| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 4, August 2022, pages 162-172
Early Colonoscopy in Hospitalized Patients With Acute Lower Gastrointestinal Bleeding: A Nationwide Analysis
Kuldeepsinh P. Atodariaa, e, Samyak Dhruvb, Joseph M. Brunoc, Brisha Bhikadiyad, Shravya R. Ginnarama, Shreeja Shaha
aInternal Medicine Department, Abington Jefferson Health, Abington, PA, USA
bInternal Medicine Department, MedStar St. Mary’s Hospital, Leonardtown, MD, USA
cGastroenterology Department, Abington Jefferson Health, Abington, PA, USA
dInternal Medicine Department, Philadelphia College of Osteopathic Medicine, Philadelphia, PA, USA
eCorresponding Author: Kuldeepsinh P. Atodaria, Internal Medicine Department, Abington Jefferson Health, Abington, PA, USA
Manuscript submitted May 9, 2022, accepted June 18, 2022, published online August 23, 2022
Short title: Early Colonoscopy in LGIB
doi: https://doi.org/10.14740/gr1536
| Abstract | ▴Top |
Background: Performing colonoscopy within 24 h of presentation to the hospital is the accepted standard of care for patients with an acute lower gastrointestinal bleed (LGIB). Previous studies have failed to demonstrate the benefit of early colonoscopy (EC) on mortality. In this study, we wanted to see if there was a change in inpatient deaths (primary outcome), length of stay (LOS), and hospitalization charges (TOTCHG) (secondary outcomes) with EC compared to previous studies.
Methods: Adults diagnosed with LGIB were identified using the International Classification of Disease 10th Revision codes from the National Inpatient Sample database for 2016 to 2019. EC was defined as the procedure performed within 24 h of hospitalization. Delayed colonoscopy (DC) was defined as a procedure performed after 24 h of presentation. The patient population was divided into EC and DC groups, and the effects of several covariates on outcomes were measured using binary logistic and multivariate regression analysis. Inverse probability treatment weighting (IPTW) was performed to adjust for confounding covariates.
Results: There were 1,549,065 cases diagnosed with LGIB, of which 285,165 cases (18.4%) received a colonoscopy. A total of 107,045 (6.9%) patients received early colonoscopies. EC was associated with decreased inpatient deaths (0.9% in EC, and 1.4% in DC, P < 0.001). However, upon IPTW, this difference was not present. EC was associated with a decreased LOS (median 3 days vs. 5 days, P < 0.001) and TOTCHG (median $32,037 vs. $44,092, P < 0.001). Weekend admissions (WA) were associated with fewer EC (31.6% in WA, and 39.5% in non-WA, P < 0.001). WA did not affect inpatient deaths.
Conclusions: EC was not associated with decreased inpatient deaths. There was no difference in endoscopic interventions in both EC and DC groups. The difference in inpatient deaths observed between the two groups was not evident upon adjusting the results for confounders. EC was associated with a decreased LOS, and TOTCHG in patients with LGIB.
Keywords: Lower gastrointestinal bleed; Early colonoscopy; Mortality; Length of stay; Hospitalization charges; Outcomes
| Introduction | ▴Top |
Lower gastrointestinal bleed (LGIB) remains a common cause of hospitalization, especially in the elderly. LGIB is defined as any bleed originating from small intestine distal to ligament of Treitz, colon, rectum or anus and presenting with melena or bright red/burgundy-colored stools [1-5]. Most common causes of LGIB include colonic diverticulosis (30%), internal hemorrhoids, ischemic colitis, and post-polypectomy bleeding. Other causes include colorectal cancer, inflammatory bowel diseases, mesenteric ischemia, anal fissures, etc. [1]. Eighty percent of LGIBs stop spontaneously, with re-bleeding occurring in 25% of cases. Identifying the source of the bleed can be challenging despite performing a colonoscopy [6, 7]. Performing colonoscopy within 24 h of presentation to the hospital is the accepted standard of care and part of the American College of Gastroenterology Guidelines [8]. American Society for Gastrointestinal Endoscopy suggests performing a colonoscopy within 24 h [9]. In this study, early colonoscopy (EC) is defined as a procedure performed within 24 h of presentation, and delayed colonoscopy (DC) is performed after 24 h. Performing colonoscopies within 24 h of presentation is not always possible. Moreover, whether EC is beneficial in mortality outcomes remains widely debated. Randomized controlled trials and prospective studies have not shown a significant correlation between EC and improved mortality outcomes [10-14]. Systematic reviews and meta-analysis have been performed that showed similar results [4, 15-20]. Retrospective studies from single and multiple institutions and national databases have reported non-significant results [21-28].
