| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 4, August 2022, pages 200-206
The Safety of the Re-Opening of an Academic Medical Center Outpatient Endoscopy Unit During the COVID-19 Pandemic
Scott Manskia, Christopher J. Schmoyera, Alice Panga, Joshua Liebermanb, Micaela Gernhardtc, Elizabeth Conna, Neveda Murugesana, Alexandra Letob, Ryan Erwina, Taylor Kavanaghb, Mitchell Conna, d
aDivision of Gastroenterology and Hepatology, Thomas Jefferson University Hospital, Philadelphia, PA, USA
bSidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA, USA
cWest Chester University of Pennsylvania, West Chester, PA, USA
dCorresponding Author: Mitchell Conn, Division of Gastroenterology and Hepatology, Thomas Jefferson University Hospital, Philadelphia, PA 19107, USA
Manuscript submitted June 15, 2022, accepted August 1, 2022, published online August 23, 2022
Short title: COVID-19 Risk Post Outpatient Endoscopy
doi: https://doi.org/10.14740/gr1551
| Abstract | ▴Top |
Background: The coronavirus disease 2019 (COVID-19) pandemic has spread globally leading to over 3,700,000 deaths. As COVID-19 cases stabilized, the re-opening of endoscopy centers potentially exposed patients and healthcare workers to viral infection. This study aims to determine risk of COVID-19 exposure among patients undergoing outpatient endoscopies in a tertiary care setting during the COVID-19 pandemic.
Methods: Patients undergoing outpatient endoscopy were contacted post-procedure for any new COVID-19 symptoms or COVID-19 test results. Patient experiences and perception of personal safety were also determined.
Results: Of the 1,584 patients who completed elective endoscopy, 996 (62.9%) completed the survey. Two patients were diagnosed with COVID-19 within 14 days of procedure. The majority (99.7%) felt safe during their procedure and apprehension regarding endoscopy decreased over time.
Conclusion: Thus, the risk of COVID-19 transmission during outpatient endoscopy is extremely low when following recommended society guidelines. Patients felt safe during the procedure and experienced less fear of exposure over time.
Keywords: COVID-19; Transmission; Endoscopy; Quality improvement
| Introduction | ▴Top |
The coronavirus disease 2019 (COVID-19) pandemic began in Wuhan, China in December 2019 and rapidly spread across the globe [1]. The first case in the United States was confirmed on January 20, 2020 [2]. It has infected over 180 million people and caused over 3 million deaths worldwide [3]. COVID-19 spreads readily via direct contact of contaminated surfaces and respiratory droplets [1]. Evidence also supports airborne spread of aerosolized viral particles, particularly during aerosol-generating procedures such as upper endoscopy [4]. Due to the unprecedented rapid spread and risk of exposure during endoscopy to patients and healthcare workers, guidelines outlining procedural safety measures were published by multiple gastrointestinal societies.
Practice guidelines from the American Society of Gastrointestinal Endoscopy (ASGE) included protocols prior to, at the time of, and after endoscopy to optimize patient safety [5]. Potential procedures were triaged as urgent, semi-urgent, and elective. For procedures that could not be delayed, patients were recommended to undergo screening for COVID-19. A questionnaire of symptoms and exposures was recommended to be performed prior to and on the day of endoscopy. In addition, laboratory testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was recommended within 48 h of a procedure [5]. Post-procedure, the ASGE advised that a follow-up call at 7 and 14 days post-endoscopy should be considered to evaluate for development of COVID-19 symptoms [6].
At the onset of the pandemic, our outpatient endoscopy center drastically reduced operations and the majority of elective procedures were cancelled. A similar occurrence was seen globally in a June 2020 survey of 252 endoscopy units from 55 countries showing an 83% decline in endoscopy volume [7]. As COVID-19 transmission peaked, we developed protocols according to gastroenterology society guidelines to safely re-open our outpatient endoscopy unit. These protocols involved scheduling, pre-procedural screening and testing, proper use of personal protective equipment, social distancing, sanitization, and post-procedure patient follow-up to assess for COVID-19 symptom development.
