| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 16, Number 1, February 2023, pages 50-55
Ischemic Reperfusion Injury After Liver Transplantation: Is There a Place for Conservative Management?
Aiman Obeda, Saqr Alsakarnehb, Mohammad Abuassic, Abdalla Bashird, Bashar Ali Ahmadd, Anwar Jarrade, Thomas Lorff, Mohammad Almeqdadig, h, i
aDepartment of Hepatobiliary and Transplant Surgery, Jordan Hospital, Amman, Jordan
bDepartment of Internal Medicine, University of Missouri - Kansas City, Kansas City, MO, USA
cDepartment of Internal Medicine, Jordan Hospital, Amman, Jordan
dDepartment of General Surgery, Jordan Hospital, Amman, Jordan
eDepartment of Hepatology, Gastroenterology and Hepatobiliary, Jordan Hospital, Amman, Jordan
fDepartment of Surgery, University of Gottingen, Gottingen, Germany
gDepartment of Transplant and Hepatobiliary Surgery, Lahey Hospital and Medical Center, Burlington, MA, USA
hDepartment of Surgery, Tufts University School of Medicine, Boston MA, USA
iCorresponding Author: Mohammad Almeqdadi, Department of Transplant and Hepatobiliary Surgery, Lahey Hospital and Medical Center, Burlington, MA, USA
Manuscript submitted October 20, 2022, accepted December 15, 2022, published online February 28, 2023
Short title: IRI After Liver Transplantation
doi: https://doi.org/10.14740/gr1584
| Abstract | ▴Top |
Ischemic reperfusion injury (IRI) after liver transplantation is a common cause of early allograft dysfunction with high mortality. The purpose of this case report series is to highlight an unusual clinical course in which complete recovery can occur following the identification of severe hepatic IRI post-transplantation and the implications of this finding on management strategies in patients with IRI post-transplant. Here, we include three cases of severe IRI following liver transplantation that are putatively resolved without retransplantation or definitive therapeutic intervention. All patients recovered until their final follow-up visits to our institution and developed no significant complications from their injury throughout the course of patient care by our institution after discharge from the hospital.
Keywords: Liver transplantation; Ischemic reperfusion; Liver injury; Post-transplant injury; Immunosuppression
| Introduction | ▴Top |
End-stage liver disease is a growing cause of mortality worldwide [1], and liver transplantation is the best practice in patients with end-stage liver disease and those with hepatic tumors [2]. However, there is a shortage of grafts, and around 15% of transplant candidates die while awaiting liver transplants [3]. As a result, this increased the use of extended criteria donor organs from older or non-heart-beating donors and organs that have been stored for prolonged periods in warm and cold temperatures. Moreover, the damage caused by procurement, preservation, and surgery makes these organs more prone to ischemic reperfusion injury (IRI).
IRI is a major deadly complication affecting the outcome of liver transplantation. IRI causes up to 10% of early organ failure, and that can lead to a higher incidence of both acute and chronic rejection [4, 5]. Indeed, IRI increases the donor organ shortage and could lead to poor early graft function and primary nonfunction, which necessitate retransplantation [6]. Especially marginal livers have a high susceptibility for IRI that often leads to retransplantation, contributing to the acute shortage of livers available for transplantation [7, 8].
The pathophysiology of hepatic IRI post-transplant is poorly understood, and many factors, such as cellular immune activation, cytoprotective functions, and immune regulation, are critically involved [9]. IRI has a complex mechanism that involves the endogenous production of reactive oxygen species, inflammation, and apoptosis. The inflammatory response first results in microvascular changes followed by immune activation, then necrosis through apoptotic pathways. This process will be further complicated if endotoxin or other toxins are involved. Epidemiological studies show that early allograft dysfunction following liver transplantation is associated with significant mortality [10].
Clinically, IRI is usually characterized by significant elevation of liver enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) combined with impaired liver function, which can be best quantified by observation of coagulation parameters such as factor V and thromboplastin time (international normalized ratio (INR)). Clinically, the manifestations of severe IRI are often indistinguishable from other causes, such as primary graft nonfunction and acute rejection. Therefore, a rapid and accurate diagnosis is vital to decide early on what clinical strategy should be used. Whenever severe IRI is suspected, immediate retransplantation may be required. However, this strategy may not be the most appropriate since it might lead to the rationing of organs and the potential loss of patients who could benefit from a period of conservative management. Here, we present three cases of severe IRI following liver transplantation with an unexpected clinical course. We discuss these cases’ clinical course, management, and outcomes (Table 1).