Most of these studies showed a decrease in length of stay (LOS) and cost of hospitalization or total charges (TOTCHG) with EC [4-6, 10-12, 19, 20, 26, 27]. Studies also report an improved ability to detect the source of the bleed with EC. Past studies using data spanning multiple years have shown increasing total hospitalizations for LGIB, with unchanging trends in patients getting EC versus DC [25].
Many of these studies had a small sample size and thus did not have statistically significant results.
We decided to investigate how previous studies have affected the approach of gastroenterologists towards acute LGIB in recent years and whether mortality outcomes have changed compared to previous studies.
| Materials and Methods | ▴Top |
Nationwide Inpatient Sample (NIS) is an administrative database and part of the Healthcare Cost and Utilization Project (HCUP). The NIS is the largest publicly available all-payer inpatient healthcare database designed to produce US regional and national estimates of inpatient utilization, access, cost, quality, and outcomes. Unweighted, it contains data from more than 7 million hospital stays each year. Weighted, it estimates more than 35 million hospitalizations nationally.
All adult patients with the International Classification of Disease 10th Revision (ICD-10) diagnosis codes for conditions associated with a LGIB were extracted from the NIS database from 2016 to 2019. Cases included were those with a primary diagnosis of anorectal bleed, bleed from diverticulosis, and unspecified gastrointestinal bleeds. Unspecified gastrointestinal bleeds include nonsteroidal anti-inflammatory drugs (NSAID) use, coagulopathies, angiodysplasia, radiation colitis, rectal varices, rectal foreign bodies, etc. Patients were further differentiated based on secondary diagnosis codes for conditions usually associated with an LGIB (Table 1) (Supplementary Material 1, www.gastrores.org). The diagnosis codes included were angiodysplasia, malignant large intestinal neoplasm, malignant anal neoplasm, malignant small intestinal neoplasm, benign neoplasms/polyps of the large intestine, ischemic bowel disease, hemorrhoids, infectious/noninfectious colitis, Crohn’s disease, ulcerative colitis, anal fissures, and fistulas of the large intestine. In theory, melena is a diagnosis of an upper gastrointestinal bleed (UGIB). In practice, melena often gets used as an all-encompassing term for bloody bowel movements, dark or otherwise. To maximize the number of cases with LGIB included in the analysis, we decided to include patients diagnosed with melena. Cases with other diagnoses of UGIB were not included in this analysis. Excluding the cases that had a procedure code for esophagogastroduodenoscopy (EGD) did not change the mortality, LOS, or TOTCHG numbers for EC and DC groups in any meaningful way. Thus, we decided not to exclude cases with an EGD during the hospitalization for regression analysis.
 Click to view | Table 1. Frequency of LGIB Etiologies Based on Presence of ICD-10 Codes, in the Total Population, and in Cases That Received a Colonoscopy (N = 1,549,605)a |
Charlson Comorbidity Index (CCI) was calculated based on the presence of diagnosis codes for 22 comorbidities that are standard for calculating CCI. The CCI is a well-validated measure of comorbidity used to predict 1-year mortality in patients [29, 30]. Race, sex, type of insurance, weekend admissions (WA), and location/teaching status of the hospital were other considered variables. Other comorbidities considered were hypovolemia/hypovolemic shock, acute renal failure/acute kidney injury (AKI), acute respiratory failure (ARF), a requirement for transfusion of any blood products, and requirement for hemodialysis. The presence of ARF was determined using ICD-10 procedure codes for ventilator support, insertion of nasopharyngeal/oropharyngeal airways, endotracheal intubation, and tracheostomy placement.
The values of age, LOS, and TOTCHG were not normally distributed. Age was transformed using the square root transformation, and LOS and TOTCHG underwent logarithmic transformation before regression analysis [31]. Cases with a Z score > 2.68 and < -2.68 (for either LOS or TOTCHG) were excluded from the regression analysis to prevent the outliers from affecting the regression model. Outliers represented 2.2% of the cases.
