| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 4, August 2023, pages 217-225
Trends in Colorectal Cancer Mortality in the United States, 1999 - 2020
Alexander Kusnika, b, Sarath Lal Mannumbeth Renjithlala, Ari Chodosa, Sanjana Chetana Shanmukhappaa, Mohamed Magdi Eida, Keerthi Mannumbeth Renjitha, Richard Alweisa
aDepartment of Internal Medicine, Unity Hospital, Rochester, NY, USA
bCorresponding Author: Alexander Kusnik, Department of Internal Medicine, Unity Hospital, Rochester, NY 14626, USA
Manuscript submitted April 23, 2023, accepted June 14, 2023, published online July 12, 2023
Short title: CRC Mortality in the United States
doi: https://doi.org/10.14740/gr1631
| Abstract | ▴Top |
Background: The United States faces a significant public health issue with colorectal cancer (CRC), which remains the third leading cause of cancer-related fatalities despite early diagnosis and treatment progress.
Methods: This investigation utilized death certificate data from the Centers for Disease Control and Prevention Wide-Ranging OnLine Data for Epidemiologic Research (CDC WONDER) database to investigate trends in CRC mortality and location of death from 1999 to 2020. Additionally, the study utilized the annual percent change (APC) to estimate the average annual rate of change over the specific time period for the given health outcome. Incorporating the location of death in this study served the purpose of identifying patterns related to CRC and offering valuable insights into the specific locations where deaths occurred.
Results: Between 1999 and 2020, there were 1,166,158 CRC-related deaths. The age-adjusted mortality rates (AAMRs) for CRC consistently declined from 20.7 in 1999 to 12.5 in 2020. Men had higher AAMR (18.8) than women (13.4) throughout the study. Black or African American patients had the highest AAMR (21.1), followed by White (15.4), Hispanic/Latino (11.8), American Indian or Alaska native (11.4), and Asian or Pacific Islanders (10.2). The location of death varied, with 41.99% at home, 28.16% in medical facilities, 16.6% in nursing homes/long-term care facilities, 7.43% in hospices, and 5.80% at other/unknown places.
Conclusion: There has been an overall improvement in AAMR among most ethnic groups, but an increase in AAMR has been observed among white individuals below the age of 55. Notably, over one-quarter of CRC-related deaths occur in medical facilities.
Keywords: Colorectal cancer; Mortality; Population; Black/African American; Hispanic/Latino; White/Caucasian
| Introduction | ▴Top |
Colorectal cancer (CRC) is a significant public health concern in the United States, with an estimated 153,020 new cases and 52,550 deaths in 2023 [1]. Despite early detection and treatment improvements, CRC remains the third leading cause of cancer-related deaths in the United States [1]. Moreover, the recent increase in early-onset colorectal cancer (EO-CRC) [2] and the consequent modification of the recommended screening protocol by the US Preventive Service Task Force (USPSTF) have emphasized the importance of routine screening for individuals aged 45 to 75 years [3]. Given that CRC remains a leading cause of death in the USA, examining the location of death is critical in identifying the necessity of bolstering home/hospice care services and implementing appropriate palliative measures to guarantee optimal end-of-life care.
This descriptive study aimed to examine CRC mortality rates in the USA from 1999 to 2020, specifically focusing on trends by sex, race/ethnicity, and death site. The objective was to analyze CRC mortality trends in diverse population groups to illustrate the incidence of death caused by CRC.
| Materials and Methods | ▴Top |
This descriptive study utilized death certificate data from the Centers for Disease Control and Prevention Wide-Ranging OnLine Data for Epidemiologic Research (CDC WONDER) database to identify deaths associated with CRC between 1999 and 2020. The CDC WONDER dataset includes information on the cause of death recorded on death certificates from all 50 states and the District of Columbia. Previously, this dataset has been utilized to assess patterns in disease mortality [4, 5]. CRC deaths were identified using the International Statistical Classification of Diseases and Related Health Problems-10th Revision (ICD-10) codes C18X (malignant neoplasm of the colon), C19 (malignant neoplasm of the rectosigmoid junction), and C20 (malignant neoplasm of the rectum). The data extracted for the study comprised population size, year, place of death and demographics. Demographic information included sex, age, race/ethnicity, and location of death, encompassing medical facilities (outpatient, emergency room, inpatient, death on arrival, or status unknown), home, hospice, and nursing home/long-term care facility. The classification of race/ethnicity was based on Hispanic and non-Hispanic White, African American, and Asian or Pacific Islanders.
This project was not subject to review by the local institutional review board since it involves the use of a deidentified government-issued public use dataset and adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The age-adjusted rate was calculated by multiplying the age-specific death rate for each age group by the corresponding weight from the specified standard population, summing across all age groups, and then multiplying this result by 100,000.
The precise formula for calculating the age-adjusted rate is as follows:
The age-specific death rate is the number of deaths for a given age group divided by the population of that age group.
The “standard population weight” for an age group is calculated by dividing the population for the age group by the sum of the populations for all of the age groups in the query [6].
The study determined both crude and age-adjusted mortality rates (AAMRs) for CRC by computing the number of deaths per 100,000 individuals. Crude mortality rates were calculated by dividing the CRC-related deaths by the corresponding US population for that year. AAMRs were standardized to the 2000 US population using the same method as previous studies, with 95% confidence intervals (CIs) [7].
Additionally, the study utilized the Joinpoint Regression Program (Joinpoint V 4.9.0.0, National Cancer Institute) to analyze the yearly national trends in CRC-related mortality. Through the use of log-linear regression models that accounted for changes over time, the Joinpoint Regression Program provided the means to calculate the annual percent change (APC) in AAMR, along with 95% CIs. This calculation method enabled the identification of significant changes in AAMR over time.
