| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 15, Number 2, April 2022, pages 106-111
An Extremely Rare Case of Rectal Signet Ring Cell Carcinoma
Woo Jin Seoga, Samyak Dhruvb, e, Kuldeepsinh P. Atodariac, Abhishek Polavarapud
aTouro College of Osteopathic Medicine, New York, NY, USA
bMedStar St. Mary’s Hospital, Leonardtown, MD, USA
cAbington Health, Abington, PA, USA
dNorman Regional Health System, Norman, OK, USA
eCorresponding Author: Samyak Dhruv, MedStar St. Mary’s Hospital, Leonardtown, MD, USA
Manuscript submitted March 5, 2022, accepted April 8, 2022, published online January xx, 2022
Short title: Rare Rectal Signet Ring Cell Carcinoma
doi: https://doi.org/10.14740/gr1516
| Abstract | ▴Top |
Signet ring cell carcinoma of the rectum is a rare variant of colorectal cancer. When found, it is often diagnosed in late stages and has poor prognosis. This case depicts a patient with a history of Crohn’s disease who presented to the hospital for perirectal abscesses. During the evaluation of both the abscesses and Crohn’s disease, he was found to have stage IV adenocarcinoma with signet ring cell features. The patient was started on chemotherapy before surgical resection was considered, however, showed little response. The patient’s family eventually pursued hospice care with comfort measures only. Colorectal signet ring cell carcinoma is rare but has poor prognosis as it is diagnosed generally at late and advanced stages. There is a need for more research in earlier detection of these rare cancers.
Keywords: Signet ring cell carcinoma; Rectum; Colorectal cancer; Chemotherapy
| Introduction | ▴Top |
Colorectal cancer (CRC) is the third most common cancer and second leading cause of death from cancer for both men and women. CRC affects approximately 135,439 new patients in the United States of America every year. The incidence of CRC has been declining almost 3% each year due to better screening guidelines and removal of precancerous lesions [1]. Mortality has been declining as well due to better screening and therapy modalities.
A majority of CRCs are adenocarcinomas which can be further differentiated in mucinous, signet ring cell, and other less common subtypes [1]. Signet ring cell carcinoma (SRCC) is defined by the presence of greater than 50% extracellular mucin by volume with the stomach being the most common site [2, 3]. Signet ring cell morphology is a rare pathological finding for CRC, making up less than 1% of CRCs and about 1.39% of rectal cancers [4, 5]. It is associated with advanced tumor stage at presentation in young patients, hence diagnosis is made usually after it has progressed, making prognosis to be poor.
| Case Report | ▴Top |
A 57-year-old male with a past medical history of cerebral palsy, autism, mental disability, and 25-year history of Crohn’s disease with perirectal abscesses presented to the emergency department (ED) complaining of rectal pain. The patient was known to have chronic rectal pain secondary to numerous perirectal abscesses and had these abscesses drained 3 months prior. A computed tomography (CT) scan was done showing increased wall thickening and pericolonic inflammation involving the rectosigmoid colon which was new compared to the CT scan performed a month ago, with several new perianal fluid collections and tracts. In the ED, an incision and drainage of the large perirectal abscess was performed at bedside. The patient was started on antibiotic treatment and was admitted for the same.
During the admission, he had episodes of hematochezia. So, gastroenterology was consulted. A flexible sigmoidoscopy was done for better evaluation of the patient’s Crohn’s disease showing necrotic, friable tissue in the entire rectosigmoid area. A colonoscopy was later done confirming a large colonic mass and necrotic friable ischemic circumferential mucosa (Fig. 1). Pathology report showed fragments of infiltrating poorly differentiated adenocarcinoma with mucinous and signet ring cell features (Fig. 2). Pathology report of the rectosigmoid mass immunohistology was cytokeratin (CK) 7 positive, CK20 positive and SATB2 positive. The tumor was positive for hMLH1, hMSH2, hMSH6, and PMS2. The tumor was shown to have normal nuclear expression of all proteins seen in tumor cell nuclei and no evidence of mismatch repair deficiency by immunohistochemical evaluation. Rectal endoscopic ultrasound (EUS) showed circumferential hypodensity at the anal verge, extending to and breaching the muscularis propria (Fig. 3) along with two lymph nodes (Fig. 4) with malignant features in the deep layers surrounding the malignancy, classifying as T3N2 disease. Of note, patient had no history of CRCs or any other malignancies in the family.
