| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 6, December 2022, pages 325-333
Transjugular Intrahepatic Portosystemic Shunt Outcomes in the Elderly Population: A Systematic Review and Meta-Analysis
Zohaib Ahmeda, j, Umer Farooqb, Syeda Faiza Arifc, Muhammad Azizd, Umair Iqbale, Ahmad Nawazf, Wade Lee-Smithg, Joyce Badalh, Asif Mahmooda, Abdallah Kobeissyd, Ali Nawrasd, Mona Hassand, Sammy Saabi
aDepartment of Internal Medicine, University of Toledo, Toledo, OH, USA
bDepartment of Internal Medicine, Loyola Medicine/MacNeal Hospital, Chicago, IL, USA
cDow University of Health Sciences, Karachi, Pakistan
dDivision of Gastroenterology and Hepatology, University of Toledo, Toledo, OH, USA
eDivision of Gastroenterology and Hepatology, Geisinger Medical Center, Danville, PA, USA
fDivision of Internal Medicine, Yale-New Haven Hospital, New Haven, CT, USA
gUniversity of Toledo Libraries, University of Toledo, Toledo, OH, USA
hUniversity of Toledo College of Medicine and Life Sciences, Toledo, OH, USA
iDepartment of Medicine and Surgery at the David Geffen School of Medicine at UCLA (University of California Los Angeles), Los Angeles, CA, USA
jCorresponding Author: Zohaib Ahmed, Department of Internal Medicine, University of Toledo, Toledo, Oh, USA
Manuscript submitted September 29, 2022, accepted November 10, 2022, published online December 1, 2022
Short title: TIPS Outcomes in Elderly Population
doi: https://doi.org/10.14740/gr1571
| Abstract | ▴Top |
Background: Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure typically utilized to treat refractory ascites and variceal bleeding. However, TIPS can lead to significant complications, most commonly hepatic encephalopathy (HE). Advanced age has been described as a risk factor for HE, as the elderly population tends to have decreased cognitive reserve and increased sarcopenia. We conducted a systematic review and meta-analysis of the available literature to summarize the association between advanced age and risk of adverse events after undergoing TIPS.
Methods: A comprehensive search strategy to identify reports of specific outcomes (HE, 30-day and 90-day mortality, and 30-day readmission due to HE) in elderly patients after undergoing TIPS was developed in Embase (Embase.com, Elsevier). We compared outcomes and performed separate data analyses for patients aged < 70 vs. > 70 years and patients aged < 65 vs. > 65 years.
Results: Six studies with a total of 1,591 patients met our inclusion criteria and were included in the final meta-analysis. Three studies divided patients by age < 65 vs. > 65 years, with a total of 816 patients who were 54% male. The remaining three studies divided patients by age < 70 vs. > 70 years, with a total of 775 patients who were 63% male. Results demonstrated a significantly lower risk of post-TIPS HE (risk ratio (RR): 0.42, confidence interval (CI): 0.185 - 0.953, P = 0.03, I2 = 49%), 30-day mortality (RR: 0.37, CI: 0.188 - 0.74, P = 0.005, I2 = 0%), and 90-day mortality (RR: 0.35, CI: 0.24 - 0.49, P = 0.001, I2 = 0%) in patients aged > 70 vs. < 70 years, as well as a trend towards lower risk of 30-day readmission due to HE. There was no significant difference in post-TIPS HE, 30-day or 90-day mortality, or 30-day readmission due to HE between patients aged < 65 vs. > 65 years.
Conclusion: Age > 70 years is associated with significantly higher rates of HE and 30-day and 90-day mortality rates in patients after undergoing TIPS, as well as a trend towards higher 30-day readmission due to HE.
Keywords: Transjugular intrahepatic portosystemic shunt; Variceal bleeding; Refractory ascites; Mortality; Morbidity; Hepatic encephalopathy; Elderly age
| Introduction | ▴Top |
Liver cirrhosis (LC) is a complex, dynamic disease that progresses through various prognostic stages, which are broadly classified as compensated or decompensated [1]. In 2017, LC was the 11th leading cause of mortality in the United States, accounting for 44,478 deaths [2]. Complications including variceal bleeding (VB) and ascites indicate the presence of decompensated cirrhosis [3]. VB is associated with a 30-day mortality of 20% in patients with LC [4].