Annual trends for CRC-related AAMR
In 1999, the AAMR for CRC was 20.7 per 100,000 population (95% CI: 20.6 - 20.9), which decreased to 12.5 (95% CI: 12.4 - 12.7) in 2020. In addition, the overall AAMR from 1999 to 2012 showed a steady APC reduction of -2.75 (95% CI: -2.9 to -2.6), and further reduction was observed from 2012 to 2020 with an APC of -1.86 (95% CI: -2.3 to -1.5) (Fig. 1).
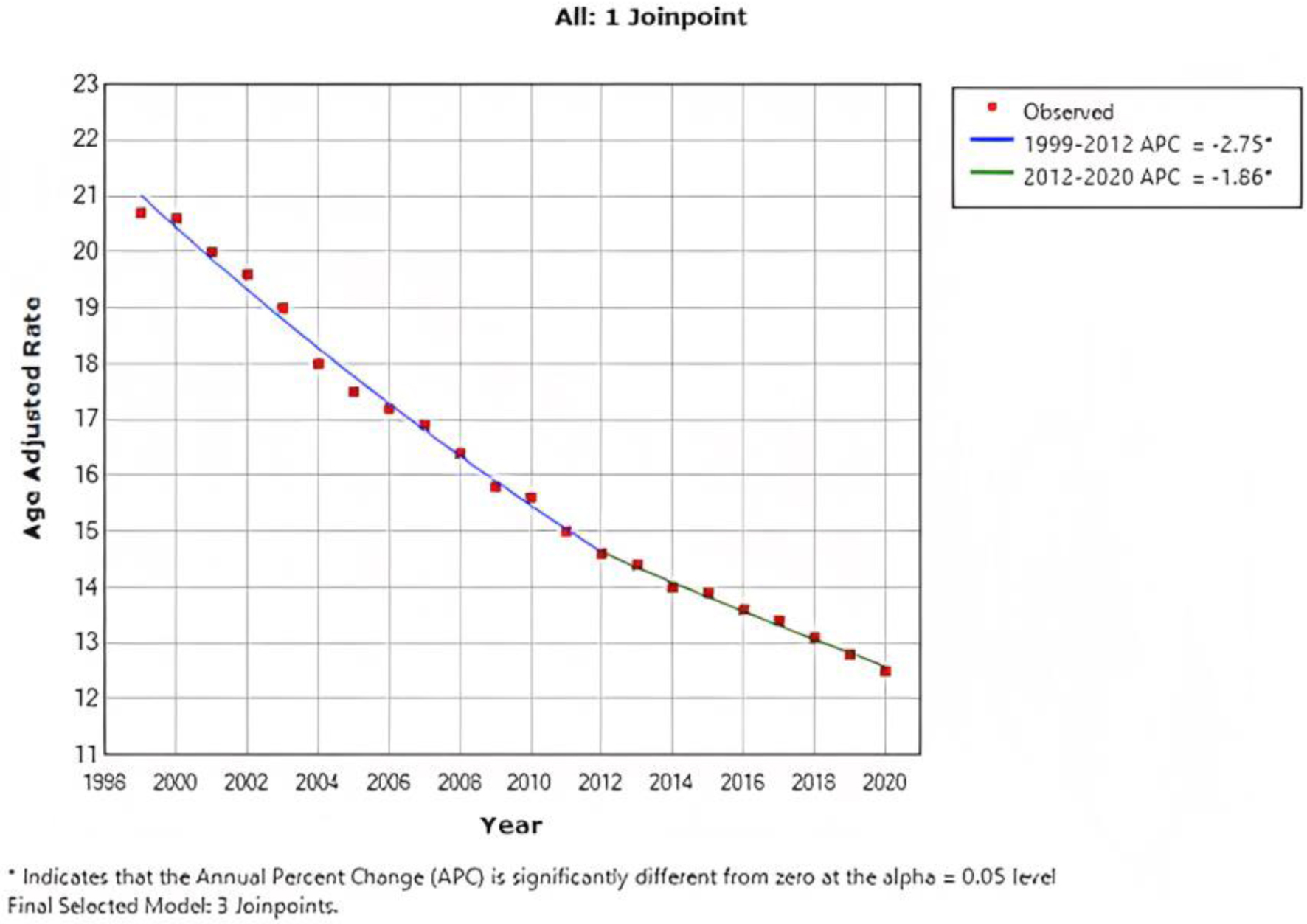 Click for large image | Figure 1. Combined AAMR for both males and females. AAMR is represented on y-axis, while x-axis indicates the years spanning from 1999 to 2020. This graph demonstrates a consistent decrease in the AAMR for CRC from 20.7 per 100,000 population in 1999 to 12.5 per 100,000 population in 2020, indicating a significant improvement in the AAMR for CRC over the specified time period. AAMR: age-adjusted mortality rate; CRC: colorectal cancer. |
CRC-related AAMR stratified by sex
The AAMR for CRC stratified by sex revealed consistently higher rates in men than in women throughout the study period (overall AAMR for men: 18.8 (95% CI: 18.7 - 18.8); women: 13.4 (95% CI: 13.3 - 13.4)). In 1999, the AAMR for men was 25.1 (95% CI: 24.8 - 25.4), which showed a decrease in 2020 to 15.1 (95% CI: 14.9 - 15.3) (APC: -2.6, 95% CI: -2.4 to -2.7) (Fig. 2a). Similarly, the AAMR for women in 1999 was 17.6 (95% CI: 17.4 - 17.8), which steadily declined to 10.4 (95% CI: 10.3 - 10.6) in 2020 (APC: -2.6, 95% CI: -2.5 to -2.7) (Fig. 2b).