Binary logistic regression was performed to predict mortality for the hospitalization using covariates that showed a statistically significant relationship with mortality independently. The covariates included were EC versus DC, age, CCI, gender, race, insurance type, AKI, respiratory failure, hypovolemia/shock, and endoscopic intervention. The goodness of fit was determined using the receiver operating curve (ROC) analysis.
Two separate multiple linear regressions were performed with LOS and TOTCHG as dependent variables, respectively, and the same variables mentioned above as independent covariates to determine their effects on LOS and TOTCHG. Finally, the predicted values obtained after regression analysis were reverse-transformed to obtain interpretable predicted outcomes.
To adjust for confounding effects of covariates namely AKI, chronic kidney disease (CKD), hemodialysis and ARF on the association between EC and inpatient death rates, we performed inverse probability treatment weighting (IPTW) analysis.
Outcomes
Primary outcome of the study was death during hospitalization or inpatient deaths. Secondary outcomes of the study were LOS and TOTCHG.
Statistical analysis
The data used for this study were de-identified patient data and were thus exempt from the Institutional Review Board process. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
All the analyses were performed using IBM SPSS Statistics 28.0.0.0 (International Business Machines Statistical Package for Social Sciences Version 28.0.0.0), which originated in Armonk, NY, USA. Chi-square tests were utilized to compare and determine the association between categorical variables like death, admission on a weekend, sex, race, type of insurance, and location of hospital/teaching status. Mann-Whitney U tests were utilized to compare continuous variables (LOS and TOTCHG) with categorical variables, and the median values were reported. However, we did also run the t-tests and reported the mean values along with standard deviations (SD) for readers that might be interested in knowing them.
The NIS database provides the discharge-level weight “DISCWT” used to statistically weigh the results to produce an estimate of discharges from hospitals at the national level [32].
| Results | ▴Top |
A total of 1,549,065 cases were identified with a diagnosis of LGIB. This number represented 1.3% of the total hospitalizations from 2016 to 2019. Of these, 285,165 patients (18.4%) received a colonoscopy. Of patients who received colonoscopies, 107,045(37.5%) patients received early colonoscopies.
Table 1 shows the distribution of patients based on etiologies of LGIB regardless of whether they received a colonoscopy. Diverticulosis was the most common cause of LGIB (17.5%). Amongst the patients that received a colonoscopy, the proportion of patients diagnosed with diverticulosis was 43%.
Table 2 shows how various demographic, insurance and hospital characteristics affected whether the patients received EC.
 Click to view | Table 2. Demographic Differences in Patients That Received Early Versus Delayed Colonoscopya (N = 285,165) |
Patients that received a colonoscopy had a significantly lower rate of inpatient deaths (1.2%) compared to patients that did not receive a colonoscopy (3.9%) (P < 0.001). In the patient population that received colonoscopies, EC was associated with a significantly lower inpatient deaths, with 1,005 inpatient deaths (0.9%) as compared to patients who received delayed colonoscopies, with 2,530 deaths (1.4%) (P < 0.001). Admission on the weekend did not affect mortality (P = 0.399).
EC was associated with a decreased LOS (median 3 days with EC and 5 days with DC, P < 0.001) and decreased total charges of hospitalization (median $32,037 with EC and $44,092 with DC, P < 0.001). The difference in median LOS between patients that received EC versus patients that received DC was 2 days. The difference in the median hospitalization charges (TOTCHG) was $12,055.
Table 3 reviews how EC and DC correlated with interventions, and adverse events, and summarizes the differences between LOS and TOTCHG for the two groups.
 Click to view | Table 3. Adverse Events and Intervention Data Reported in Patients With Lower Gastrointestinal Bleeding That Received Early Versus Delayed Colonoscopy (N = 285,150) |
On binary logistic regression analysis, the model that was generated was able to predict the observed outcome (death during hospitalization) with 99.0% accuracy (P < 0.001). Furthermore, the ROC curve made using the predicted and observed values for death showed 0.867 area under the curve (P < 0.001), which represents excellent discrimination, or in other words, a good fit (Table 4, Fig. 1). Despite the statistically significant difference in inpatient deaths observed between the EC and DC groups, some covariates, namely AKI, CKD, hemodialysis and ARF had very significant odds ratios which could be confounding the results of the regression analysis. When adjusted for using IPTW, the difference in inpatient deaths between the two groups disappeared (odds ratio of 0.945, 95% CI: 0.877 - 1.019, P = 0.142).