Hemodynamic stabilization, prophylactic antibiotics, vasoactive medications including vasopressin and somatostatin analogs, and endoscopic therapy are standard treatments for VB [5]. Endoscopic variceal ligation (EVL) and endoscopic sclerotherapy (EST) are typically used after resuscitation [6]. EVL is generally preferred over EST, as the average number of sessions necessary to achieve variceal obliteration in patients undergoing EVL is 3.6 compared to 5.4 in those undergoing EST. Overall, EVL has a lower rate of adverse effects than EST, including esophageal laceration or perforation, transient dysphagia, retrosternal pain, and esophageal strictures [7, 8]. Gastric fundal varices are primarily treated with glue or thrombin injections [9]. Advances in medical and endoscopic treatment have lowered mortality in patients with VB from approximately 50% in the 1980s to 20% in the early 2000s [10]. Medical treatment for ascites includes salt restriction, diuretics, and therapeutic paracentesis [11]. Definitive long-term treatment of refractory ascites and VB usually involves liver transplantation or TIPS creation [12]. Refractory VB refers to active bleeding nonresponsive to pharmacological or endoscopic therapy [13]. Refractory ascites describes ascites that fails to resolve with therapeutic paracentesis combined with sodium restriction and diuretic therapy [14].
TIPS is typically utilized for treatment of refractory VB or refractory ascites [15], and it reduces the portal pressure gradient by more than 50% in most patients. Stent diameter determines the amount decrease in portal pressure [16, 17]. TIPS is a relatively effective, safer, and less invasive option than liver transplant in high-risk patient groups [18-20]. However, TIPS can lead to significant complications, most commonly hepatic encephalopathy (HE). HE develops in 5-35% of patients following TIPS creation [21, 22]. Advanced age has been described as a risk factor for HE, as the elderly population tends to have decreased cognitive reserve and increased sarcopenia [23]. Prior single-center studies have also reported that age is associated with negative outcomes after TIPS, including morbidity, mortality, and overall survival [24-26]. Therefore, we conducted a systematic review and meta-analysis of the available literature to summarize the association between advanced age and risk of adverse events after undergoing TIPS.
| Materials and Methods | ▴Top |
Systematic review
A comprehensive search strategy to identify reports of three specific outcomes (HE, 30- and 90-day mortality, and readmission due to HE) in elderly patients after undergoing TIPS was developed in Embase (Embase.com, Elsevier) by an experienced health sciences librarian (WLS) using truncated keywords, phrases, and subject headings. This strategy was translated to MEDLINE (PubMed platform, NCBI), Cochrane Central Register of Controlled Trials (CochraneLibrary.com, Wiley), and the Web of Science Core Collection (Web of Science platform, Clarivate) with all searches performed from January 1960 till 25 January 2022 (Supplementary Material 1, www.gastrores.org). No publication date or language limits were used. Results were uploaded to the citation management software EndNote 20 (Clarivate, Philadelphia, PA, USA), and duplicates were removed by EndNote algorithms and manual inspection. No human or animal subjects were utilized in this study and meta-analysis. Institutional Review Board approval was not required.
Inclusion/exclusion criteria
We employed the following criteria for study inclusion: 1) patients with a history of liver cirrhosis who underwent TIPS; 2) studies reporting adverse events in patients aged < 65 vs. > 65 and < 70 vs. > 70; and 3) studies reporting outcomes including overall 30-day and 90-day mortality, post-TIPS hepatic encephalopathy, and 30-day all-cause readmission. All available retrospective and prospective studies that reported the above outcomes were included. We excluded all other study designs, including case reports, review articles, case series, and editor letters.
Screening and data collection
Studies were screened by two independent reviewers (ZA and AN). Titles and abstracts were used for the initial screening, then complete texts of pertinent publications were examined. Next, the data were extracted by two reviewers (ZA and AN). Discrepancies in study selection and data extraction were settled by mutual conversation. Finally, data on demographics (age and sex), procedure indications, Model for End-Stage Liver Disease (MELD) and Child-Pugh scores, and outcomes were collected and summarized using Microsoft Excel.
Data synthesis and statistical analysis
The random-effects model and DerSimonian-Laird technique were employed as a priori to pool and compare results due to the presumption of study heterogeneity. The risk ratios (RRs), 95% confidence intervals (CIs), and P-values for binary products were calculated. The I2 statistic from the Cochrane handbook for systematic reviews was used to gauge the degree of study heterogeneity. An I2 of more than 50% was used to define significant heterogeneity. For each measured outcome, a P-value of ≤ 0.05 was deemed statistically significant. The outcomes were calculated using comprehensive meta-analysis software, Open Meta Analyst (CEBM, University of Oxford, Oxford, United Kingdom).