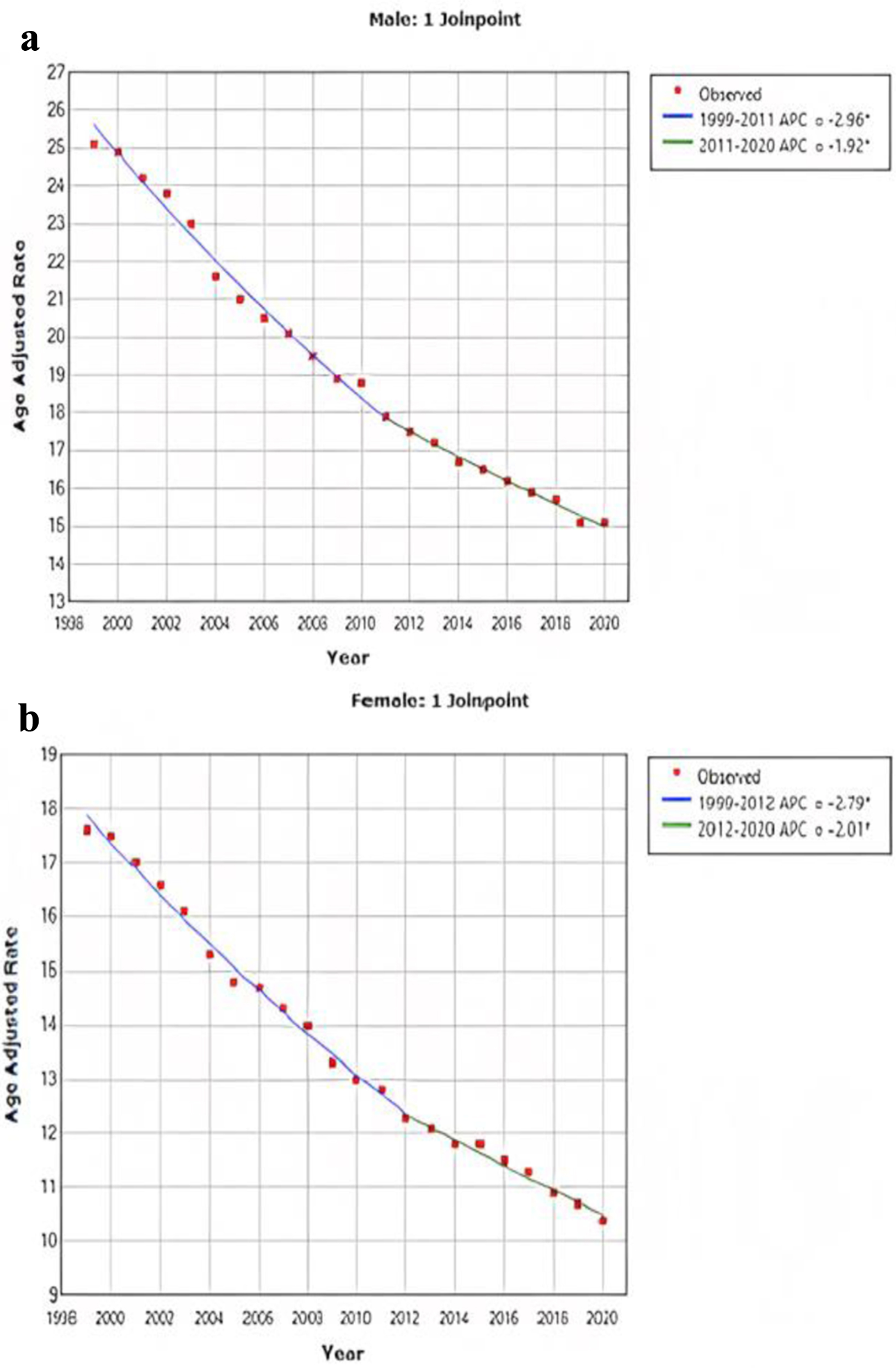 Click for large image | Figure 2. AAMR for CRC is stratified for male (a) and female (b). Y-axis on both graphs represents AAMR, while x-axis represents the years spanning from 1999 to 2020. Both graphs demonstrate a significant improvement in the AAMR. For men, the AAMR decreased from 25.1 to 15.1, and for women, it decreased from 17.6 to 10.4. AAMR: age-adjusted mortality rate; CRC: colorectal cancer. |
CRC-related AAMR stratified by race/ethnicity
When analyzed by race and ethnicity, the highest all-cause AAMR was observed in African American patients, with a rate of 21.1 (95% CI: 21.0 - 21.3), followed by White patients with a rate of 15.4 (95% CI: 15.4 - 15.4), Hispanic/Latino patients with a rate of 11.8 (95% CI: 11.7 - 11.8), American Indian or Alaska Native patients with a rate of 11.4 (95% CI: 11.1 - 11.7), and Asian or Pacific Islander patients with a rate of 10.2 (95% CI: 10.1 - 10.4).
In essence, the AAMR among African American patients decreased from 28.2 (95% CI: 27.5 - 28.9) in 1999 to 16.1 (95% CI: 15.7 - 16.5) in 2020, with a significant average APC of -2.8 (95% CI: -2.9 to -2.7). Similarly, the AAMR among White patients decreased from 20.3 (95% CI: 20.1 - 20.4) in 1999 to 12.4 (95% CI: 12.2 - 12.5) in 2020, with an APC of -2.47 (95% CI: -2.6 to -2.3). American Indian or Alaska Native patients also exhibited a trend of improvement in AAMR, with a rate of 13.4 (95% CI: 11.2 - 15.6) in 1999 and a rate of 9.7 (95% CI: 8.7 - 10.7) in 2020, with an APC of -1.75 (95% CI: -1.8 to -2.3). The AAMR among Asian and Pacific Islander patients decreased from 12 (95% CI: 11.1 - 12.8) in 1999 to 8.7 (95% CI: 8.3 - 9.1) in 2020, with an APC of -1.94 (95% CI: -2.2 to -1.7). A similar trend was observed in the Hispanic population, with a rate of 14.2 (95% CI: 13.6 - 14.9) in 1999 and 10.3 (95% CI: 10.0 - 10.6) in 2020, with an APC of -1.67(95% CI: -1.8 to -1.5) (Fig. 3).
