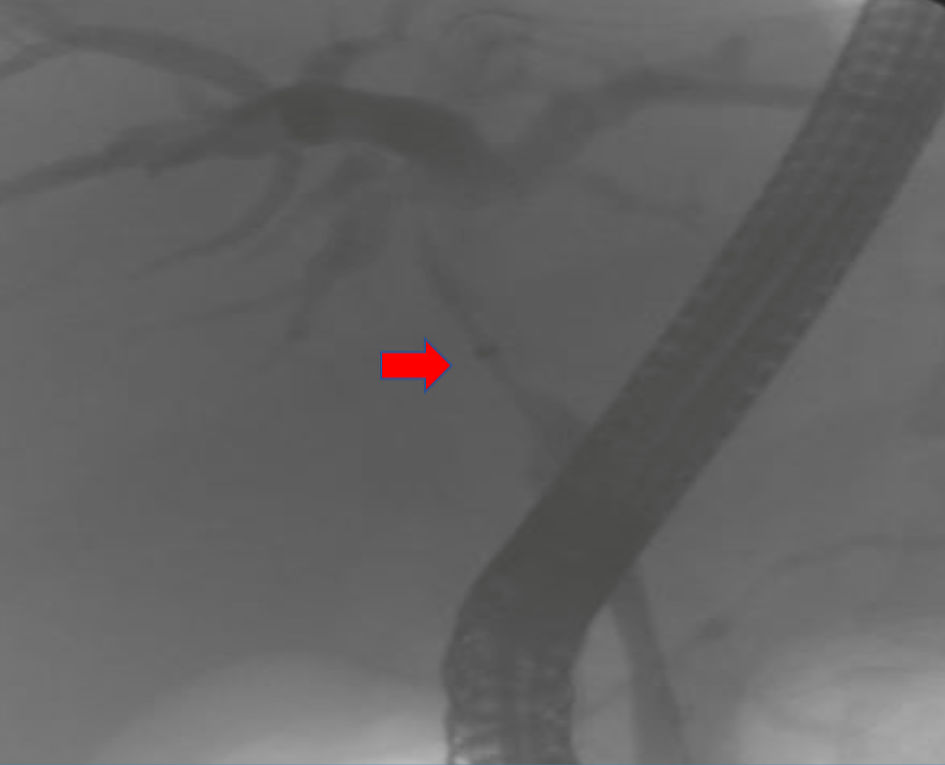 Click for large image | Figure 4. Endoscopic retrograde cholangiopancreatography (ERCP). The arrow shows high-grade biliary stricture 3 - 4 cm involving the proximal common bile duct and common hepatic duct with significant intrahepatic dilation. |
| Discussion | ▴Top |
Primary squamous cell carcinoma remains extremely rare, with scarce literature to guide diagnosis and treatment. Several theories have been suggested to clarify how malignant cells develop. The most widely accepted theory is malignant transformation under chronic insult to biliary cells, leading to metaplasia, and subsequently, malignant transformation. The majority of available literature reports nonspecific presenting symptoms; in our case, the patient sought attention for relatively common gastrointestinal symptoms (i.e., weight loss, postprandial abdominal pain, and early satiety). Such presentations, with nonspecific laboratory findings, make the diagnosis more challenging and solely rely on pathological findings after excluding other sites of primary origin. In the case of our patient, cancer antigen 125 (CA-125) was mildly elevated at 64.3 U/mL, and cancer antigen 19-9 (CA 19-9) was within normal limits (9.5 U/mL). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were also unremarkable (34/38 U/L), with elevated alkaline phosphatase (354 U/L). Radiographically, the patient’s CT scan showed a large, heterogeneous mass with an area of central necrosis. Extracapsular extension and invasion into the large bowel were also present. Most patients exhibited low-density masses with arterial phase enhancements, which persisted through the portal and delayed phases. Interestingly, this patient’s biopsies demonstrated mixed results, highlighted by positive CK19 indicating biliary duct origin of the malignant cells, in addition to being positive for CK7 and pan keratin indicating squamous epithelial origin. The disparity in these results is unclear. However, Adenomatous squamous metaplasia has the potential to evolve into several different types of cells, one of which could be SCBC. Existing reports of histological fusion of adenocarcinoma and SCBC support this potential [15]. The prognosis, unfortunately, continues to be poor; as for our patient, shortly after her diagnosis, her case was complicated by biliary obstruction requiring stenting, followed by developing a liver abscess and a duodenal fistula. In terms of treatment, no standard protocol has yet been established. Available literature has demonstrated that radical surgery provided a longer mean duration of survival in comparison to partial resection, drainage, chemotherapy, radiation, or palliative treatment [16]. Based on prior literature, there is only one documented case of primary intrahepatic squamous cell carcinoma, which showed a histological fusion of adenocarcinoma that involves the right hepatic duct [17]. Our case is the first in the literature to describe an SCBC on the proximal common bile duct extending to the proximal common hepatic duct with liver metastasis along with histological collision of adenocarcinoma and squamous cell carcinoma. There was one case of SCBC described on the left hepatic duct [18].