Bias assessment
The risk of bias within each study was determined by the Methodological Index for Non-Randomized Studies (MINORS) for cohort studies [27].
Quality assessment
MINORS was used to assess the quality of the studies (Table 1) [28-33]. Non-comparative studies were graded on eight MINORS criteria, with each item ranging from 0 to 2 (0 if not reported; 1 if reported but inadequate; 2 if reported and adequate), and a global score of 16 for non-comparative studies and 24 for comparative analyses considered ideal. Two authors (ZA and UF) independently completed the quality assessment, and discrepancies were handled by a third reviewer (AN).
 Click to view | Table 1. Quality Assessment of Studies via the MINORS Scale |
| Results | ▴Top |
Six studies with a total of 1,591 patients met our inclusion criteria and were included in the final meta-analysis [28-33]. Three studies divided patients by age < 65 vs. > 65 years, with a total of 816 patients who were 54% male. The remaining three studies divided patients by age < 70 vs. > 70 years, with a total of 775 patients who were 63% male. The PRISMA flow diagram (Fig. 1) elaborates our systematic literature search process. Baseline characteristics, including patient demographics and indications for TIPS, are reported in Tables 2 and 3 [28-33]. Publication bias was not assessed due to the low number of studies.
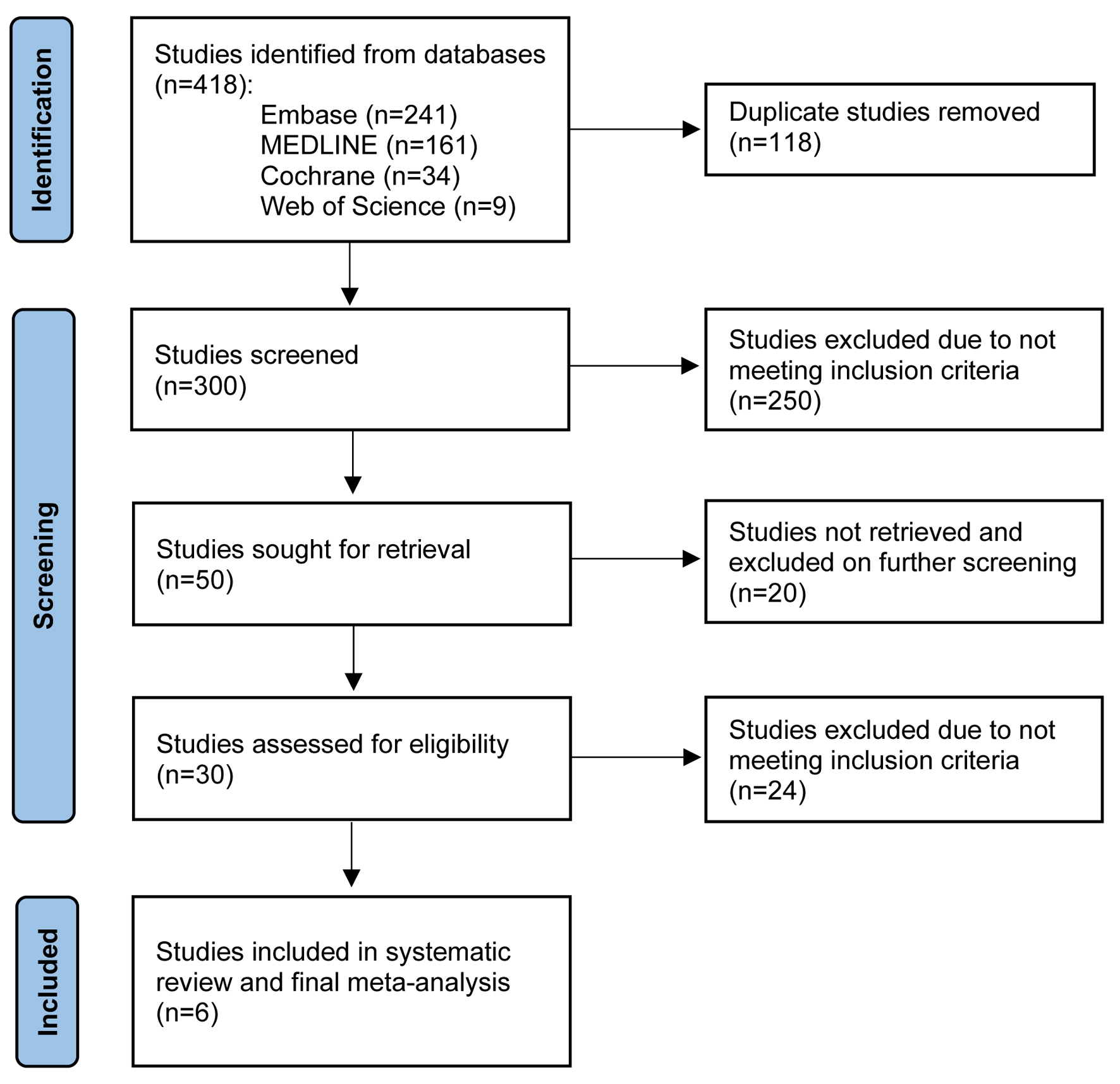 Click for large image | Figure 1. PRISMA flow diagram of the literature review process. |