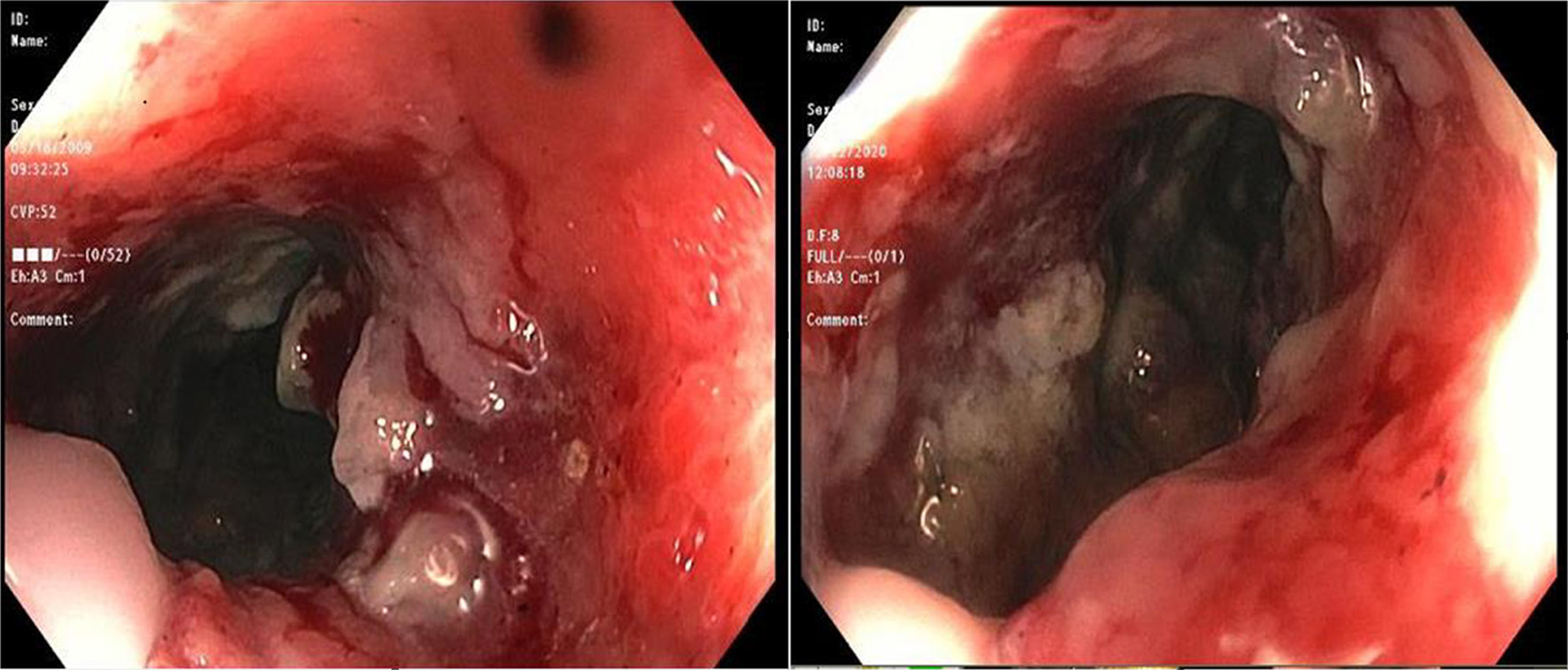 Click for large image | Figure 1. A colonoscopic image showing large circumferential ischemic, necrotic and friable areas in rectum. |
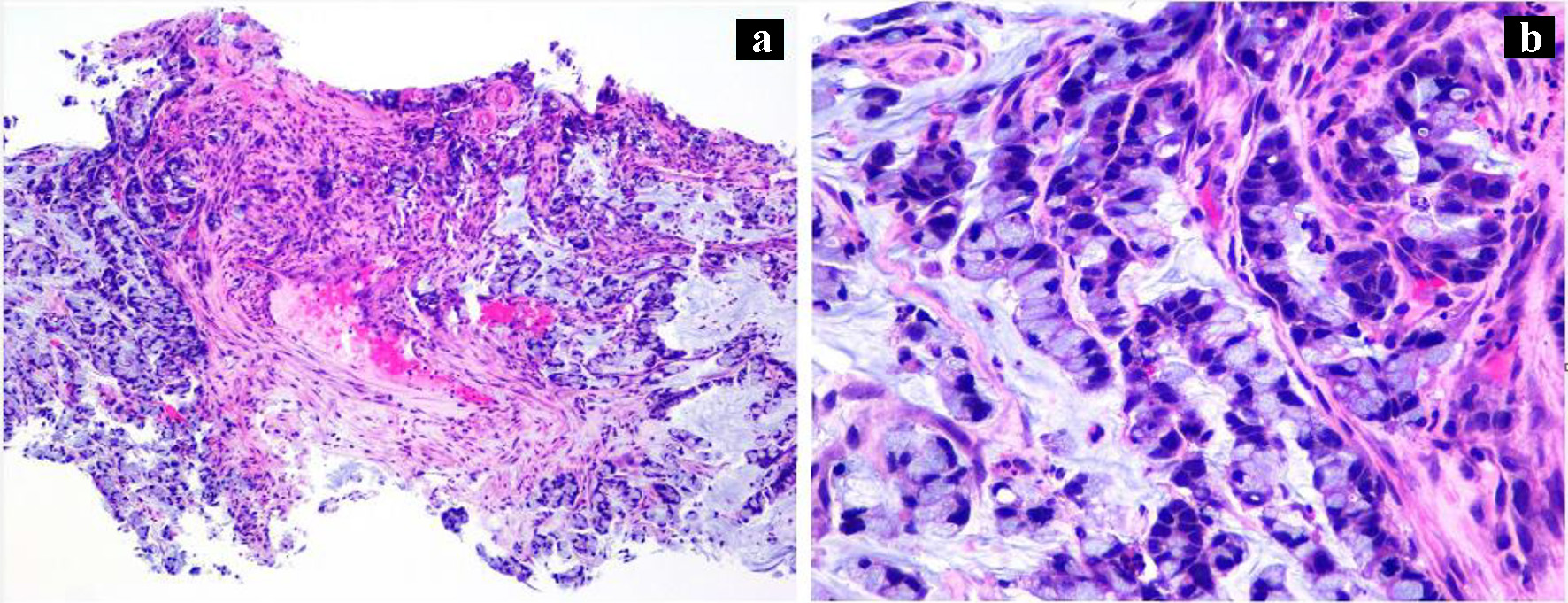 Click for large image | Figure 2. (a) Rectosigmoid biopsy (H&E, × 100) showing infiltrating malignant glands, poorly differentiated in the background of necrotic debris. (b) High power (H&E, × 400) image showing mucinous and signet ring features. |