 Click to view | Table 1. Comparison Between the Course, Management, and Outcomes of Three Cases of IRI After Liver Transplantation |
| Case Reports | ▴Top |
Case 1
A 32-year-old woman with a past medical history of cirrhosis and biopsy-proven autoimmune hepatitis (AIH) type 1 presented with altered mental status and lower extremities edema. On examination, she had ascites, bilateral lower extremity edema, and severe asterixis. Initial labs showed acute kidney injury with blood urea nitrogen (BUN) at 89 mg/dL and creatinine at 3.8 mg/dL. Additionally, her INR was 4.0, albumin 2.3 g/dL, and elevated liver enzymes of AST at 448 IU/L and ALT at 576 IU/L. Moreover, the titer for antinuclear antibody (ANA) was positive at 1:320. She was diagnosed with acute liver failure complicated by severe hepatic encephalopathy and acute kidney injury. This was the third time in 6 months that the patient presented with decompensation of her liver cirrhosis. Given the progressive nature of her disease, her worsening liver function, and her Model for End-stage Liver Disease (MELD) score of 34, she was listed for liver transplantation. She was included in the extracorporeal liver support program with molecular absorbent recirculating system (MARS) as a bridging therapy. Three weeks later, she received a deceased-donor liver transplant.
The donor was a 44-year-old man with normal liver function tests who expired due to cerebral hemorrhage following lysis therapy due to thrombosis of the basilar vein. Cold ischemia time was 11 h 22 min, and warm ischemia time was 32 min. Both donor and recipient were cytomegalovirus (CMV) IgG seropositive. Liver transplantation was performed in standard technique with the replacement of retrohepatic vena cava. No venovenous bypass was used intraoperatively, and there was no need for blood transfusions. Intraoperatively, the patient received 1,000 mg of methylprednisolone immediately after reperfusion, and the same dose was continued for the next 2 days.
Postoperative immunosuppression, a tacrolimus-sparing regimen was used in the setting of her worsening acute kidney injury. The first laboratory study 2 h after reperfusion in intensive care unit (ICU) revealed elevated transaminase with AST at 1,309 U/L, ALT at 663 U/L, lactate dehydrogenase (LDH) 2,947 U/L, bilirubin of 3.06 mg/dL and INR 2.56. Creatinine was normal at 0.8 mg/dL with 6,000 mL urine production on the first day under continuous infusion of 2.5 mg/h furosemide. The patient was extubated 5 h after the operation and has been awake since then in regular neurologic status.
About 24 h after liver transplantation, serum biochemistry findings increased to AST of 13,160 U/L, ALT of 7,580 U/L, LDH of 14,760 U/L, and total bilirubin of 6.13 mg/dL. Coagulation had diminished to INR 3.37, factor V had dropped to 12%, whereas renal parameters persisted at normal levels. The patient’s neurologic status and cardio-pulmonary condition were still inconspicuous. Later during the day, anti-thrombin III declined to 52%, and 500 IU were replaced. Duplex sonography was performed to exclude an occlusion of the portal vein or hepatic artery, which revealed normal flow in both vessels, including the retrohepatic vena cava.
Concerning the overall clinical findings described above, we decided to take a wait-and-see approach instead of considering retransplantation for severe IRI. Nevertheless, we performed close scheme laboratory studies, including liver function test, complete metabolic panel, LDH, INR, and lipid and coagulation profile to respond to changes in the patient’s condition. Transaminases and LDH declined within 24 h after the maximum amount reached values of LDH 565 U/L, AST 4,602 U/L, and ALT 2,584 U/L. The coagulation status stabilized with an INR of 2.66.
On postoperative day 7, bilirubin increased to 13.7 g/dL. acute cellular rejection (ACR) was suspected. A liver biopsy revealed parenchymal neutrophilic infiltration, hepatocellular necrosis, and apoptotic cells. The differential diagnosis at that time was ACR versus IRI. Therefore, empiric treatment was given with methylprednisolone 500 mg intravenously for 3 days. Serological parameters kept improving until the patient’s discharge on day 18 after transplantation. Given the rapid recovery without the need for long-term immunosuppression, we believe the diagnosis was most likely to be severe IRI. Figure 1 shows the trend of ALT postoperatively.