 Click to view | Table 2. Study Characteristics for Patients Aged < 70 vs. > 70 Years |
 Click to view | Table 3. Study Characteristics for Patients Aged < 65 vs. > 65 Years |
Age < 70 vs. > 70 years
Post-TIPS HE
There was a significantly lower risk of post-TIPS HE in patients aged < 70 vs. > 70 years (RR: 0.42, CI: 0.185 - 0.953, P = 0.03, I2 = 49%) (Fig. 2a, Table 4).
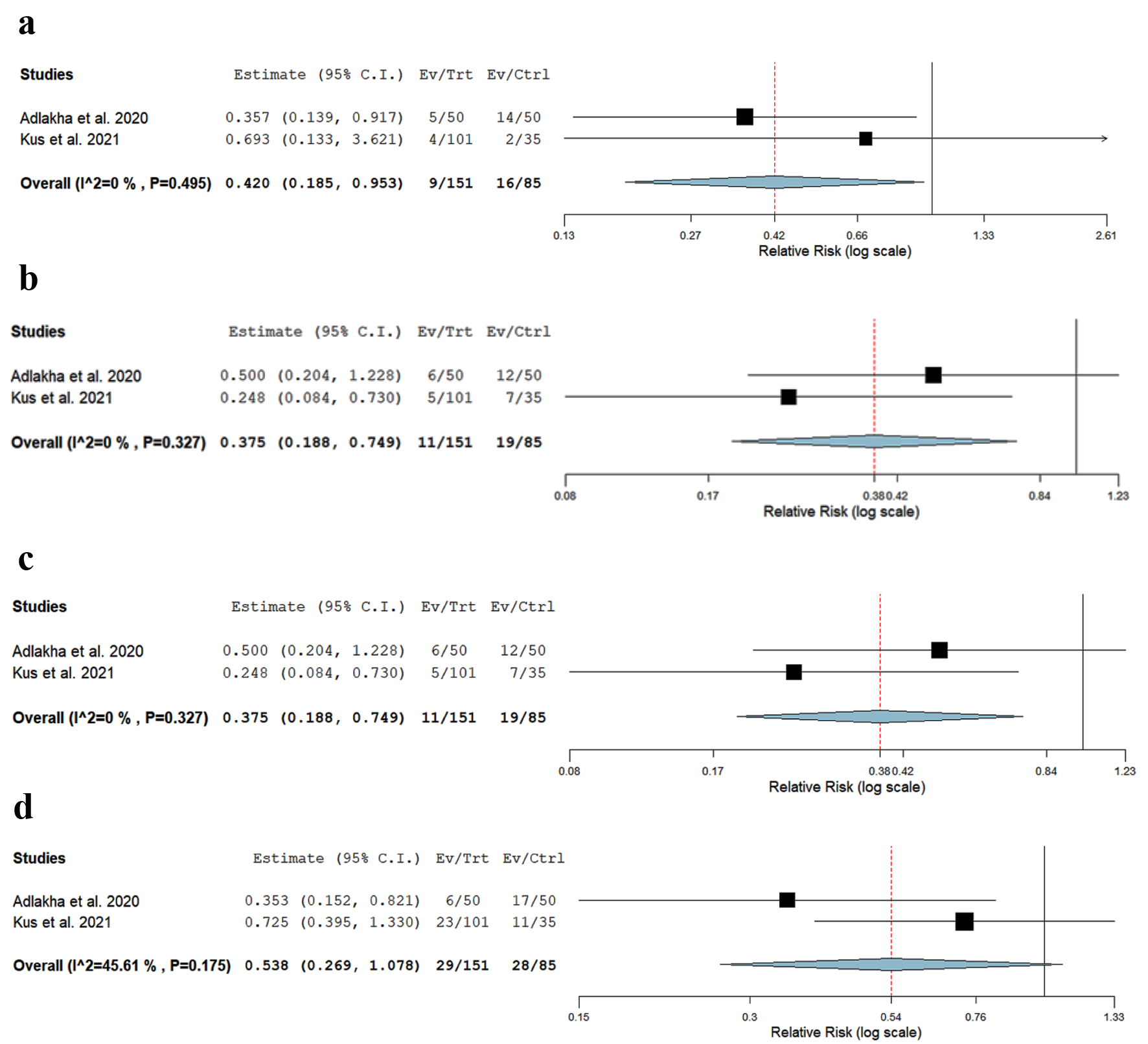 Click for large image | Figure 2. Forest plot of (a) post-TIPS HE in patients age < 70 vs. > 70, (b) 30-day mortality in patients age < 70 vs. > 70, (c) 90-day mortality in patients age < 70 vs. > 70, and (d) 30-day readmission rates due to HE in patients age < 70 vs. > 70. HE: hepatic encephalopathy; TIPS: transjugular intrahepatic portosystemic shunt. |
 Click to view | Table 4. Results Comparing Outcomes of Patients Aged < 70 vs. > 70 Years |
The 30-day mortality
There was a significantly lower risk of 30-day mortality in patients aged < 70 vs. > 70 years (RR: 0.37, CI: 0.188 - 0.74, P = 0.005, I2 = 0%) (Fig. 2b).
The 90-day mortality
There was a significantly lower risk of 90-day mortality in patients aged < 70 vs. > 70 years (RR: 0.35, CI: 0.24 - 0.49, P = 0.001, I2 = 0%) (Fig. 2c).
The 30-day readmission due to HE
There was a non-significant trend towards lower risk of 30-day readmission due to HE in patients aged < 70 vs. > 70 years (RR: 0.538, CI: 0.269 - 1.078, P = 0.08, I2 = 45%) (Fig. 2d).