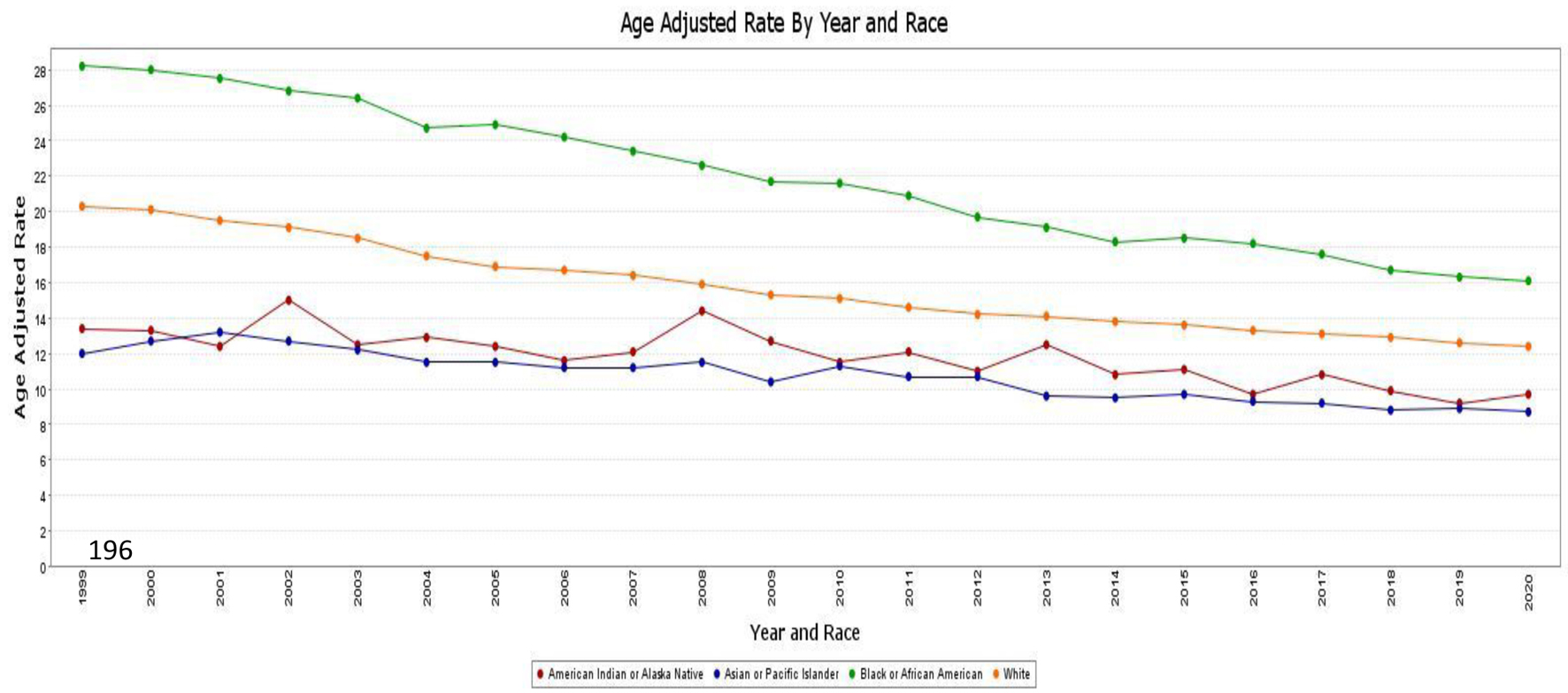 Click for large image | Figure 3. AAMR associated with CRC is stratified by race/ethnicity, with x-axis representing the year and race and y-axis indicating the AAMR. Green line: African American patients (AAMR decreased from 28.2 in 1999 to 16.1 in 2020). Orange line: White patients (AAMR decreased from 20.3 in 1999 to 12.4 in 2020). Red line: American Indian or Alaska native patients (AAMR decreased from 13.4 in 1999 to 9.7 in 2020). Blue line: Asian and Pacific Islander patients (AAMR decreased from 12 in 1999 to 8.7 in 2020). AAMR: age-adjusted mortality rate; CRC: colorectal cancer. |
CRC in younger patients
From 1999 to 2020, patients below the age of 55 accounted for 138,652 (11.8%) of the total deaths attributed to CRC.
Regarding annual trends for CRC AAMR, the AAMR per 100,000 population was 2.6 (95% CI: 2.5 - 2.7) in 1999 and 2.9 (95% CI: 2.8 - 2.9) in 2020. The AAMR from 1999 to 2002 showed an APC increase of 1.20 (95% CI: -0.8 to 3.2), followed by a reduction from 2002 to 2005 with an APC of -2.6 (95% CI: -6.6 to 1.4). From 2005 to 2013, the APC increase was at 1.4 (95% CI: 0.9 - 2.0), and from 2013 to 2020, the APC increase was 0.3 (95% CI: -0.2 to 0.8).
CRC in younger patients stratified by sex and race
In the age group < 55 years, men consistently exhibited higher AAMR than women throughout the study period (overall AAMR for men: 3.1 (95% CI: 3.0 - 3.1); women: 2.3 (95% CI: 2.3 - 2.3)). The AAMR for men decreased from 2.9 (95% CI: 2.8 - 3.0) in 1999 to 2.7 (95% CI: 2.6 - 2.8) in 2005 with an APC of -1.8 (95% CI: -3.4 to -0.2), but steadily increased from 2006 to 3.4 (95% CI: 3.3 - 3.5) in 2020 with an APC of 1.54 (95% CI: 1.2 - 1.9). The AAMR for women increased from 2.3 (95% CI: 2.2 - 2.4) in 1999 to 2.4 (95% CI: 2.3 - 2.5) in 2020 with an APC of 0.3 (95% CI: 0.1 - 0.5).