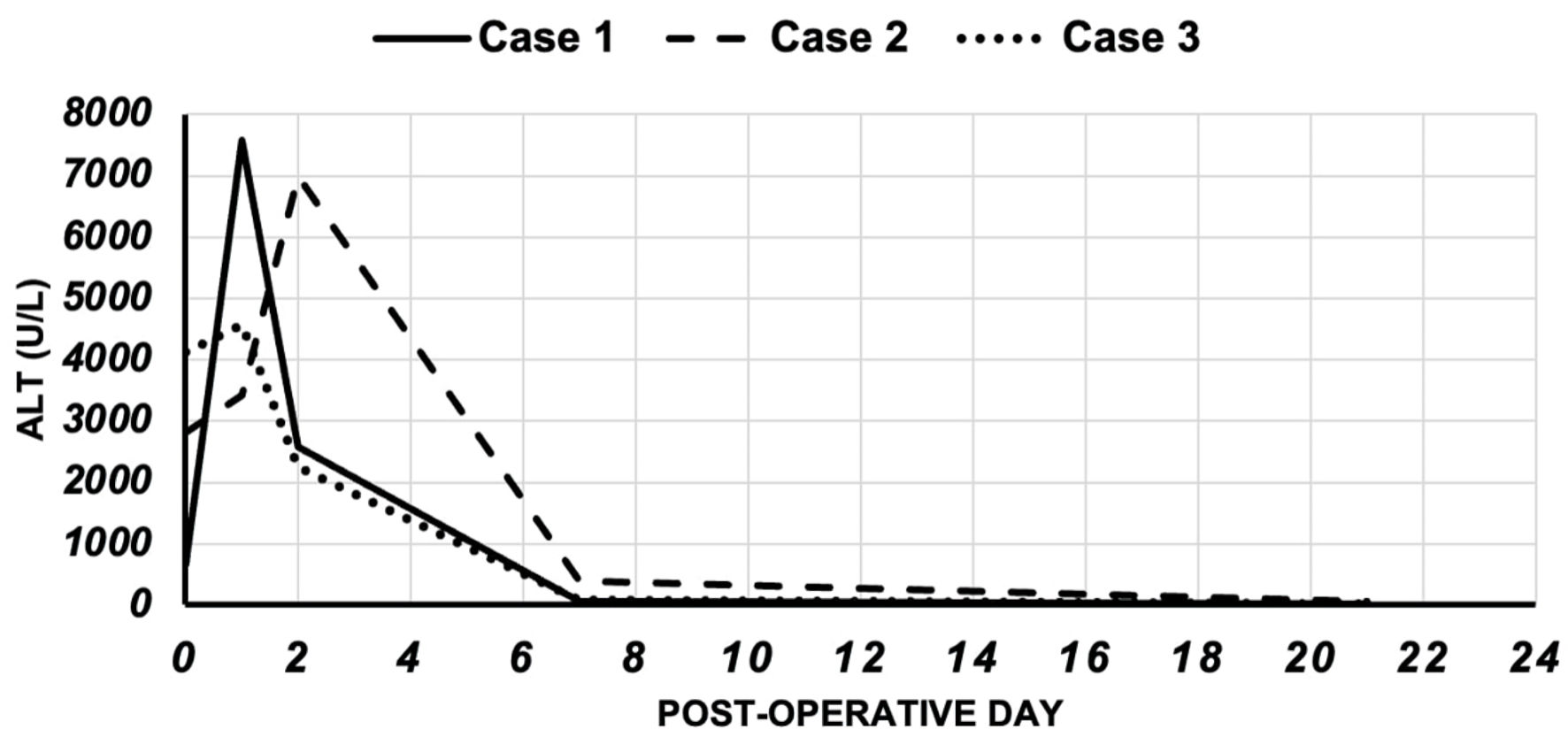 Click for large image | Figure 1. The trend of ALT in the cases. ALT: alanine aminotransferase. |
Case 2
A 57-year-old woman with a past medical history of alcoholic-associated cirrhosis and no history of hepatitis B, C, or metabolic liver diseases presented with altered mentation, drowsiness, and asterixis. On physical exam, her vitals were within normal limits, and the exam was pertinent for a disoriented patient with asterixis, consistent with hepatic encephalopathy. Alcohol levels were 0.0%. Labs showed creatinine of 3.2 mg/dL, INR of 3.3, AST of 320 IU/L, ALT of 378 IU/L, alkaline phosphatase (ALP) of 65, and total bilirubin of 2.5 mg/dL. Her liver biopsy showed cirrhosis without malignancy, confirming the alcoholic-associated cirrhosis diagnosis. Soon after admission, her mental status deteriorated, and she was mechanically ventilated. Additionally, her INR worsened from 2.1 on access to 4.3. Therefore, for emergency liver transplantation, she was included in the extracorporeal liver support program with MARS.