Age < 65 vs. > 65 years
Post-TIPS HE
There was no significant difference in post-TIPS HE in patients aged < 65 vs. > 65 years (RR: 0.923, CI: 0.632 - 1.43, P = 0.680, I2 = 34%) (Table 5).
 Click to view | Table 5. Results Comparing Outcomes of Patients Aged < 65 vs. > 65 Years |
The 30-day mortality
There was no significant difference in 30-day mortality in patients aged < 65 vs. > 65 years (RR: 0.87, CI: 0.35 - 2.1, P = 0.76, I2 = 81%).
The 90-day mortality
There was no significant difference in 90 day-mortality in patients aged < 65 vs. > 65 years (RR: 0.67, CI: 0.413 - 1.1, P = 0.115, I2 = 63%).
The 30-day readmission due to HE
There was no significant difference in 30-day readmission due to HE in patients aged < 65 vs. > 65 years (RR: 0.907, CI: 0.75 - 1.095, P = 0.308, I2 = 0%).
| Discussion | ▴Top |
This is the first systematic review and meta-analysis conducted to summarize the risk of post-TIPS adverse events in the elderly. We found that patients aged > 70 years had a significantly increased risk of developing post-TIPS HE, as well as 30-day and 90-day mortality, compared to those aged < 70 years. There was a trend towards increased risk of 30-day readmission due to HE in patients aged > 70 years compared to < 70 years, although this did not reach statistical significance. There was no significant difference in risk of post-TIPS adverse events in patients < 65 vs. > 65 years.