When stratified by race/ethnicity, Black or African American patients had an AAMR at 3.9 (95% CI: 3.9 - 4.0), followed by White patients at 2.5 (95% CI: 2.5 - 2.5), Hispanic/Latino patients at 1.9 (95% CI: 1.9 - 1.9), American Indian or Alaska native patients at 1.9 (95% CI: 1.8 - 2.1), and Asian or Pacific Islanders patients at 1.9 (95% CI: 1.8 - 1.9). Over a 20-year period, the mortality rate of Black or African American patients in the age group < 55 years improved from 4.2 (95% CI: 3.9 - 4.4) to 3.7 (95% CI: 3.5 - 3.9) with an APC of -0.91 (95% CI: -1.1 to -0.7), while the mortality rate in white patients increased from 2.4 (95% CI: 2.3 - 2.4) to 2.8 (95% CI: 2.7 - 2.8) with an APC of 0.94 (95% CI: 0.7 - 1.2) (Fig. 4).
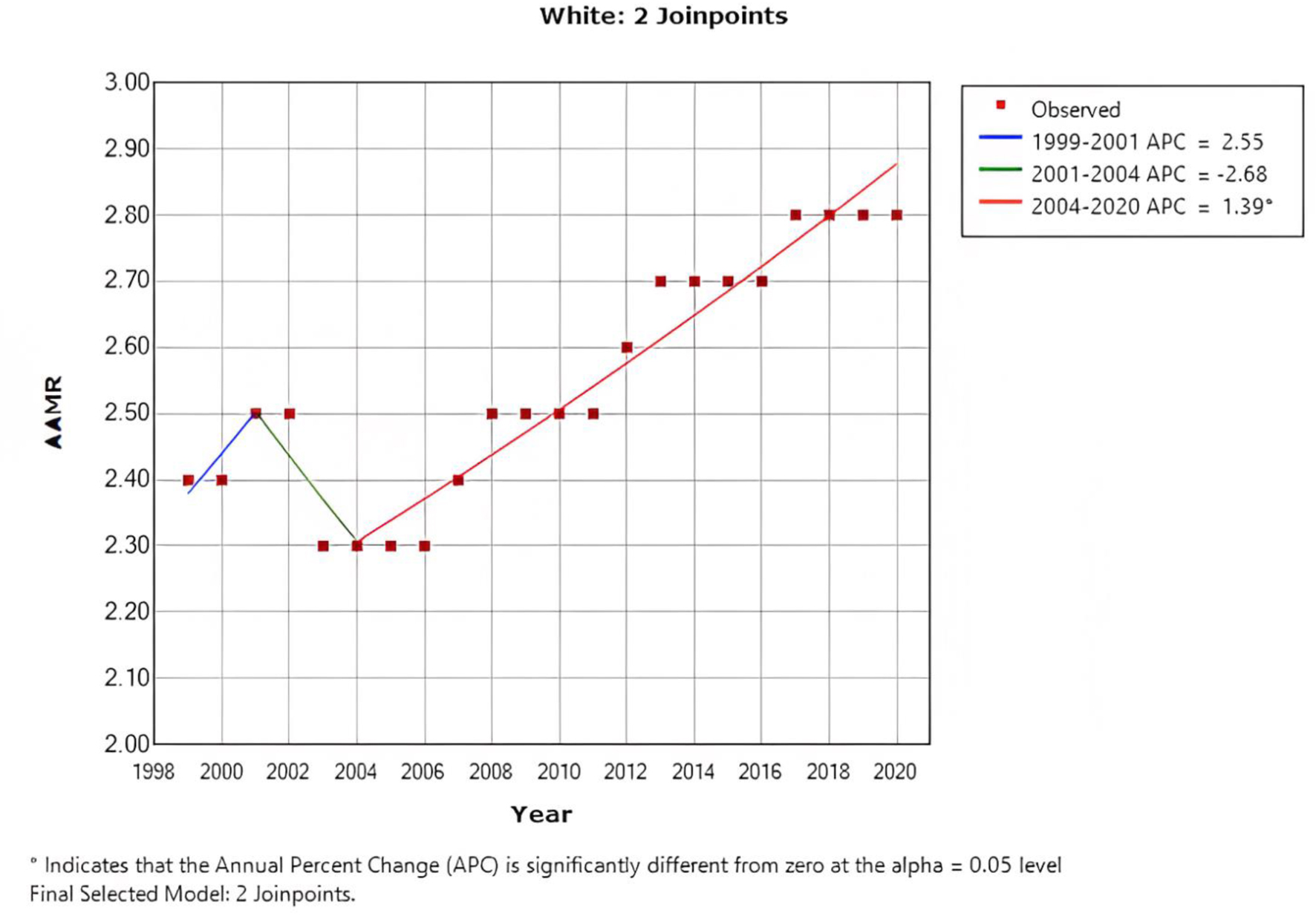 Click for large image | Figure 4. AAMR in white patients below the age of < 55 years, indicating an increase in the mortality rate from 2.4 to 2.8. AAMR: age-adjusted mortality rate. |
| Discussion | ▴Top |
Our investigation has yielded several noteworthy results pertaining to 20-year mortality data sourced from the CDC WONDER database. Firstly, a consistent downward trend in the annual mortality rate of CRC was observed from 2002 to 2012, with a steady reduction in APC of -2.75. This decline persisted until 2020, although with a slightly lower APC of -1.86 between 2012 and 2020. Additionally, it was observed throughout the 20-year period that men exhibited a consistently higher AAMR than women. Secondly, African Americans demonstrated the highest AAMR, followed by White patients, those of Hispanic/Latino origin, American Indian or Alaska native patients, and finally, Asian or Pacific Islanders. Differences in the frequency of risk factors and access to high-quality medical care contribute to varying CRC outcomes among different racial or ethnic groups [8]. The increased incidence of colon cancer among Black men compared to White individuals is thought to be the outcome of a combination of multiple factors, including genetic, lifestyle, socioeconomic, and health disparity factors that interact in a complex manner.