The donor was a 59-year-old female with an anoxic brain injury resulting in brain death. Cold and warm ischemia time was 8 h and 47 min, respectively. Liver transplantation was performed in standard technique with retrohepatic vena cava replacement. No venovenous bypass was used intraoperatively, and there was no need for blood transfusions. The graft was reperfused well, and no biopsies were taken. Intravenous methylprednisolone (500 mg) was administered intraoperative, and postoperatively the patient received induction with tacrolimus (2 mg) twice daily.
The first laboratory study 2 h after reperfusion in ICU revealed elevated transaminase with AST of 3,365 U/L, ALT of 2,808 U/L, LDH of 1,836 U/L, total bilirubin of 2.1 mg/dL, creatinine of 0.93 mg/dL, and an INR of 1.1. The patient was extubated 12 h after the operation and has been awake since then in regular neurologic status.
Ultrasonography with Doppler revealed a patent hepatic artery and portal vein, as well as patent hepatic veins. Her clinical status improved, and our clinical approach was only observation and repeated investigation.
Two days after the operation, serum AST surged to 10,160 U/L, serum ALT 9,669 U/L, and ammonia was elevated at 115 µg/dL. A repeated liver function test 1 week after the operation showed AST 115 U/L and ALT 579 U/L and kept improving after that. However, the renal function test was normal, and the neurological status was unremarkable. The patient was managed conservatively for severe IRI.
Three weeks after the operation, liver function normalized with serum AST of 42 U/L, serum ALT of 47 U/L, and INR 1.0. Figure 1 shows the trend of ALT postoperatively.
Case 3
The patient is a 43-year-old male with a history of cryptogenic cirrhosis who underwent a deceased-donor liver transplantation. The donor was a 42-year-old male who suffered an intracranial injury following a motor vehicle collision. During procurement, the liver was found well perfused with no focal injuries and macroscopic evidence of steatosis.
The recipient underwent uncomplicated conventional liver transplantation without using a venovenous bypass. There were no periods of hypoxia or severe hypotension during transplantation. The cold ischemic time of the graft was 9 h and 40 min, while the warm ischemic time was 42 min. The graft was reperfused well, and no biopsies were taken. Intravenous methylprednisolone (500 mg) was administered intraoperatively, and postoperatively the patient received induction with tacrolimus (3 mg).
The first laboratory study 2 h after reperfusion in ICU revealed elevated transaminase with AST 17,778 U/L, ALT 4,113 U/L, LDH 6,204 U/L, bilirubin 4.6 mg/dL, and INR 1.3. Creatinine was normal at 0.63 mg/dL with 750 mL urine production on the first day under continuous infusion of 2 mg/h furosemide. The patient was extubated 24 h after the operation and has been awake since then in regular neurologic status.
Ultrasonography with Doppler revealed a patent hepatic artery and portal vein, as well as patent hepatic veins. His clinical status improved, and our clinical approach was only observation and repeated investigation.
One day after the transplantation, serum AST surged to 20,248 U/L, serum ALT 4,570 U/L, and ammonia was elevated at 185 µg/dL. One week later, a repeated liver function test showed AST 58 U/L and ALT 91 U/L and kept improving after that. However, the renal function test was normal, and the neurological status was unremarkable. The patient was managed conservatively for severe IRI.
On follow-up, 3 weeks later, liver function normalized with serum AST of 15 U/L, serum ALT of 9 U/L, and INR 1.0. Figure 1 shows the trend of ALT postoperatively.
Table 2 and Figure 1 compare the trend of both AST and ALT for 21 days post-transplant. All three patients were followed for at least 5 years after performing the liver transplantation without any complications in relation to the early-occurred reperfusion injury.