 Click to view | Table 4. Multivariate Analysis of Predictors of Mortality for Patients That Received a Colonoscopy During Hospitalization for an LGIB |
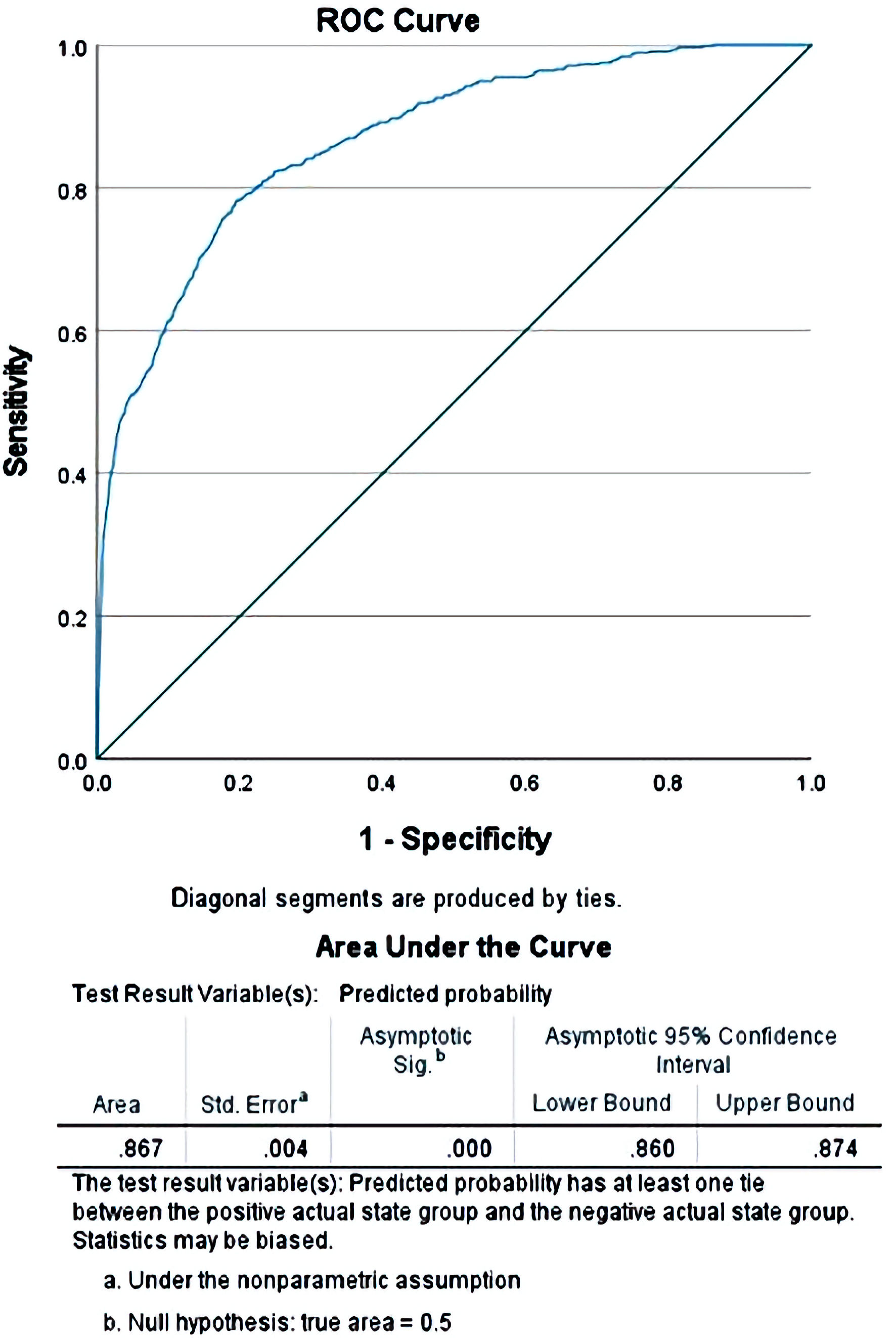 Click for large image | Figure 1. Receiver operating curve (ROC) comparing the predicted values of mortality obtained after the binary regression when compared with the observed values for the cases in the study. |
On linear regression analysis, predicted unstandardized values of the LOS and TOTCHG were obtained. The predicted median LOS for patients who received an EC was 3.90 days versus 5.92 days for patients that received a DC. The difference between these two values was 2.02 days, which closely resembles the 2-day difference in median values observed in the actual data.