Current data suggest that in the past, there has been a disparity between Black individuals and White individuals in terms of CRC screening rates, although this gap appears to be gradually narrowing [9]. As a result, the recent decrease in CRC cases among African Americans can largely be attributed to an increased adoption of colonoscopic screening within this population [10]. The persistently higher incidence of CRC among African Americans can be attributed to various factors, including a lower likelihood of having awareness about their ancestral cancer history compared to White patients [11], as well as a general reluctance among family members to share information about the identification of colonic polyps with others [12]. Another factor that contributes to this is the increased probability of African American individuals being diagnosed with CRC before the age of 50, in contrast to the overall population. This highlights the inadequacy of the previous screening guidelines, which commenced screening at the age of 50, as it potentially overlooked a considerable portion of the African American population compared to the general population [13, 14].
Several other factors have been recognized to contribute to the risk of CRC within the African American population, including genetic predisposition and phenotypic differences in presentation. As an instance, a study by Mendelsohn et al [15] discovered that advanced adenomas were more prevalent among African Americans, although these findings were not confirmed in similar studies [16, 17]. Furthermore, a historical observation has revealed that African Americans have shown a higher propensity for presenting with advanced metastatic colon cancer upon diagnosis. One possible explanation for this trend is the elevated likelihood of African Americans having proximal CRC, which primarily affects the right side of the colon. It is worth noting that colonoscopy exhibits reduced effectiveness in detecting proximal CRCs in comparison to distal CRCs [17, 18]. These findings align with the research conducted by Lieberman et al [19], which indicates a higher prevalence of advanced polyps in the proximal location among African Americans (53.3%) compared to White Americans (50.6%).
The decline in CRC incidence rate among American Indians has been slower in comparison to African Americans. This can be partially attributed to the lower life expectancy within the American Indian population, which is influenced by the burden of conditions like diabetes mellitus and metabolic syndrome. As a result, there has been relatively less time available for the development of CRC due to the overall lower life expectancy in this population [20, 21].
Historically, Asian Americans and Pacific Islanders have exhibited lower rates of CRC screening in comparison to other groups, such as White individuals or African Americans. It is important to recognize that within the Pacific and Asian Islander categorization, there are distinct racial and ethnic entities. While there may be cultural and geographical intersections in specific regions, these groups are acknowledged as separate entities. Furthermore, they often consist of unique subgroups that have diverse factors influencing the observed variations in AAMRs related to CRC [22].
A study conducted by Lee et al examined the disparities in CRC screening among Asian Americans and Pacific Islanders. The findings indicated that the overall CRC screening rate for this population was 46.8%, which was lower compared to the rate among White individuals (57.7%). However, significant differences were observed when further analyzing the various existing subgroups. For instance, Koreans exhibited the lowest CRC screening rate at 32.7%, while Japanese had the highest rate at 59.8%. These trends within different subpopulations may potentially mask worsening screening rates among smaller subgroups, as improvements in screening rates occur in other larger groups [23].
Considering that each subgroup within the Asian American and Pacific Islander population has variations in lifestyles, health beliefs and behaviors, as well as socioeconomic statuses, further research is necessary to analyze and address the heterogenous disease patterns within each subgroup individually [24, 25].
The US Multi-Society Task Force on Colorectal Cancer recommended in 2008 that individuals of average risk should begin screening for CRC at age 50 [26]. However, other organizations, such as the American College of Gastroenterology in 2009 and the American College of Physicians in 2012, had already suggested earlier colon cancer screening in African Americans aged 45 and 40, respectively [27, 28]. More recently, the USPSTF updated their guidelines for CRC screening in May 2021, recommending that individuals of average risk should start screening at 45 instead of 50 [29]. The studies mentioned earlier, as well as the collaborative efforts, have led to a remarkable improvement in the AAMR of colon cancer in most patients’ groups, particularly in African American patients, with a decrease in AAMR from 28.2 (95% CI: 27.5 - 28.9) to 16.1 (95% CI: 15.7 - 16.5) in 2020 within the last two decades. Additionally, advancements in treatment modalities such as surgery, chemotherapy, radiation, and immunotherapy have improved survival rates and decreased morbidity [30-32]. Also, there are several other contributing factors to the continued decline in the AAMR of CRC. These factors may include advancements in screening techniques, such as fecal occult blood tests, computed tomographic colonography, sigmoidoscopy, and colonoscopy [33].
Women consistently had a lower AAMR during the study period than men. While the underlying factors contributing to this difference are likely multifaceted and diverse, estrogen is thought to be a contributing factor in lowering the risk of CRC in women. However, the precise mechanism by which estrogen confers this protection remains to be elucidated [34].
Thirdly, we analyzed the trends for CRC in patients below the age of 55 between 1999 and 2020. EO-CRC is the term used for cases diagnosed before the age of 50 [35]. Despite the apparent improvement in CRC trends in patients aged 50 and above, there has been a continual rise in the incidence of EO-CRC in recent years which is likely going to continue for the next decade [36]. In general, men had higher AAMRs than women in the age group under 55 years, with an overall AAMR of 3.1 (95% CI: 3.0 - 3.1) for men and 2.3 (95% CI: 2.3 - 2.3) for women. From our data, it can be observed that the mortality rate of African American patients in the age group of less than 55 years improved from 4.2 (95% CI: 3.9 - 4.4) to 3.7 (95% CI: 3.5 - 3.9) over a 20-year period. The estimated APC was -0.91 (95% CI: -1.1 to -0.7), indicating a decrease in mortality rates during the study period. Conversely, the mortality rate in White patients increased from 2.4 (95% CI: 2.3 - 2.4) to 2.8 (95% CI: 2.7 - 2.8) over the same 20-year period, with an estimated APC of 0.94 (95% CI: 0.7 - 1.2). Our study corroborates the results of previous research, which suggest a particular increase in EO-CRC, especially in White individuals, likely related to a rise in rectal cancer [37, 38].