Resumption of outpatient procedures raised safety concerns given the potential exposure of patients and providers to COVID-19. One early study from Italy evaluated 802 patients 14 days after endoscopy. While only one patient was found to be COVID-19-positive, seven other cases developed COVID-19 symptoms. Of those, three tested negative and the other four were not tested. None of the eight patients were hospitalized. The authors of this retrospective study concluded that there is negligible risk of COVID-19 infection in patients undergoing endoscopy [8]. While transmission was low in this study, real world data on the safety and outcomes of viral transmission have been sparse. This study aims to determine the rates of COVID-19 infection after outpatient endoscopy at a tertiary care center in the United States.
| Materials and Methods | ▴Top |
After Pennsylvania State approval, our tertiary care center re-opened its outpatient endoscopy unit May 2020 and was operating at capacity by July 2020. Patient safety protocols were followed in accordance with available multi-society guidelines. All patients underwent pre-procedure SARS-CoV-2 testing within 72 h of their procedure. On the day of the procedure, all patients and staff were screened for symptoms of or possible exposure to COVID-19. Personal protective equipment (PPE) including gowns, gloves, and N95 masks or powered air-purifying respirators were used. Social distancing was adhered to as appropriate. Sanitization of the equipment and room after each case and a terminal clean at the end of the day were performed per guidelines.
This was a retrospective study among all patients who had an elective endoscopic procedure at Thomas Jefferson University Hospital between May 2020 and July 2020. The study design, methodology, and aims were reviewed and approved by Thomas Jefferson University’s Institutional Review Board (IRB) prior to study initiation. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Only adult patients 18 years of age or older were included. Patients were contacted at a median of 14 days (range 13 - 62 days) after their procedure. The first and second quartile of patients were contacted by 14 days; the third quartile was contacted by 16 days post-procedure (Fig. 1). Those who were unable to be reached via phone following at least two attempts were excluded from the study. Patients were contacted and assessed for the development of symptoms potentially caused by acquisition of COVID-19. Symptoms specifically queried included fever, chills, shortness of breath, muscle aches, loss of taste or smell, sore throat, congestion or rhinorrhea, nausea or vomiting, and diarrhea. Those who endorsed symptoms were asked follow-up questions including whether symptoms were persistent, a recent history of sick contacts or travel, and whether medical attention was sought. The result of any subsequent COVID-19 testing was obtained. Patient experiences were also documented, including apprehension about