The median predicted TOTCHG for patients who received an EC was $31,659.87, compared to patients with a DC for whom the median TOTCHG was $43,208.76. The difference in the predicted total charges for early versus late colonoscopies was $11,548.89, which again was close to the actual observed difference of TOTCHG at $12,055. The coefficients obtained from the regression analyses are reported in Tables 5 and 6.
 Click to view | Table 5. Multivariate Analysis of Predictors of Length of Stay (LOS) for Patients That Received a Colonoscopy During Hospitalization for an LGIB |
 Click to view | Table 6. Multivariate Analysis of Predictors of Total Charges (TOTCHG) for Patients That Received a Colonoscopy During Hospitalization for an LGIB |
The median age for both EC and DC groups was 73 years. The mean CCI for EC and DC was 4.62 (SD 2.37) and 5.122 (SD 2.48) (P < 0.001), respectively.
WA, on Saturday or Sunday, made the patient less likely to receive an EC (31.6% for WA, and 39.5% for non-WA, P < 0.001). WA was not associated with increased inpatient deaths (1.3% for WA and 1.2% for non-WA, P = 0.399). Being admitted on the weekend did not affect the median LOS (4 days for both groups) and TOTCHG ($39,018 for WA and $39,258 for non-WA). Mean LOS (5.90 days for WA and 5.94 days for non-WA, P = 0.183) and mean TOTCHG ($66,037 for WA and $67,051 for non-WA, P = 0.043).
| Discussion | ▴Top |
On initial analysis, we noticed a decrease in inpatient death rates (which was the primary outcome) in patients in the EC group compared to the DC group. However, when adjusted for confounders, this difference disappeared. There was no difference in the proportion of patients that received endoscopic intervention to control the source of the bleed in the two groups. The patients in the DC group were more likely to have other complications such as AKI, CKD, requirement for hemodialysis, or respiratory failure requiring intubation and mechanical ventilation. This suggests that the patients in this group are inherently more unstable, requiring additional time for initial resuscitation, thus preventing them from receiving an EC. This also explains the higher inpatient deaths observed in the DC group of patients before adjusting for confounders. Furthermore, pre-procedure preparation is difficult to finish in an unstable patient in a timely fashion, thus providing another reason why performing EC in such patients would be challenging [8]. The absence of a significant difference in inpatient deaths between the EC and DC groups was consistent with the findings of the previous studies [10-28]. The secondary outcomes of our study were LOS and TOTCHG. We noticed a significantly decreased LOS and TOTCH among the patients in the EC group when compared to the DC group. These findings are consistent with previous studies [4-6, 10-12, 15, 26, 27]. The difference in the CCI between the EC and DC groups although statistically significant, was very small. This suggests that the acuity of the patients’ presentation was more important in whether the patient received an EC, as compared to the patients’ past histories of comorbidities.
Another important finding of our study was the decreased number of patients in the EC group requiring transfusion of blood products compared to patients in the DC group. This result was again consistent with the findings of previous studies [13, 22-24, 33]. It is difficult to ascertain the exact reason for this decreased requirement for transfusion in the EC group. It could be because the patients in the EC group were relatively more stable, thus requiring fewer transfusions, or it could be because the patients in the EC group received the endoscopy earlier, allowing for a quicker diagnosis of the bleeding etiology and achievement of hemostasis. However, given that we did not observe a difference in the proportion of patients that received interventions during the endoscopy between the EC and DC groups, the latter explanation seems less likely.
Data for the newer years showed a shift away from performing EC (37.5%) compared to older data, where 45% of cases received EC [25]. This could represent a shift in the preferences of the gastroenterologists when it comes to performing EC, based on the findings of several past studies that failed to show mortality benefits with EC.
WA were less likely to receive EC, but this did not increase inpatient deaths, LOS, or TOTCHG. The reason for fewer EC being performed for WA is likely because of decreased availability of medical personnel in the hospital during the weekends. However, the fact that this does not increase inpatient deaths, LOS or TOTCHG is reassuring, and perhaps further strengthens the claim that EC does not have a mortality benefit in case of LGIB.