Published literature regarding outcomes of TIPS in the elderly population is scarce. The pooled results from the analysis in our study revealed that 30-day and 90-day mortality was significantly higher in patients aged > 70 years. Additionally, different investigators have reached different conclusions about the impact of age on post-TIPS outcomes. Syed et al reported TIPS was a successful treatment for refractory complications of portal hypertension in elderly patients [34]. Adlakha and Russo reported a higher 30-day mortality rate in patients aged > 70 vs. < 70 years (24% vs. 12%), although the finding did not achieve statistical significance (P = 0.19) [32]. Similarly, in a retrospective review, Suraweera et al reported trends towards a higher 90-day mortality rate (OR: 3.3, 95% CI: 0.78 - 14.01, P = 0.182) and a higher 90-day hospitalization rate (OR: 1.76, 95% CI: 0.52 - 5.95, P = 0.546) in the elderly aged ≥ 65 years, although these also did not reach statistical significance [26]. Pan et al found age > 70 years was significantly associated with poor survival at 90 days and 1 year after TIPS creation (P < 0.05) in their retrospective analysis [24]. Lee et al also reported patients aged > 70 years have 1.28 times higher odds of in-hospital mortality than their younger counterparts, based on data from the National Inpatient Service, which included 83,884 patients who underwent TIPS creation and were admitted to a United States hospital between 1998 and 2012 [35]. In contrast, other studies have shown that the effect of age is statistically insignificant when compared to bilirubin and other assessments of liver function, such as the Child-Pugh score [36, 37]. A retrospective study by Parvinian et al found that age but not MELD score was related to a higher 90-day mortality rate post-TIPS among patients with MELD scores of 18 - 25; however, this study did not account for comorbidities and only included 23 participants aged > 54 years [38]. TIPS is an effective alternative to frequent large-volume paracentesis for refractory ascites; however, hospital readmission after TIPS poses a significant burden on healthcare systems [39, 40]. Age represents an independent predictor for 30-day readmission for patients with liver cirrhosis irrespective of the severity of the disease or MELD score [41]. The elderly population is at increased risk for HE (a frequent cause of readmission in decompensated cirrhosis) regardless of TIPS status, and TIPS creation further enhances this risk [34].
A strength of our meta-analysis includes a systematic search of all available comparative studies in the current literature, with well-defined inclusion criteria and careful exclusion of redundant studies. However, our meta-analysis has several limitations. First, our study is susceptible to selection bias, as all the studies included in the final analysis were retrospective. Second, a few of the studies that met our inclusion criteria could not be included in the final analysis due to the unavailability of raw data. Third, the severity grading of encephalopathy was not standardized, nor routinely documented across the studies. Only a few studies reported HE using the West Haven classification, and HE was more frequently documented as present or absent. Fourth, there was inconsistent reporting across studies regarding variables including rules for transplant allocation in patients aged > 70 years, stent type (covered or bared, new generation-controlled expansion versus old generation), and stent graft diameters. Fifth, our study did not control for existing comorbidities in patients aged > 70 years, which could potentially confound morbidity and mortality outcomes.
The findings of this meta-analysis are important when considering the individualized risks and benefits of TIPS, and may be useful for providers and patients aged > 70 years when discussing treatment options and informed consent for the procedure. Age may be a helpful prognosticator for post-TIPS adverse events, whose clinical utility may be maximized if used in combination with other potential indicators such as bilirubin, serum creatinine, and prothrombin time, which have been used in calculating the MELD score and predicting the risk of post-TIPS mortality [42, 43]. Our results suggest caution should be used when considering TIPS in patients > 70 years, although the applicability of our study results is limited by the factors described above.
In conclusion, age > 70 years is associated with significantly higher rates of HE and 30-day and 90-day mortality rates, as well as a trend towards higher 30-day readmission due to HE, in patients after undergoing TIPS creation. No significant differences in adverse event rates were found between patients aged < 65 and > 65 years. Advanced age may be useful for clinicians to consider when evaluating individualized patient risk of adverse events after TIPS. However, further studies investigating age and other risk factors for post-TIPS adverse outcomes are warranted.
| Supplementary Material | ▴Top |
Suppl 1. Full search strategies (all searched performed January 25, 2022).
Acknowledgments
None to declare.
Financial Disclosure
No financial support was received for the preparation of this manuscript.