undergoing endoscopy due to COVID-19 and their impression of safety on the day of their procedure. In an effort to screen for asymptomatic COVID-19 post-procedure patients, a second round of telephone calls were performed between September and November 2020. All patients were asked whether they were tested for COVID-19 since their procedure regardless of symptoms. If so, results and date of the test as well as whether symptoms were absent or present at the time were assessed. The primary objective was to assess the risk of COVID-19 transmission and infection in patients undergoing outpatient endoscopy. The secondary objective was to document patient impressions and experiences of undergoing an endoscopy during a pandemic.
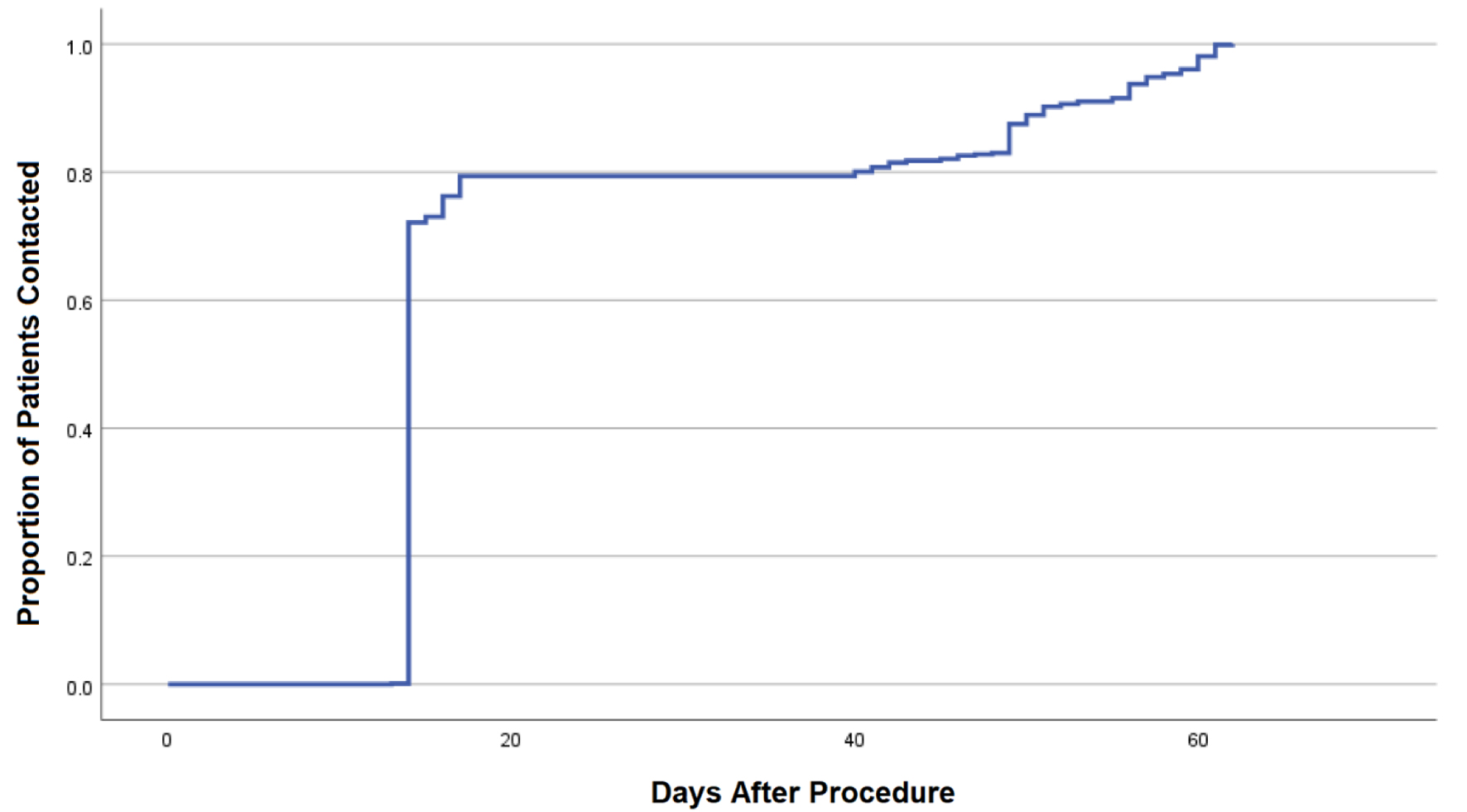 Click for large image | Figure 1. Kaplan-Meier curve demonstrating time from endoscopic procedure to successful phone call and documentation of COVID-19 survey. COVID-19: coronavirus disease 2019. |
Categorical data were reported using frequency and percentage. Continuous variables were presented as a mean with corresponding standard deviation (SD). All statistical analyses were performed using SPSS v. 28 (SPSS Inc., Chicago, IL, USA).
| Results | ▴Top |
From May to July 2020, 1,641 patients underwent pre-procedure COVID-19 testing for outpatient endoscopy. Twelve of these patients tested positive (0.7%) and were cancelled. Of the 1,584 patients who completed an outpatient endoscopy, 996 (62.9% of total patients) were successfully contacted post-procedure during the first round of calls (Table 1). These patients were 53.8% female, predominantly middle-aged (mean age 58.7, SD 14.9), and typically overweight with a mean body mass index (BMI) of 28.5 (SD 7.6). The majority were Caucasian (70.3%) and African American (20.7%). Nearly half (52.1%) reported no previous tobacco use history. Common comorbidities included hypertension (45.1%), hyperlipidemia (37.8%), and diabetes (18.2%). A total of 525 upper endoscopies, 330 lower endoscopies, and 141 same day upper and lower endoscopies were performed.
 Click to view | Table 1. Baseline Characteristics |
Sixty-six patients (6.46%) self-reported the onset of new symptoms seen in COVID-19 within 2 weeks of an elective endoscopic procedure (Table 2). The most frequently reported symptoms were nausea or vomiting (27, 2.7%), diarrhea (19, 1.9%), and shortness of breath (16, 1.6%). Fever, chills, body aches, sore throat, rhinorrhea, and loss of taste or smell were reported at lower rates. Patients undergoing upper endoscopy were most likely to report any post-procedure symptom (45, 8.6%). Thirty-six of these patients stated their symptoms were persistent and 24 (2.4%) sought medical attention. Within 14 days of their procedure, only one patient was in direct contact with a confirmed case of COVID-19. Three patients were required to travel within 14 days post-procedure and one patient was in close contact with someone who travelled during that time.