Strengths of the study
Our study included newer data from 2016 to 2019, making it more relevant to the present day. Patients included were from various demographic, economic, race, and socio-cultural backgrounds. Thus, the results obtained would be generalizable to the population of the USA. We had a large sample size and detected significant associations between different variables. The newer NIS data uses ICD-10 diagnosis and procedural codes that are more descriptive in terms of details of medical conditions and interventions than ICD-9 codes used for the older data reported in the previous studies. We also used advanced statistical methods to address the non-normalcy of the data using transformation, and to adjust for the confounding variables using IPTW.
Limitations of the study
NIS is an administrative database. All the diagnoses, treatments, and procedures were detected using the ICD-10 diagnostic and procedure codes. The accuracy of the results depends on the accuracy with which data were reported in NIS. Clinical details, laboratory values, and procedure reports of the patients were not available. Longitudinal follow-up of the patients and occurrence of re-bleeds could not be obtained. The presence of anticoagulation in these patients was not studied. Further studies are needed to address the limitations of this study.
Conclusions
EC did not decrease inpatient deaths in patients presenting to the hospital with LGIB. The difference in inpatient deaths between the EC and DC groups that was observed on initial analysis was not evident when adjusted for confounders. EC was associated with a decreased LOS and TOTCHG in these patients. Thus, EC would be warranted in patients with LGIB with the goal to decrease the LOS and TOTCHG. WA received fewer EC, but this did not affect inpatient deaths.
| Supplementary Material | ▴Top |
Suppl 1. Secondary diagnosis codes for conditions usually associated with an LGIB.
Acknowledgments
None to declare.
Financial Disclosure
We hereby acknowledge that all the funding for this project, including the acquisition of the data, its analysis, writing of the manuscript, editing, and reviewing was funded by the authors themselves. No financial aid was obtained from any third-party companies or entities.
Conflict of Interest
We do not have any conflict of interest to disclose.
Informed Consent
The data used in the study is a nationally available de-identified database. The question of obtaining consent from study subjects is not applicable to this study.
Author Contributions
Kuldeepsinh P. Atodaria MD: conceptualization, data acquisition, statistical analysis, writing original draft, review, and editing. Samyak Dhruv MD: statistical analysis, review, and editing. Joseph M. Bruno MD: conceptualization, review, and editing. Brisha Bhikadiya DO: writing original draft, review, and editing. Shravya R. Ginnaram MD: writing original draft, review, and editing. Shreeja Shah MD: review and editing.
Data Availability
The result files of the statistical analysis (which was performed using IBM SPSS 28.0) were available to the reviewers of the manuscript from the corresponding author upon reasonable request.
Abbreviations
LGIB: lower gastrointestinal bleed; EC: early colonoscopy; DC: delayed colonoscopy; HCUP: Healthcare Utilization Project; NIS: National (Nationwide) Inpatient Sample; ICD-10: International Classification of Disease 10th Edition; ICD-9: International Classification of Disease 9th Edition; CCI: Charlson Comorbidity Index; LOS: length of stay; TOTCHG: total charges of hospitalization; AKI: acute kidney injury; SD: standard deviation; ROC: receiver operating curve; WA: weekend admissions
| References | ▴Top |
- Ghassemi KA, Jensen DM. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333.
doi pubmed - Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92(3):419-424.
- Zuckerman GR, Prakash C. Acute lower intestinal bleeding: part I: clinical presentation and diagnosis. Gastrointest Endosc. 1998;48(6):606-617.
doi - Waye JD. Diagnostic endoscopy in lower intestinal bleeding. Gastrointestinal bleeding. New York: Igaku Shoin Medical Publishers. 1992;14(2):30-41.
- Davila RE, Rajan E, Adler DG, Egan J, Hirota WK, Leighton JA, Qureshi W, et al. ASGE Guideline: the role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc. 2005;62(5):656-660.
doi pubmed - Edelman DA, Sugawa C. Lower gastrointestinal bleeding: a review. Surg Endosc. 2007;21(4):514-520.
doi pubmed - Imdahl A. Genesis and pathophysiology of lower gastrointestinal bleeding. Langenbecks Arch Surg. 2001;386(1):1-7.
doi pubmed - Strate LL, Gralnek IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474.
doi pubmed - Asge Standards of Practice Committee, Pasha SF, Shergill A, Acosta RD, Chandrasekhara V, Chathadi KV, Early D, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79(6):875-885.
doi pubmed - Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100(11):2395-2402.