 Click to view | Table 2. The Course of AST and ALT for 21 Days After Liver Transplantation |
| Discussion | ▴Top |
Liver graft failure following liver transplantation is often caused by IRI (Fig. 2) [11, 12]. Several strategies, like optimizing the preservation solution, minimizing ischemia time, and avoiding grafts with severe steatosis, have been developed to diminish this injury [8, 13]. Furthermore, a better understanding of the underlying molecular mechanisms of IRI led to the development of chemical strategies for its prevention. Various methods have been used so far to manage IRI [14]. These include the noninvasive and more advanced approaches encompassing several types of liver conditioning and machine perfusion [15, 16].
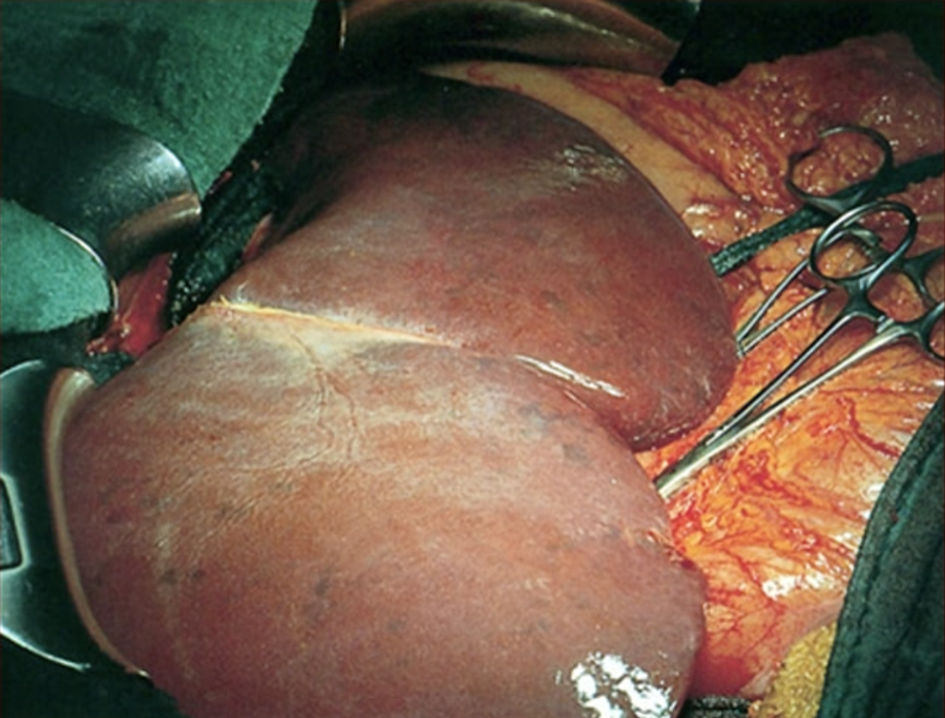 Click for large image | Figure 2. Liver during reperfusion phase of liver transplantation. Blue spots on liver surface indicate areas with impaired perfusion. |
Animal experiments with glutathione (GSH) as antioxidant therapy and atrial natriuretic peptide (ANP) to reduce reactive oxygen species (ROS)-related injury showed promising results [17, 18]. However, these therapies failed to improve the overall clinical outcome of liver transplantation. Other strategies to shorten ischemic times and improve organ allocation pathways, like ischemic preconditioning, are currently under clinical investigation [19, 20].
One important differential diagnosis of IRI is primary hepatic nonfunction, which in most cases requires consideration for retransplantation. This is important as we show that even in severe IRI, the resolution does not necessitate retransplantation and only requires supportive measures. Given the lack of reliable biomarkers, it is difficult to truly differentiate between primary nonfunction and graft injury secondary to IRI. As far as we know, beyond clinical judgment, there are no established algorithms for this decision in the reported literature. Further research is necessary to establish reliable biomarkers, predictors, or algorithms for differentiating primary nonfunction and IRI.
Upon diagnosis, the outcome of IRI after liver transplantation is usually poor without retransplantation. Furthermore, the available strategies to avoid and treat IRI, despite constantly improving, are still lacking a gold standard method [21]. Interestingly, these three cases described in this article serve as an example of an unusual course of IRI following liver transplantation, given that all of them improved with supportive measures alone. Although liver function test following liver transplantation is commonly accepted as indicators of post-transplant liver injury, these cases show that the absolute level of transaminases is an insufficient parameter to assess the functional outcome of a dysfunctional graft. Moreover, these cases highlight the importance of taking into account other parameters, such as the clinical picture and radiological findings, before determining the outcome of IRI following a liver transplant. Even in severe IRI following liver transplantation, a more observant strategy is justified in cases with stable hemodynamic status, unimpaired renal function, absence of severe signs of neurologic disturbance, and mild coagulation disorders.