 Click to view | Table 2. COVID-19-Related Symptoms and Outcomes After Elective Endoscopy |
A total of 693 patients responded to questions regarding COVID-19 testing and diagnosis following elective endoscopy. Three hundred sixty patients (51.9%) underwent COVID-19 testing after their procedure. Of these, 15 were positive indicating a new COVID-19 diagnosis. However, only two patients (0.6%) tested positive within 2 weeks of their procedure while the remaining 13 (3.9%) became positive ≥ 15 days after endoscopy (Table 3). Both of the patients testing positive within 2 weeks of endoscopy were asymptomatic and incidentally identified prior to another elective procedure or travel. Among all who tested positive following endoscopy, 10 had symptoms consistent with COVD-19 including fever, headache, fatigue/malaise, respiratory symptoms, and/or loss of taste or smell.
 Click to view | Table 3. COVID-19 Testing After Elective Endoscopy |
Almost all (99.7%) patients felt safe during elective endoscopy and believed hospital staff used appropriate safety measures during their procedure throughout the study period (Fig. 2, Supplementary Material 1, www.gastrores.org).
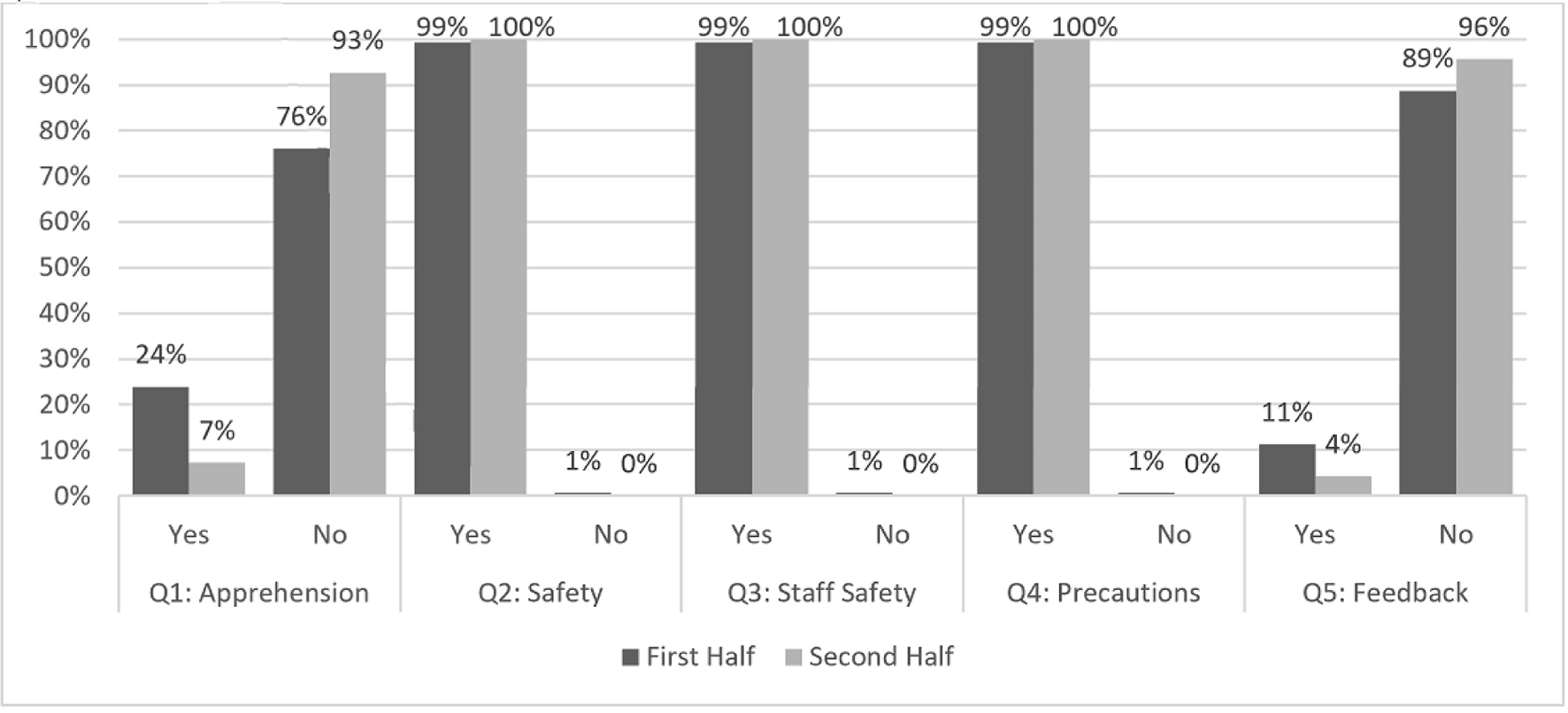 Click for large image | Figure 2. Patient reported measures during outpatient endoscopy, stratified between the first and second half of the observed time period, during the COVID-19 pandemic. COVID-19: coronavirus disease 2019. |
While 113 (23.9%) patients were apprehensive to undergo a procedure in May to late June, only 38 (7.3%) shared this worry in the months of late June to July. Of the 74 patients who provided constructive feedback, several negative comments were related to the logistics of pre-procedure COVID-19 testing.