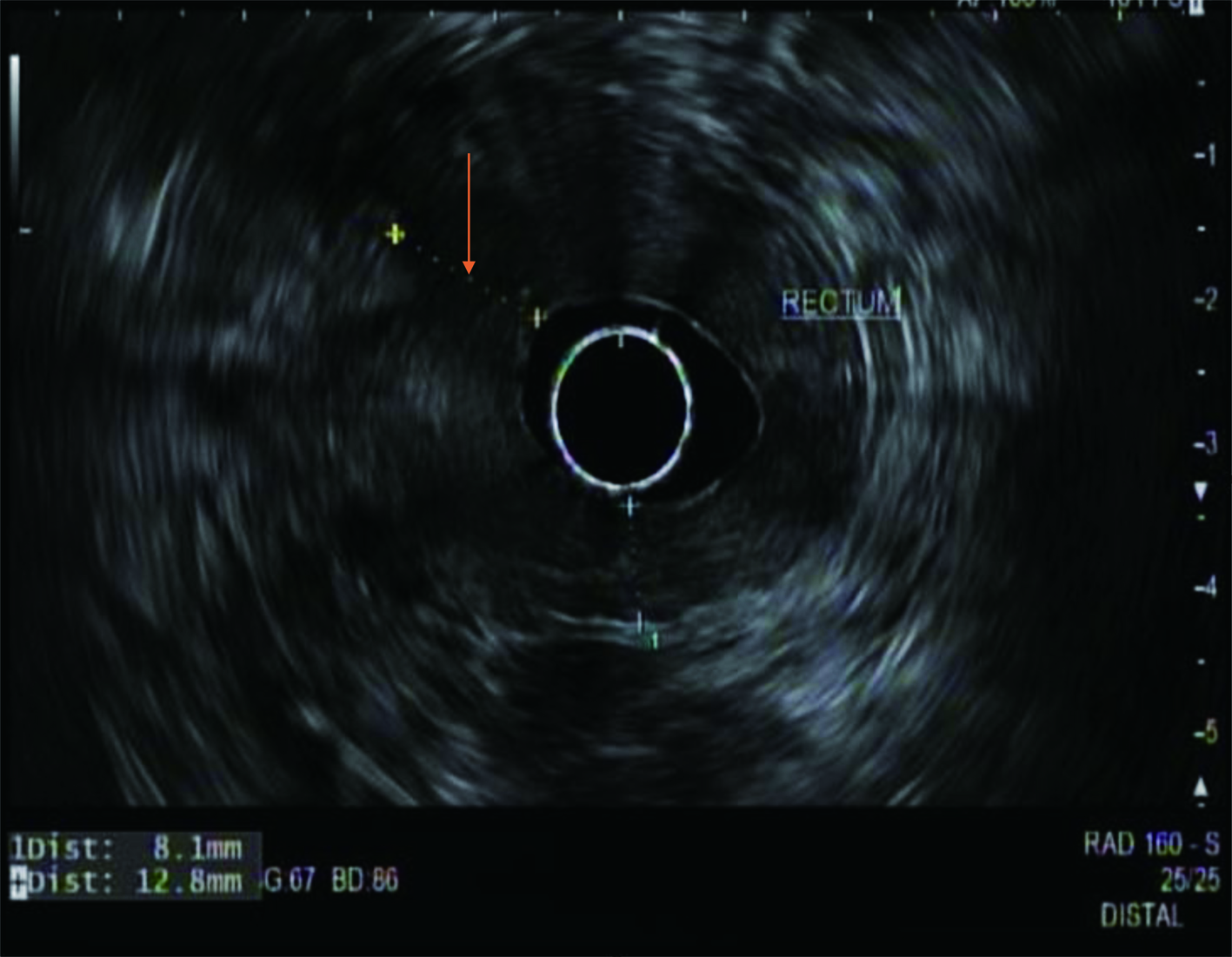 Click for large image | Figure 3. A radial rectal endoscopic ultrasound image showing an irregular hypodense rectal mass (arrow) invading the muscularis propria. |
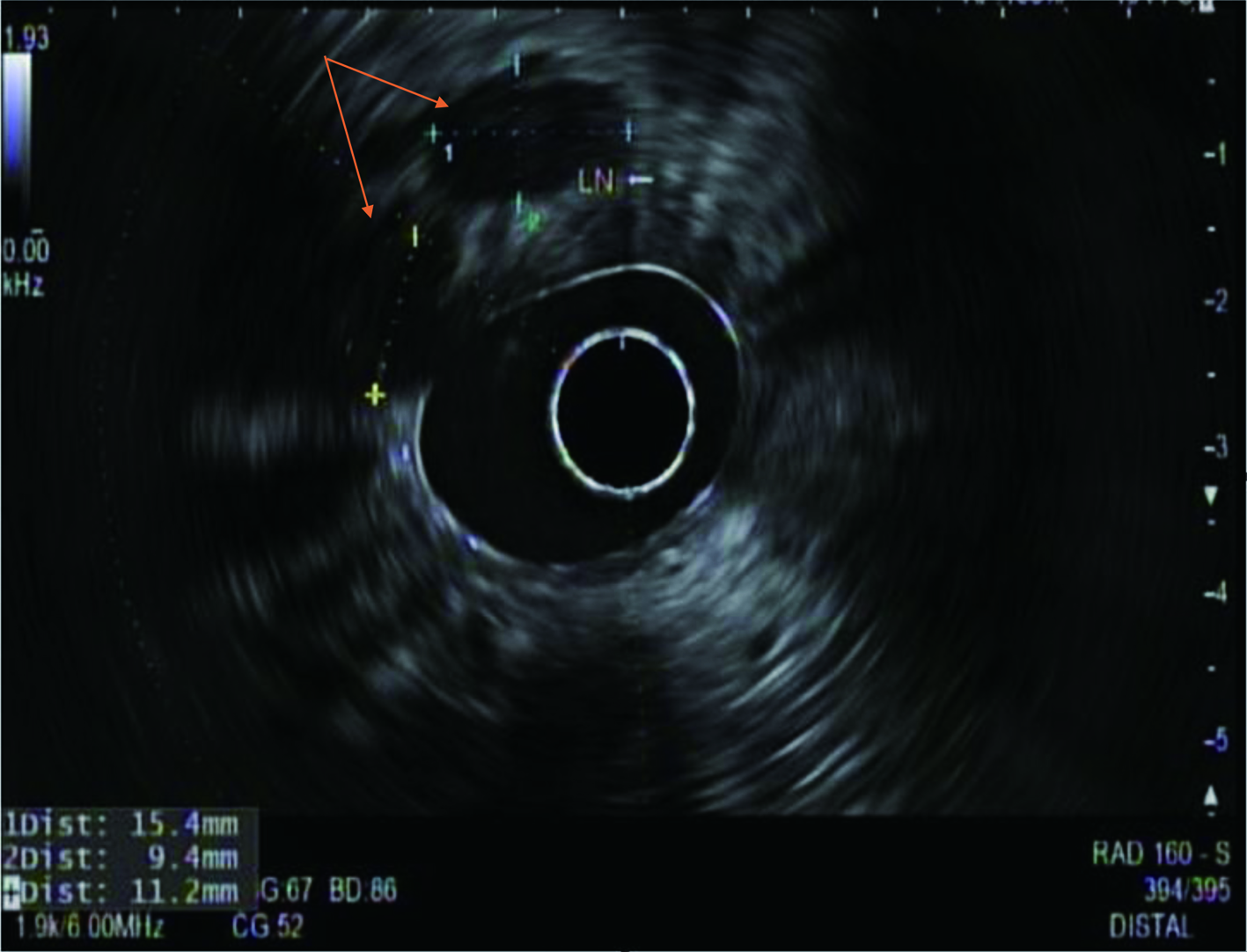 Click for large image | Figure 4. A radial rectal endoscopic ultrasound image showing two enlarged perirectal lymph nodes (arrows) with sizes approximately 15 and 11 mm. |
CT scan of abdomen had shown increased perirectal soft tissue thickening and rectosigmoid wall thickening with pericolonic inflammation extending to the level of the distal descending colon with some evidence of pelvic lymph node enlargement but an absence of any lesions in the stomach. Upon conversation with surgery, mass was likely a rectal based cancer, extending up to the rectosigmoid area. CT of chest showed no evidence of metastatic disease to the lungs or evidence of lesions in esophagus. Magnetic resonance imaging (MRI) of the abdomen was done and showed thickening and abnormal signaling of the posterior urinary bladder wall along with partially visualized distal bilateral hydroureter, suspicious for bladder invasion. There was suspected metastasis in the peritoneum as the tumor was seen to abut the peritoneal reflection with suspected invasion. MRI also showed deposits along the right pelvic sidewall and irregular contour of the levator ani and puborectalis muscle. Decreased signal intensity along the anterior sacrum was also noted. Distance from inferior border of tumor to anal verge was 1 cm. MRI showed at least stage T4a, suspected T4b disease. Rectal examination under anesthesia showed the lesion extending to the prostate and coccyx.