Conclusions
Biliary squamous cell carcinoma is a rare entity that continues to carry many unknowns in the field of gastrointestinal oncology. Although the exact prognosis remains unclear, it is known that squamous cell cholangiocarcinoma displays aggressive behavior and tends to precipitate poor outcomes. Diagnosis requires a high degree of clinical suspicion due to a lack of effective screening tools, and pathologic evaluation of a tissue sample remains the gold standard in diagnosis. Therefore, many patients are diagnosed in the advanced stages of the disease. Given the paucity of literature in this field, treatments are determined individually. Further investigation is required in order to establish standard treatment protocols.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
There is no actual or potential conflict.
Informed Consent
Informed patient consent was obtained for publication of the case details.
Author Contributions
Mohamad Khaled Almujarkesh, as the corresponding Author and article guarantor, contributed to the parts of Introduction, Case Report, Discussion, and Conclusions. Anirudh R. Damughatla contributed to the Introduction, Case Report, Discussion, Conclusions, References, and Figures creation. Dana LaBuda contributed to obtaining pathology images and review of manuscript. Samer Alkassis contributed to the parts of Introduction, Case Report, Discussion, and Conclusions. Mohammed Najeeb Al Hallak, as the principal senior author, supervised and guided the entire manuscript writeup.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Cabot RC. Case records of the Massachusetts General Hospital. NEJM. 1930;202:1260-1262.
- Abbas R, Willis J, Kinsella T, Siegel C, Sanabria J. Primary squamous cell carcinoma of the main hepatic bile duct. Can J Surg. 2008;51(4):E85-86.
pubmed pmc - Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc. 2015;90(6):791-800.
doi pubmed pmc - Weimann A, Klempnauer J, Gebel M, Maschek H, Bartels M, Ringe B, Pichlmayr R. Squamous cell carcinoma of the liver originating from a solitary non-parasitic cyst case report and review of the literature. HPB Surg. 1996;10(1):45-49.
doi pubmed pmc - Lim JH. Liver flukes: the malady neglected. Korean J Radiol. 2011;12(3):269-279.
doi pubmed pmc - Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145(3):521-536.
doi pubmed pmc - Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924-933.
doi pubmed - Lowe RC, Afdhal NH, Anderson CD. Epidemiology, pathogenesis, and classification of cholangiocarcinoma. Available from: http://www.uptodate.com/contents/epidemiology-pathogenesis-and-classification-of-cholangiocarcinoma.
- Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729245.
doi pubmed pmc - Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, Hamilton SR. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102(6):1980-1982.
doi pubmed - Sanabria JR, Croxford R, Berk TC, Cohen Z, Bapat BV, Gallinger S. Familial segregation in the occurrence and severity of periampullary neoplasms in familial adenomatous polyposis. Am J Surg. 1996;171(1):136-140; discussion 140-131.
doi pubmed - O'Boyle MK. Gallbladder wall mass on sonography representing large-cell non-Hodgkin's lymphoma in an AIDS patient. J Ultrasound Med. 1994;13(1):67-68.
doi pubmed - Devos M, Gilbert B, Denecker G, Leurs K, Mc Guire C, Lemeire K, Hochepied T, et al. Elevated DeltaNp63alpha levels facilitate epidermal and biliary oncogenic transformation. J Invest Dermatol. 2017;137(2):494-505.
doi pubmed - Hoshimoto S, Hoshi S, Hishinuma S, Tomikawa M, Shirakawa H, Ozawa I, Wakamatsu S, et al. Adenosquamous carcinoma in the biliary tract: association of the proliferative ability of the squamous component with its proportion and tumor progression. Scand J Gastroenterol. 2017;52(4):425-430.
doi pubmed - Morito K, Kai K, Miyoshi A, Kubo H, Ide T, Azama S, Irie H, et al. Primary squamous cell carcinoma of the liver concomitant with primary colon cancer: report of a case. Clin J Gastroenterol. 2013;6(2):134-138.
doi pubmed - Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5(5):61.
doi pubmed - Tamaoka K, Tanemura M, Furukawa K, Mikamori M, Saito T, Ohtsuka M, Suzuki Y, et al. Primary intrahepatic squamous cell carcinoma with histological collision of adenocarcinoma and squamous cell carcinoma: a case report. Am J Case Rep. 2018;19:1184-1191.
doi pubmed pmc - Lubana SS, Singh N, Seligman B, Tuli SS, Heimann DM. First reported case of primary intrahepatic cholangiocarcinoma with pure squamous cell histology: a case report. Am J Case Rep. 2015;16:438-444.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