| Discussion | ▴Top |
The COVID-19 pandemic affected all aspects of life and required drastic changes in how the medical community provided patient care. At the beginning of the pandemic, the perceived risk of infection and transmission led to the delay or cancellation of the majority of outpatient endoscopies. As COVID-19 infection rates stabilized, endoscopy centers throughout the United States gradually began re-opening. Standardized protocols to maintain safety of patients and staff during the pandemic were published in joint gastrointestinal society guidelines. This study shows that rates of transmission of COVID-19 were extremely low when these guidelines are adhered to in real world practice.
Following elective endoscopy, 66 of 996 patients reported at least one symptom possibly attributable to COVID-19. However, a number of these symptoms overlap with underlying gastrointestinal diseases or adverse effects of the procedure itself. This is supported by the finding that the most frequently reported symptoms were nausea, vomiting, or diarrhea. While 15 patients tested positive for COVID-19 during follow-up, only two were positive within 14 days of their procedure. Given our understanding of COVID-19 viral transmission, it is highly unlikely the remaining 13 positive cases could be attributed to elective endoscopy. The two patients testing positive within 14 days of elective endoscopy resulted in a 0.6% positive rate.
This was similar to the rate of positive cases found incidentally on pre-procedure testing of 0.7%. Of note, other studies have found variable rates of positive cases on pre-endoscopic testing. Between April and May 2020 at the University of Miami, Florida, the rate of positive asymptomatic pre-procedure testing was 0.25% compared to a prevalence of 12.7% in Miami-Dade County. A similar study found a 0.2% positive rate [9]. However, it is difficult to compare different populations and geographic regions of the United States who have experienced distinct rates of SARS-CoV-2 prevalence over time.
A total of 15 patients tested positive for COVID-19 within 51 days of their elective endoscopy. However, only two patients were positive within 14 days. As most individuals with COVID-19 remain infectious for up to 10 days after the onset of symptoms, these two patients could have potentially been exposed during their elective procedure [10]. Given our understanding of viral transmission, the remaining 13 patients who tested positive outside the 14-day window were exposed through an alternative source. Our findings are similar to that of a study in Italy, which found only one patient who tested positive for COVID-19 within 14 days after their procedure out of 802 patients [8]. As the 0.6% rate of infection following elective endoscopy in our study mirrored the 0.7% rate of infection among those undergoing pre-procedure screening, there is no evidence that elective endoscopy introduces undue risk of COVID-19 exposure to our patients.
Self-reported outcomes showed a larger portion of patients were apprehensive about undergoing a procedure during the early months of the pandemic. These concerns alleviated with time. This is likely a reflection of the general fear and uncertainty surrounding COVID-19 among the general population and healthcare workers alike. In early 2020, the medical community was in the early stages of learning to identify, test for, and treat COVID-19. As our knowledge and public health infrastructure developed, patients became more comfortable and familiar with infectious control measures and in-person medical care.