During a multidisciplinary discussion during tumor board, the plan was decided to give chemotherapy with folinic acid (leucovorin)-fluorouracil-oxaliplatin (mFOLFOX6) and then repeat imaging in 3 months to assess for response. Then based on the response, go for surgical resection. Patient soon started chemotherapy with mFOLFOX6 and was to receive chemotherapy every 2 weeks for 3 - 6 months. He was to be reevaluated with imaging and colonoscopy, and based on the response, the patient was to undergo surgical resection. One month later, the patient later developed acute kidney injury secondary to obstructive hydronephrosis. On cystoscopy, it was seen that cancer had spread to the bladder, with the apical portion of bladder encased with cancer and no visualization of the trigone. Biopsy showed fragments of urothelial mucosa showing extensive replacement by mucinous adenocarcinoma, consistent with the involvement by CRC.
One month later, when the patient was on cycle 6 of chemotherapy, a repeat CT scan of the abdomen and pelvis was done, showing evidence of progression with little response. CT of chest remained unremarkable. After discussion with the family, the decision was made to place the patient on hospice with comfort measures only.
| Discussion | ▴Top |
Colorectal SRCC is a rare form of CRC. In a 2012 study (N = 244,794), SRCC made up 1% of colorectal carcinomas [4]. The incidence of SRCC makes up 1.39% of rectal cancers [5]. The clinical symptoms of colorectal SRCC are often similar to those of most CRCs such as abdominal pain, hematochezia, changes in bowel habits, change in appetite and unintentional weight loss [6]. On colonoscopy, the gross appearance of colorectal SRCC often presents with more diffuse circumferential thickening of the bowel wall than the gland forming adenocarcinoma [7]. The location of the mass from SRCC can vary. In a 2020 population-based study (N = 3,278), primary colorectal SRCC was most seen in the cecum-transverse colon (60.49%), following the rectum (19.52%), then descending colon-sigmoid (15.71%) [8]. Primary rectal SRCC often presents in young males [5]. In a retrospective study comparing the clinical data of rectal SRCC with non-signet ring cell mucinous rectal adenocarcinomas (NSMR) and non-mucinous rectal adenocarcinomas (NMR), primary rectal SRCC had a male predominancy of 67.2% and the average age of patients of 48.1 years compared to 57.4 years for NSMR and 62.6 years for NMR [5]. Some general risk factors for CRC such as inflammatory bowel disease, hereditary risk factors such as Lynch syndrome and familial adenomatous polyposis, cigarette smoking, low fiber consumption, low physical activity, and high BMI could be possible risk factors for SRCC [9, 10]. However, due to the limited number of cases and instances of SRCC in patients with no history of familial cancers, there may be other yet unknown risk factors or unidentified correlations to certain environmental risks.
The most common immunostainings for colorectal carcinoma are CK20, CK7 and CDX2. Positive CK20 and negative CK7 are usually the findings for colorectal origin [11]. Positive SATB also favors lower gastrointestinal tract primary [12]. However, these immunostainings may not be specific enough to identify colorectal SRCC. SRCC has a unique immunohistology involving CK20, CDX2, MUC2, and MUC5AC; variable MUC1 and HepPar1 [13]. In colonic SRCC, negative Hep Par 1, homogenous CDX2 nuclear positivity and diffuse cytoplasmic MUC2 and MUC5AC positivity is seen. This staining profile can also be used to differentiate SRCC of gastric origin which will have positive Hep Par 1 and heterogeneous CDX2 staining [13]. Primary urinary bladder SRCC and primary colorectal SRCC overlap for CK7, CK20, and CDX2. However, it was shown that the absence of nuclear positivity for β-catenin is more specific for urinary SRCC [14]. SRCC should also be differentiated from benign processes with signet ring cell change, such as pseudomembranous colitis. In this case, benign signet ring cell change will be positive for E-cadherin and negative for p53 and Ki-67, while SRCC will be positive for p53 and absent/weak positivity of E-cadherin [15]. When comparing genomic alterations for colorectal SRCC versus non-signet ring cell colorectal adenocarcinoma, colorectal SRCC was shown to have low rates of KRAS, PIK3CA and APC mutations while amplified Bcl-2 [16, 17].