Other factors contributing to the increase in CRC among White individuals are multifaceted and not fully comprehended, and research has indicated that genetic mutations in DNA mismatch repair (MMR) genes may play a role in the development of certain types of CRCs [39]. Furthermore, it is important to consider that other factors may be contributing to the observed trends in CRC mortality. For instance, research has suggested that type II diabetes mellitus [40] and childhood obesity [41] are prevalent among various racial and ethnic groups, particularly with a recent increase within the white population, which could be exacerbating the current situation [42]. In general, an increase in EO-CRC has been documented in multiple Western countries, such as Australia, Slovakia, Canada, and New Zealand. The steepest rise in EO-CRC was observed in South Korea [35, 42]. Additionally, this trend has been noted among various ethnic groups, particularly among Hispanic/Latinos [43]. However, our assessment using the CDC WONDER database did not reveal a clear visualization of this trend in this subgroup. Overall, the underlying mechanisms responsible for the development of EO-CRC are not entirely comprehended.
Finally, between 1999 and 2020, a total of 1,166,158 deaths related to CRC were reported. Out of these cases, the majority, 489,693 (41.99%), occurred at individuals’ residences, while 328,447 (28.16%) took place within medical facilities. Additionally, 193,608 (16.6%) deaths were recorded in nursing homes or long-term care facilities, 86,722 (7.43%) occurred in hospices, and 67,688 (5.80%) deaths were classified as occurring in other or unknown locations.
Limited information is available regarding the specific location of death associated with CRC. Only one study, conducted by Jones et al in the United Kingdom in 2006 [44], explored the care pathway and place of death among CRC patients, taking into account hospitalization duration. The study involved 671 patients categorized into three groups: those who underwent tumor resection, non-resectional surgery for defunctioning or bypassing the tumor, and a non-surgical group. The findings suggested that the majority of CRC-related deaths occurred within hospices, although statistical significance was not observed. Furthermore, individuals who underwent surgical resection demonstrated an extended period of time between their diagnosis and death, during which they were primarily hospitalized. Moreover, this group exhibited a greater probability of experiencing mortality within a hospital setting when compared to the other treatment groups. However, it is challenging to directly apply the results from this study to our data due to advancements in surgical treatments, chemotherapy, and overall different healthcare system. Additionally, our study lacks comprehensive information regarding the involvement of palliative care in the home setting.
This topic is highly relevant as the landscape of CRC is changing, with an increasing number of younger patients being diagnosed before the age of 50. Further research is needed to identify patients who are at risk of mortality and to effectively categorize them in order to ensure that their preferred end-of-life care is appropriately provided if needed. This is particularly important as the provision of end-of-life care, which has traditionally been preferred at home for individuals with cancer, may require a more nuanced approach [45, 46].
Limitations
To begin with, the use of ICD codes and death certificates may lead to an under-representation of CRC as a cause of death. Additionally, the dataset relies on information reported by healthcare providers, which may be subject to errors or inaccuracies, which is particularly pertinent with regard to essential variables such as race and ethnicity.
Furthermore, the lack of detailed information on individual risk factors or potential confounders that may influence the development or progression of CRC may also restrict the capacity to explore complex relationships between variables comprehensively. According to our data, there was an uptick in AAMR among White individuals under the age of 55. Even though EO-CRC typically refers to cases diagnosed before the age of 50, our findings showed a notable increase and were included in the manuscript, despite not meeting the full criteria for EO-CRC. We acknowledge the challenges associated with grouping different populations, such as the Pacific and Asian Islander categorization, as distinct subgroups within this classification can exhibit varying outcomes in mortality rates. However, it is important to note that this analysis may not fully capture these variations due to the grouping of these subgroups.
Conclusion
From 1999 to 2020, the AAMR for CRC showed a steady decrease from 20.7 to 12.5. Men consistently had a higher AAMR than women, and Black or African American patients had the highest AAMR during the study period. Although most subgroups experienced a decline in AAMR, there was a notable increase among White individuals under the age of 55, with mortality rates rising from 2.4 (95% CI: 2.3 - 2.4) to 2.8 (95% CI: 2.7 - 2.8) over a 20-year span and an estimated APC of 0.94 (95% CI: 0.7 - 1.2). Additionally, the location of death varied, with 41.99% of deaths occurring at home, 28.16% within medical facilities, and 16.6% taking place in nursing homes or long-term care facilities, highlighting the diverse settings in which individuals pass away from CRC.
Acknowledgments
We would like to thank the Internal Medicine Department at Rochester Regional Health - Unity Hospital for the significant support and help in completing this project.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Dr. Alweis supervised the entire project including the design and the analysis. Dr. Kusnik and Dr. Renjithlal designed the study, performed data analysis, and wrote the final manuscript. Dr. Kusnik and Dr. Renjithlal were responsible for interpretation of data for the work. Dr. Chodos, Dr. Shanmukhappa, Dr. Eid and Dr. Renjiith critically revised the manuscript for important intellectual content. Final approval of the version to be published was collected from each author before submission. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The CDC WONDER database is a publicly available online system developed by the CDC in the United States. This database offers a vast collection of data related to public health, which can be used to track disease outbreaks, identify trends, and evaluate the success of public health interventions. The CDC WONDER database is accessible for free, and users can easily access the data via the CDC WONDER website.
| References | ▴Top |
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254.
doi pubmed - Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7(3):262-274.
doi pubmed - Gupta S. Screening for colorectal cancer. Hematol Oncol Clin North Am. 2022;36(3):393-414.
doi pubmed pmc - Yeo YH, He X, Ting PS, Zu J, Almario CV, Spiegel BMR, Ji F. Evaluation of trends in alcohol use disorder-related mortality in the US before and during the COVID-19 pandemic. JAMA Netw Open. 2022;5(5):e2210259.
doi pubmed pmc - Mirzazadeh A, Facente SN, Burk K, Kahn JG, Morris MD, End Hep CS. Hepatitis C mortality trends in San Francisco: can we reach elimination targets? Ann Epidemiol. 2022;65:59-64.
doi pubmed pmc - Available from: https://wonder.cdc.gov/wonder/help/mcd.html#.