Conflict of Interest
The authors have disclosed no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Zohaib Ahmed: study conception, study planning and conduction, data collection and interpretation, manuscript drafting and revision. Umer Farooq and Syeda Faiza Arif: data collection, manuscript drafting; Muhammad Aziz: statistical analysis. Umair Iqbal, Ahmad Nawaz and Abdallah Kobeissy: manuscript revision. Wade Lee-Smith: study search strategy, data collection, manuscript drafting. Joyce Badal: data collection, manuscript revision. Asif Mahmood, Ali Nawras, Mona Hassan, and Sammy Saab: critical manuscript revision.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CI: confidence interval; EST: endoscopic sclerotherapy; EVL: endoscopic variceal ligation; HE: hepatic encephalopathy; LC: liver cirrhosis; LT: liver transplant; MELD: Model for End-Stage Liver Disease; MINORS: Methodological Index for Non-Randomized Studies; RR: risk ratio; TIPS: transjugular intrahepatic portosystemic shunt; VB: variceal bleeding
| References | ▴Top |
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749-1761.
doi - Yoon YH, Chen CM. Surveillance report #114 liver cirrhosis mortality in the United States: national, state, and regional trends. 2000-2017. 2019.
- Rahimi RS, Rockey DC. Complications of cirrhosis. Curr Opin Gastroenterol. 2012;28(3):223-229.
doi pubmed - Wright AS, Rikkers LF. Current management of portal hypertension. J Gastrointest Surg. 2005;9(7):992-1005.
doi pubmed - Fortune B, Garcia-Tsao G. Current Management Strategies for Acute Esophageal Variceal Hemorrhage. Curr Hepatol Rep. 2014;13(1):35-42.
doi pubmed - Kapoor A, Dharel N, Sanyal AJ. Endoscopic Diagnosis and Therapy in Gastroesophageal Variceal Bleeding. Gastrointest Endosc Clin N Am. 2015;25(3):491-507.
doi pubmed - Cordon JP, et al. Endoscopic management of esophageal varices. World Journal of Gastrointestinal Endoscopy. 2012;4(7):312.
doi pubmed - Zargar SA, Javid G, Khan BA, Shah OJ, Yattoo GN, Shah AH, Gulzar GM, et al. Endoscopic ligation vs. sclerotherapy in adults with extrahepatic portal venous obstruction: a prospective randomized study. Gastrointest Endosc. 2005;61(1):58-66.
doi - Frost JW, Hebbar S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc Int Open. 2018;6(6):E664-E668.
doi pubmed - D'Amico G, De Franchis R, Cooperative Study G. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38(3):599-612.
doi pubmed - Pedersen JS, Bendtsen F, Moller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6(3):124-137.
doi pubmed - Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18(11):1166-1175.
doi pubmed - Shao XD, Qi XS, Guo XZ. Esophageal stent for refractory variceal bleeding: a systemic review and meta-analysis. Biomed Res Int. 2016;2016:4054513.
doi pubmed - Siqueira F, Kelly T, Saab S. Refractory ascites: pathogenesis, clinical impact, and management. Gastroenterology & Hepatology. 2009;5(9):647-656.
- Boyer TD, Haskal ZJ, American Association for the Study of Liver D. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51(1):306.
doi pubmed - Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rossle M, Panther E, Wiest R, et al. Prevention of Rebleeding From Esophageal Varices in Patients With Cirrhosis Receiving Small-Diameter Stents Versus Hemodynamically Controlled Medical Therapy. Gastroenterology. 2015;149(3):660-668.e661.
doi pubmed - Rossle M. TIPS: 25 years later. J Hepatol. 2013;59(5):1081-1093.
doi pubmed - Perry BC, Kwan SW. Portosystemic Shunts: Stable Utilization and Improved Outcomes, Two Decades After the Transjugular Intrahepatic Portosystemic Shunt. J Am Coll Radiol. 2015;12(12 Pt B):1427-1433.
doi pubmed - Saab S, Nieto JM, Lewis SK, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2006;4:CD004889.
doi pubmed - Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, Nicolini A, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40(3):629-635.
doi pubmed - Suhocki PV, Lungren MP, Kapoor B, Kim CY. Transjugular intrahepatic portosystemic shunt complications: prevention and management. Semin Intervent Radiol. 2015;32(2):123-132.
doi pubmed - Boyer TD, Haskal ZJ, American Association for the Study of Liver D. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41(2):386-400.
doi pubmed - Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and Risk Factors for Hepatic Encephalopathy in a Population-Based Cohort of Americans With Cirrhosis. Hepatol Commun. 2019;3(11):1510-1519.
doi pubmed - Pan JJ, Chen C, Caridi JG, Geller B, Firpi R, Machicao VI, Hawkins IF, Jr., et al. Factors predicting survival after transjugular intrahepatic portosystemic shunt creation: 15 years' experience from a single tertiary medical center. J Vasc Interv Radiol. 2008;19(11):1576-1581.