Primary colorectal SRCC also has a poor prognosis as it frequently presents at late stages with rapid progression [18]. In a 2016 retrospective study (N = 170), it was seen that 91.2% of patients diagnosed with SRCC presented in stage III and IV of disease [19]. Patients with primary SRCC usually present late, hence had a worse 5-year prognosis in comparison to mucinous or adenocarcinoma of the colon [20]. The overall SCCR survival rates at 1, 2 and 5 years are 73.9%, 36.3%, and 23.3%, respectively [5]. According to a multivariate adjusted survival analysis from the National Cancer Data Base (NCDB), signet ring cell histology for both colon and rectal cancers is associated with 57% higher risk of death relative to non-signet ring adenocarcinoma [4]. Much of the poor prognosis of primary colorectal SRCC is related to its highly malignant biological behavior. It has characteristic peritoneal seeding and low incidence of hepatic metastasis [19, 21]. In a 2016 retrospective study (N = 170), about 86% of patients with stage IV rectal SRCC had isolated peritoneal metastasis. Up to 83% of patients with rectal SRCC had peritoneal metastasis [19]. SRCC was shown to have reduced expression of E-cadherin which causes a loss in epithelial differentiation and acquisition of more invasive and motile properties. This allows the cancer cells to detach from their surroundings and become more metastasizing [22, 23]. The down regulation of E-cadherin reduces cell adhesion of high mucus content areas and promotes tumor scattering [24]. This characteristic contributes to advancing tumor stages and is an indication of its malignant potential. SRCC with peritoneal involvement was shown to have rapid progression and poor prognosis despite systemic and intraperitoneal chemotherapy [25].
Effectiveness of chemotherapy and surgical intervention for colorectal SRCC is limited. There has been evidence that patients with stage III and high microsatellite instability were more responsive to single agent 5-FU adjuvant therapy [26]. However, many of the advanced SRCCs are microsatellite stable and are classified as high grade, hence associated with poor outcome [24]. Surgical resection is offered to these patients but because the patients present very late in disease course, usually only palliative surgery is possible. Surgical intervention is often aggressive and overall survival ranges from 19 to 57 months [21, 27]. Low survival chances and reduced chances of curative resection are due to the delay in diagnosis and possibility of local or distal metastasis [28].
Our patient’s SRCC occurred in the rectum and had metastasized to peritoneum, coccyx, prostate and bladder. Due to late disease of stage IVb and microsatellite stability in our patient, mFOLFOX6 was initiated to delay tumor progression. However, due to some of these late features discussed, the cancer was too aggressive, and chemotherapy was not effective.
Conclusion
SRCC of the rectum is a rare variant of CRC. Compared to other forms of CRC, SRCC has a younger onset and is diagnosed in later stages. It is a highly malignant cancer with poor prognosis even with optimal treatment. This indicates that more research in detection and treatment therapies should be promoted to allow early detection to improve prognosis in these patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed patient consent was obtained to write and publish the case report.
Author Contributions
Woo Jin Seog has written the manuscript. Samyak Dhruv has written the manuscript and edited it. Abhishek Polavarapu has provided the images.
Data Availability
The authors declare that data supporting the findings of the study are available within the article.
| References | ▴Top |
- Recio-Boiles A, Cagir B. Colon cancer. In: StatPearls. Treasure Island (FL), 2022.
- Sung CO, Seo JW, Kim KM, Do IG, Kim SW, Park CK. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol. 2008;21(12):1533-1541.
doi pubmed - Krsteska B, Jovanovic R, Eftimov A, Ilievski B, Hadzi-Mancev D, Osmani B, Kostadinova-Kunovska S. Signet ring cell carcinoma of rectum metastasizing to synchronous renal cell carcinoma: a case report. J Med Case Rep. 2021;15(1):123.
doi pubmed - Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, Rodriguez-Bigas MA, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19(9):2814-2821.
doi pubmed - Chen JS, Hsieh PS, Hung SY, Tang R, Tsai WS, Changchien CR, Lin PY, et al. Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis. 2004;19(2):102-107.
doi pubmed - Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11(1):164.
doi pubmed - Zhou JL, Zhao XY, Lin GL, Qiu HZ, Xiao Y, Wu B, Lu JY, et al. [Clinicopathological characteristics, diagnosis, and treatment of 29 cases of signet ring cell carcinoma of the rectum and sigmoid colon]. Zhonghua Zhong Liu Za Zhi. 2020;42(10):897-902.