- Sidney S, Quesenberry CP, Jr., Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1(5):594-599.
doi pubmed - Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960-2005. Am J Public Health. 2010;100(10):1912-1916.
doi pubmed pmc - Rutter CM, Knudsen AB, Lin JS, Bouskill KE. Black and white differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30(1):3-12.
doi pubmed pmc - Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
doi pubmed - Kupfer SS, McCaffrey S, Kim KE. Racial and gender disparities in hereditary colorectal cancer risk assessment: the role of family history. J Cancer Educ. 2006;21(1 Suppl):S32-S36.
doi pubmed - Murff HJ, Peterson NB, Fowke JH, Hargreaves M, Signorello LB, Dittus RS, Zheng W, et al. Colonoscopy screening in African Americans and Whites with affected first-degree relatives. Arch Intern Med. 2008;168(6):625-631.
doi pubmed pmc - Carethers JM. Should African Americans be screened for colorectal cancer at an earlier age? Nat Clin Pract Gastroenterol Hepatol. 2005;2(8):352-353.
doi pubmed pmc - Carethers JM. Clinical and genetic factors to inform reducing colorectal cancer disparitites in African Americans. Front Oncol. 2018;8:531.
doi pubmed pmc - Mendelsohn RB, Winawer SJ, Jammula A, Mills G, Jordan P, O'Brien MJ, Close GM, et al. Adenoma prevalence in blacks and whites having equal adherence to screening colonoscopy: the national colonoscopy study. Clin Gastroenterol Hepatol. 2017;15(9):1469-1470.
doi pubmed pmc - Friedenberg FK, Singh M, George NS, Sankineni A, Shah S. Prevalence and distribution of adenomas in black Americans undergoing colorectal cancer screening. Dig Dis Sci. 2012;57(2):489-495.
doi pubmed pmc - Schroy PC, 3rd, Coe A, Chen CA, O'Brien MJ, Heeren TC. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159(1):13-20.
doi pubmed pmc - Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
doi pubmed - Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3(8):798-805.
doi pubmed - Gbd Us Health Disparities Collaborators. Life expectancy by county, race, and ethnicity in the USA, 2000-19: a systematic analysis of health disparities. Lancet. 2022;400(10345):25-38.
doi pubmed pmc - Carethers JM. Racial and ethnic disparities in colorectal cancer incidence and mortality. Adv Cancer Res. 2021;151:197-229.
doi pubmed pmc - McCracken M, Olsen M, Chen MS, Jr., Jemal A, Thun M, Cokkinides V, Deapen D, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57(4):190-205.
doi pubmed - Lee HY, Lundquist M, Ju E, Luo X, Townsend A. Colorectal cancer screening disparities in Asian Americans and Pacific Islanders: which groups are most vulnerable? Ethn Health. 2011;16(6):501-518.
doi pubmed - Gu M, Thapa S. Colorectal cancer in the United States and a review of its heterogeneity among Asian American subgroups. Asia Pac J Clin Oncol. 2020;16(4):193-200.
doi pubmed - Choe JH, Koepsell TD, Heagerty PJ, Taylor VM. Colorectal cancer among Asians and Pacific Islanders in the U.S.: survival disadvantage for the foreign-born. Cancer Detect Prev. 2005;29(4):361-368.
doi pubmed - Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595.
doi pubmed - Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, American College of G. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104(3):739-750.
doi pubmed - Qaseem A, Denberg TD, Hopkins RH, Jr., Humphrey LL, Levine J, Sweet DE, Shekelle P, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156(5):378-386.
doi pubmed - Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. U.S. Preventive services task force evidence syntheses, formerly systematic evidence reviews. Screening for colorectal cancer: an evidence update for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021.
- Murphy CC, Harlan LC, Lund JL, Lynch CF, Geiger AM. Patterns of colorectal cancer care in the United States: 1990-2010. J Natl Cancer Inst. 2015;107(10):djv198.
doi pubmed pmc - Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409-436.
doi pubmed - Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, Poston G, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015.
doi pubmed pmc - Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687-696.
doi pubmed pmc - Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, Ho GY, et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst. 2015;107(10):djv210.
doi pubmed pmc - Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179-2185.
doi pubmed - Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158(2):341-353.
doi pubmed pmc - Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Lund JL, Sandler RS. Patterns of sociodemographic and clinicopathologic characteristics of stages II and III colorectal cancer patients by age: examining potential mechanisms of young-onset disease. J Cancer Epidemiol. 2017;2017:4024580.
doi pubmed pmc - Murphy CC, Wallace K, Sandler RS, Baron JA. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology. 2019;156(4):958-965.
doi pubmed pmc - Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, Bacher J, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(4):464-471.
doi pubmed pmc - Koopman RJ, Mainous AG, 3rd, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med. 2005;3(1):60-63.
doi pubmed pmc - Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292-2299.
doi pubmed pmc - Muller C, Ihionkhan E, Stoffel EM, Kupfer SS. Disparities in early-onset colorectal cancer. Cells. 2021;10(5):1018.
doi pubmed pmc - Rahman R, Schmaltz C, Jackson CS, Simoes EJ, Jackson-Thompson J, Ibdah JA. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med. 2015;4(12):1863-1870.
doi pubmed pmc - Jones OM, John SK, Horseman N, Lawrance RJ, Fozard JB. Cause and place of death in patients dying with colorectal cancer. Colorectal Dis. 2007;9(3):253-257.
doi pubmed - Tay RY, Choo RWK, Ong WY, Hum AYM. Predictors of the final place of care of patients with advanced cancer receiving integrated home-based palliative care: a retrospective cohort study. BMC Palliat Care. 2021;20(1):164.
doi pubmed pmc - Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3(3):287-300.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