Conclusions
IRI is a major cause of early liver graft failure after transplantation, presenting nonspecific signs and symptoms. An unusual clinical course such as the one described in these cases highlights that a patient who recovers from severe IRI Liver transplant requires close observation before deciding to retransplant the patient in case they could potentially improve without retransplantation. Future studies are necessary to establish a definitive diagnosis by providing reliable biomarkers of IRI and predictors or algorithms for the differentiation between primary nonfunction and IRI.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents were obtained from all patients in the study.
Author Contributions
AO and SA analyzed the cases and wrote the manuscript. M. Abuassi performed the literature review. AB, BAA, AJ, and TL contributed to writing the manuscript. M. Almeqdadi supervised, revised, and contributed to writing the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38(Suppl 1):2-6.
doi pubmed - Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11(9):1773-1784.
doi pubmed - van der Meulen JH, Lewsey JD, Dawwas MF, Copley LP, Uk, Ireland Liver Transplant A. Adult orthotopic liver transplantation in the United Kingdom and Ireland between 1994 and 2005. Transplantation. 2007;84(5):572-579.
doi pubmed - Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49(1):103-107.
doi pubmed - Fellstrom B, Akuyrek LM, Backman U, Larsson E, Melin J, Zezina L. Postischemic reperfusion injury and allograft arteriosclerosis. Transplant Proc. 1998;30(8):4278-4280.
doi pubmed - Ishii D, Matsuno N, Gochi M, Iwata H, Shonaka T, Nishikawa Y, Obara H, et al. Beneficial effects of end-ischemic oxygenated machine perfusion preservation for split-liver transplantation in recovering graft function and reducing ischemia-reperfusion injury. Sci Rep. 2021;11(1):22608.
doi pubmed - Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury—a fresh look. Exp Mol Pathol. 2003;74(2):86-93.
doi pubmed - Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181(2):160-166.
doi pubmed - Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10(2):79-89.
doi pubmed - Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39(5):788-801.
doi pubmed - Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, et al. Malfunction of the liver after transplantation: an analysis of potential risk factors. Transplant Proc. 1993;25(1 Pt 2):1659-1661.
- Henderson JM. Liver transplantation and rejection: an overview. Hepatogastroenterology. 1999;46(Suppl 2):1482-1484.
- Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20(4 Pt 1):829-838.
doi pubmed - Masior L, Grat M. Methods of attenuating ischemia-reperfusion injury in liver transplantation for hepatocellular carcinoma. Int J Mol Sci. 2021;22(15):8229.
doi pubmed - Nishizawa N, Ito Y, Eshima K, Ohkubo H, Kojo K, Inoue T, Raouf J, et al. Inhibition of microsomal prostaglandin E synthase-1 facilitates liver repair after hepatic injury in mice. J Hepatol. 2018;69(1):110-120.
doi pubmed - Choi EK, Jung H, Jeon S, Lim JA, Lee J, Kim H, Hong SW, et al. Role of remote ischemic preconditioning in hepatic ischemic reperfusion injury. Dose Response. 2020;18(3):1559325820946923.
doi pubmed - Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79(2):115-136.
doi pubmed - Gerbes AL, Vollmar AM, Kiemer AK, Bilzer M. The guanylate cyclase-coupled natriuretic peptide receptor: a new target for prevention of cold ischemia-reperfusion damage of the rat liver. Hepatology. 1998;28(5):1309-1317.
doi pubmed - Azoulay D, Del Gaudio M, Andreani P, Ichai P, Sebag M, Adam R, Scatton O, et al. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft's preservation and function: the ying and the yang. Ann Surg. 2005;242(1):133-139.
doi pubmed - Kim D, Choi JW, Han S, Gwak MS, Kim GS, Jeon SY, Ryu S, et al. Ischemic preconditioning protects against hepatic ischemia-reperfusion injury under propofol anesthesia in rats. Transplant Proc. 2020;52(10):2964-2969.
doi pubmed - Gazia C, Lenci I, Manzia TM, Martina M, Tisone G, Angelico R, Abenavoli L, et al. Current strategies to minimize ischemia-reperfusion injury in liver transplantation: a systematic review. Rev Recent Clin Trials. 2021;16(4):372-380.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