- Yang LL, Wang M, He P. Clinicopathological characteristics and survival in colorectal signet ring cell carcinoma: a population-based study. Sci Rep. 2020;10(1):10460.
doi pubmed - Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, Berry DA. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207-1222.
doi pubmed - Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044-2058.
doi pubmed - Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: pathologic aspects. J Gastrointest Oncol. 2012;3(3):153-173.
- Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, Naicker K, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35(7):937-948.
doi pubmed - Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121(6):884-892.
doi - Rao Q, Williamson SR, Lopez-Beltran A, Montironi R, Huang W, Eble JN, Grignon DJ, et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: extended immunohistochemical profiles emphasizing novel markers. Mod Pathol. 2013;26(5):725-732.
doi pubmed - Wang K, Weinrach D, Lal A, Musunuri S, Ramirez J, Ozer O, Keh P, et al. Signet-ring cell change versus signet-ring cell carcinoma: a comparative analysis. Am J Surg Pathol. 2003;27(11):1429-1433.
doi pubmed - Korphaisarn K, Morris V, Davis JS, Overman MJ, Fogelman DR, Kee BK, Dasari A, et al. Signet ring cell colorectal cancer: genomic insights into a rare subpopulation of colorectal adenocarcinoma. Br J Cancer. 2019;121(6):505-510.
doi pubmed - Borger ME, Gosens MJ, Jeuken JW, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Signet ring cell differentiation in mucinous colorectal carcinoma. J Pathol. 2007;212(3):278-286.
doi pubmed - Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol. 1996;3(4):344-348.
doi pubmed - Tamhankar AS, Ingle P, Engineer R, Bal M, Ostwal V, Saklani A. Signet ring colorectal carcinoma: Do we need to improve the treatment algorithm? World J Gastrointest Oncol. 2016;8(12):819-825.
doi pubmed - Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48(6):1161-1168.
doi pubmed - Kaoutar L, et al. Ordinary rectal adenocarcinoma vs. primary rectal signet-ring cell carcinoma. Annals of Oncology. 2014;25:ii101.
doi - Kim HC, Kim HJ, Kim JC. Reduced E-cadherin expression as a cause of distinctive signet-ring cell variant in colorectal carcinoma. J Korean Med Sci. 2002;17(1):23-28.
doi pubmed - Wang R, Ma X, Li Y, He Y, Huang D, Cai S, Peng J. The characteristics and prognostic effect of E-cadherin expression in colorectal signet ring cell carcinoma. PLoS One. 2016;11(8):e0160527.
doi pubmed - Nitsche U, Zimmermann A, Spath C, Muller T, Maak M, Schuster T, Slotta-Huspenina J, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258(5):775-782; discussion 782-773.
doi pubmed - Khan M, Korphaisarn K, Saif A, Foo WC, Kopetz S. Early-onset signet-ring cell adenocarcinoma of the colon: a case report and review of the literature. Case Rep Oncol Med. 2017;2017:2832180.
doi pubmed - Sargent DJ, et al. Prognostic impact of deficient mismatch repair (dMMR) in 7,803 stage II/III colon cancer (CC) patients (pts): A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J Clin Oncol. 2014;32(15_suppl):3507.
doi - Belli S, Aytac HO, Karagulle E, Yabanoglu H, Kayaselcuk F, Yildirim S. Outcomes of surgical treatment of primary signet ring cell carcinoma of the colon and rectum: 22 cases reviewed with literature. Int Surg. 2014;99(6):691-698.
doi pubmed - Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol. 1996;91(10):2195-2199.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