This study does have some limitations. The retrospective nature of data acquisition is dependent on patient recall for presence and timing of symptoms and testing. We attempted to account for asymptomatic COVID-19-positive patients, though a smaller number of patients were able to be reached at a later time period on a second round of calls. Also, only 62.9% of all patients who underwent endoscopy completed the survey. It is possible a handful of patients could not be reached because they developed COVID-19 infection, possibly leading to hospitalization or inability to answer their phone. Given this, it is possible we are underestimating the degree of transmission from elective endoscopy. Still, this was a large study with a relatively high survey completion rate.
Recommendations have continued to further change as vaccination for COVID-19 has become more prevalent and spread has been controlled. This is reflected in updated 2021 recommendations from the American Gastroenterological Association (AGA). Routine pre-procedure testing for SARS-CoV-2 infection is not recommended for patients undergoing endoscopy irrespective of vaccination status due to similar data of low rates of infection described in our current study. However, centers should continue to implement screening of patients for COVID-19 symptoms and implement testing based on responses [11]. In conclusion, this study provides real world data from a critical timepoint during the COVID-19 pandemic, demonstrating endoscopy was a safe procedure and a positive patient experience.
| Supplementary Material | ▴Top |
Suppl 1. Questions (Q1-5) included patient apprehension regarding endoscopy during the pandemic, their perception of safety and whether staff provided a sense of safety and appropriate precautions, and an opportunity for feedback.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The approval allowed for a retrospective chart review and analysis of de-identified data that did not require informed consent.
Author Contributions
SM: study design, data acquisition; analysis and interpretation of data, drafting and revision of manuscript; CJS: data acquisition; analysis and interpretation of data, drafting and revision of manuscript; AP: data acquisition; analysis and interpretation of data; JL, MG, NM, AL, RE, and TK: data acquisition; MC: project oversight, study design, analysis and interpretation of data, and revision of manuscript.
Data Availability
Data supporting the findings of this study are available within the article as well as upon request from the corresponding author.
Abbreviations
ASGE: American Society of Gastrointestinal Endoscopy; BMI: body mass index; SD: standard deviation; PPE: personal protective equipment; AGA: American Gastroenterological Association
| References | ▴Top |
- Lui RN, Wong SH, Sanchez-Luna SA, Pellino G, Bollipo S, Wong MY, Chiu PWY, et al. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020;35(5):749-759.
doi pubmed - Corral JE, Hoogenboom SA, Kroner PT, Vazquez-Roque MI, Picco MF, Farraye FA, Wallace MB. COVID-19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc. 2020;92(3):524-534.e526.
doi pubmed - Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. World Health Organization. www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- Sagami R, Nishikiori H, Sato T, Tsuji H, Ono M, Togo K, Fukuda K, et al. Aerosols produced by upper gastrointestinal endoscopy: a quantitative evaluation. Am J Gastroenterol. 2021;116(1):202-205.
doi pubmed - Hennessy B, Vicari J, Bernstein B, Chapman F, Khaykis I, Littenberg G, Robbins D. Guidance for resuming GI endoscopy and practice operations after the COVID-19 pandemic. Gastrointest Endosc. 2020;92(3):743-747.e741.
doi pubmed - Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, Carrara S, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92(1):192-197.
doi pubmed - Parasa S, Reddy N, Faigel DO, Repici A, Emura F, Sharma P. Global impact of the COVID-19 pandemic on endoscopy: an international survey of 252 centers from 55 countries. Gastroenterology. 2020;159(4):1579-1581.e1575.
doi pubmed - Repici A, Aragona G, Cengia G, Cantu P, Spadaccini M, Maselli R, Carrara S, et al. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020;69(11):1925-1927.
doi pubmed - Sultan S, Siddique SM, Altayar O, Caliendo AM, Davitkov P, Feuerstein JD, Francis D, et al. AGA institute rapid review and recommendations on the role of pre-procedure SARS-CoV-2 testing and endoscopy. Gastroenterology. 2020;159(5):1935-1948.e1935.
doi pubmed - Walsh KA, Spillane S, Comber L, Cardwell K, Harrington P, Connell J, Teljeur C, et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect. 2020;81(6):847-856.
doi pubmed - Sultan S, Siddique SM, Singh S, Altayar O, Caliendo AM, Davitkov P, Feuerstein JD, et al. AGA rapid review and guideline for SARS-CoV2 testing and endoscopy post-vaccination: 2021 update. Gastroenterology. 2021;161(3):1011-1029.e1011.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