doi pubmed - Laine L, Shah A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am J Gastroenterol. 2010;105(12):2636-2641; quiz 2642.
doi pubmed - van Rongen I, Thomassen BJW, Perk LE. Early Versus Standard Colonoscopy: A Randomized Controlled Trial in Patients With Acute Lower Gastrointestinal Bleeding: Results of the BLEED Study. J Clin Gastroenterol. 2019;53(8):591-598.
doi pubmed - Saino M, Aoyama T, Yamane M, Masuda S, Nomura R, Shigita K, Asayama N, et al. Optimal candidates for early colonoscopy in the management of acute lower gastrointestinal bleeding. J Gastroenterol Hepatol. 2022.
doi pubmed - Niikura R, Nagata N, Yamada A, Honda T, Hasatani K, Ishii N, Shiratori Y, et al. Efficacy and Safety of Early vs Elective Colonoscopy for Acute Lower Gastrointestinal Bleeding. Gastroenterology. 2020;158(1):168-175.e166.
doi pubmed - Richter JM, Christensen MR, Kaplan LM, Nishioka NS. Effectiveness of current technology in the diagnosis and management of lower gastrointestinal hemorrhage. Gastrointest Endosc. 1995;41(2):93-98.
doi - Farrell JJ, Friedman LS. Review article: the management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2005;21(11):1281-1298.
doi pubmed - Kherad O, Restellini S, Almadi M, Strate LL, Menard C, Martel M, Roshan Afshar I, et al. Systematic review with meta-analysis: limited benefits from early colonoscopy in acute lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2020;52(5):774-788.
doi pubmed - Roshan Afshar I, Sadr MS, Strate LL, Martel M, Menard C, Barkun AN. The role of early colonoscopy in patients presenting with acute lower gastrointestinal bleeding: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2018;11:1756283X18757184.
doi pubmed - Sengupta N, Tapper EB, Feuerstein JD. Early versus delayed colonoscopy in hospitalized patients with lower gastrointestinal bleeding: a meta-analysis. J Clin Gastroenterol. 2017;51(4):352-359.
doi pubmed - Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86(1):107-117.e101.
doi pubmed - Angtuaco TL, Reddy SK, Drapkin S, Harrell LE, Howden CW. The utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: a 2-year experience from a single center. Am J Gastroenterol. 2001;96(6):1782-1785.
doi pubmed - Nigam N, Patel P, Sengupta N. Outcomes of early versus delayed colonoscopy in lower gastrointestinal bleeding using a hospital administrative database. J Clin Gastroenterol. 2018;52(8):721-725.
doi pubmed - Navaneethan U, Njei B, Venkatesh PG, Sanaka MR. Timing of colonoscopy and outcomes in patients with lower GI bleeding: a nationwide population-based study. Gastrointest Endosc. 2014;79(2):297-306 e212.
doi pubmed - Chaudhry V, Hyser MJ, Gracias VH, Gau FC. Colonoscopy: the initial test for acute lower gastrointestinal bleeding. Am Surg. 1998;64(8):723-728.
- Devani K, Radadiya D, Charilaou P, Aasen T, Reddy CM, Young M, Brahmbhatt B, et al. Trends in hospitalization, mortality, and timing of colonoscopy in patients with acute lower gastrointestinal bleeding. Endosc Int Open. 2021;9(6):E777-E789.
doi pubmed - Schmulewitz N, Fisher DA, Rockey DC. Early colonoscopy for acute lower GI bleeding predicts shorter hospital stay: a retrospective study of experience in a single center. Gastrointest Endosc. 2003;58(6):841-846.
doi - Strate LL, Syngal S. Timing of colonoscopy: impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol. 2003;98(2):317-322.
doi pubmed - Mosli M, Aldabbagh A, Aseeri H, Alqusair S, Jawa H, Alsahafi M, Qari Y. The diagnostic yield of urgent colonoscopy in acute lower gastrointestinal bleeding. Acta Gastroenterol Belg. 2020;83(2):265-270.
- Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42(1):125-132.
doi - D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49(12):1429-1433.
doi - Pek J, Wong O, Wong ACM. How to address non-normality: a taxonomy of approaches, reviewed, and illustrated. Front Psychol. 2018;9:2104.
doi pubmed - HCUP-NIS [database]: https://www.hcup-us.ahrq.gov/db/vars/discwt/nisnote.jsp6.
- Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342(2):78-82.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