doi pubmed - Casadaban LC, Parvinian A, Minocha J, Lakhoo J, Grant CW, Ray CE, Jr., Knuttinen MG, et al. Clearing the Confusion over Hepatic Encephalopathy After TIPS Creation: Incidence, Prognostic Factors, and Clinical Outcomes. Dig Dis Sci. 2015;60(4):1059-1066.
doi pubmed - Suraweera D, Jimenez M, Viramontes M, Jamal N, Grotts J, Elashoff D, Lee EW, et al. Age-related Morbidity and Mortality After Transjugular Intrahepatic Portosystemic Shunts. J Clin Gastroenterol. 2017;51(4):360-363.
doi pubmed - Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716.
doi pubmed - Kus J, Fonseca A, Ness J, Brown M, Choudhari R, Gjeluci A, Amin P. Abstract No. 462 Outcomes in patients over 70-years undergoing TIPS procedure: a single-center 10-year retrospective review. Journal of Vascular and Interventional Radiology. 2021;32(5):S115.
doi - Madhok IK, et al. Su369 transjugular intrahepatic shunt (TIPS) outcomes with respect to age. Gastroenterology. 2021;160(6):S867.
doi - Bisht RU, Liu MC, Koblinski JE, Kang P, Wong MN, Little EC. Is 70 the new 50? Complications and outcomes of transjugular intrahepatic portosystemic shunt in older versus younger patients. Abdom Radiol (NY). 2021;46(6):2789-2794.
doi pubmed - Stockhoff L, Schultalbers M, Tergast TL, Hinrichs JB, Gerbel S, Meine TC, Manns MP, et al. Safety and feasibility of transjugular intrahepatic portosystemic shunt in elderly patients with liver cirrhosis and refractory ascites. PLoS One. 2020;15(6):e0235199.
doi pubmed - Adlakha N, Russo MW. Outcomes after transjugular intrahepatic portosystemic shunt in cirrhotic patients 70 years and older. J Clin Med. 2020;9(2):381.
doi pubmed - Saad N, Rude MK, Darcy M, Hanin JB, Wentworth A, Korenblat KM. Older age is associated with increased early mortality after transjugular intrahepatic portosystemic shunt. Ann Hepatol. 2016;15(2):215-221.
- Syed MI, Karsan H, Ferral H, Shaikh A, Waheed U, Akhter T, Gabbard A, et al. Transjugular intrahepatic porto-systemic shunt in the elderly: Palliation for complications of portal hypertension. World J Hepatol. 2012;4(2):35-42.
doi pubmed - Lee EW, Kuei A, Saab S, Busuttil RW, Durazo F, Han SH, El-Kabany MM, et al. Nationwide trends and predictors of inpatient mortality in 83884 transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2016;22(25):5780-5789.
doi pubmed - Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, McCashland T, et al. The North American study for the treatment of refractory ascites. Gastroenterology. 2003;124(3):634-641.
doi pubmed - Rajan DK, Haskal ZJ, Clark TW. Serum bilirubin and early mortality after transjugular intrahepatic portosystemic shunts: results of a multivariate analysis. J Vasc Interv Radiol. 2002;13(2 Pt 1):155-161.
doi - Parvinian A, Shah KD, Couture PM, Minocha J, Knuttinen MG, Bui JT, Gaba RC. Older patient age may predict early mortality after transjugular intrahepatic portosystemic shunt creation in individuals at intermediate risk. J Vasc Interv Radiol. 2013;24(7):941-946.
doi pubmed - Vozzo CF, Singh T, Bullen J, Sarvepalli S, McCullough A, Kapoor B. Hospital readmission following transjugular intrahepatic portosystemic shunt: a 14-year single-center experience. Gastroenterol Rep (Oxf). 2020;8(2):98-103.
doi pubmed - Kuei A, Lee EW, Saab S, Busuttil RW, Durazo F, Han SH, ElKabany M, et al. Inpatient cost assessment of transjugular intrahepatic portosystemic shunt in the USA from 2001 to 2012. Dig Dis Sci. 2016;61(10):2838-2846.
doi pubmed - Chirapongsathorn S, Poovorawan K, Soonthornworasiri N, Pan-Ngum W, Phaosawasdi K, Treeprasertsuk S. Thirty-day readmission and cost analysis in patients with cirrhosis: a nationwide population-based data. Hepatol Commun. 2020;4(3):453-460.
doi pubmed - Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864-871.
doi pubmed - Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


