| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 15, Number 2, April 2022, pages 100-105
Extensive Aortic Thrombosis and Renal Infarction in Association With an Active Flare-Up of Crohn’s Disease
Eltaib Saada, c, Abdalaziz Awadelkarimb, Mohamed Agaba, Akram Babkira
aDepartment of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL, USA
bDepartment of Internal Medicine, Detroit Medical Center/Wayne State University, Detroit, MI, USA
cCorresponding Author: Eltiab Saad, Department of Internal Medicine, Saint Francis Presence Hospital, Evanston, IL, USA
Manuscript submitted January 29, 2022, accepted February 23, 2022, published online March 12, 2022
Short title: Aortic Thrombosis and Renal Infarction in CD
doi: https://doi.org/10.14740/gr1504
| Abstract | ▴Top |
Venous thromboembolism (VTE) is a recognized extraintestinal manifestation of inflammatory bowel disease (IBD), with deep venous thrombosis (DVT) and pulmonary embolism being reported as the most frequent vascular complications in IBD patients. Much less frequently, arterial thromboembolic events may also be associated with greater morbidity and mortality. Aortic mural thrombosis is a rare phenomenon described in patients with IBD that often results in serious consequences such as visceral infarction and acute ischemia of the lower extremities. We described an unusual case of a female patient with Crohn’s disease (CD) who presented with generalized abdominal pain and vomiting. Imaging showed an active flare-up of intestinal CD as well as two mural thrombi in the distal descending thoracic aorta and the abdominal aorta at the level of the left renal artery, respectively, with a left renal infarction. The mesenteric angiogram revealed a patent celiac axis and mesenteric arteries. The patient was therapeutically anticoagulated, and she underwent a right hemicolectomy for the perforated ileal disease. A comprehensive diagnostic workup for hypercoagulability and thrombophilia was negative for an underlying etiology, and the active CD flare-up was considered the main culprit triggering the aortic thrombosis in this reported patient. Our case highlighted the occurrence of aortic thrombosis in a patient with IBD and that entails careful attention. Early recognition and timely management with a multidisciplinary team is the key to improving the outcome of aortic events that coincide with the active flare-up of IBD.
Keywords: Aortic mural thrombosis; Aortic disease; Crohn’s disease; Hypercoagulability
| Introduction | ▴Top |
Patients with inflammatory bowel disease (IBD) are at substantial risk of thromboembolic events [1, 2], with venous diseases accounting for the vast majority (about 85%) of thromboembolism, which usually manifested as deep venous thrombosis (DVT) and pulmonary embolism [1, 2]. Arterial events are very much less frequently encountered with IBD, but they are associated with significantly worse outcomes [1-3]. Aortic mural thrombosis is an exceedingly rare phenomenon being reported among a small series of patients with IBD in the lack of underlying aortic pathologies that usually predispose to thrombosis (such as ulcerated atherosclerotic plaques, aneurysm, and dissection) [1, 3-9]. These aortic thrombi carry an inherently high risk of embolization that may result in serious consequences such as mesenteric infarction and acute ischemia of the lower extremities [1-4]. Herein, we report an unusual case of a female patient with Crohn’s disease (CD) who presented with acute abdomen and was found to have active Crohn’s ileal disease and an extensive mural thrombosis of the aorta that was complicated by a left renal infarction. A comprehensive hematological workup was unrevealing for an underlying cause for aortic lesions, and the aortic mural thrombosis was attributed to the active flare-up of intestinal CD.
| Case Report | ▴Top |
A 61-year-old female patient was brought to the emergency department (ED) with a sudden onset of abdominal pain and bilious vomiting that started 3 days prior to presentation. The pain was associated with left flank pain and dysuria for 1 day. She denied fevers or rigors, diarrhea, and rectal bleeding. The review of systems was negative for other pertinent symptoms. She was diagnosed with CD with stenosing ileal disease, and her regular medications included mesalamine 800 mg twice daily, methotrexate 15 mg weekly, and infliximab 5 mg/kg injection every 8 weeks, but she stopped taking her regular medications due to complex psycho-social issues. Notably, the patient had previously declined elective laparoscopic surgery for the ileal disease. Her past medical history also included bipolar disorders and fibromyalgia. The patient was an active smoker, and she never used oral contraceptives or hormonal replacement therapy.
The patient was confused, febrile to 104 °F, hypotensive to 90/55 mm Hg, and tachycardiac to 140 beats per minute. Abdominal examination revealed right lower quadrant tenderness with focal guarding as well as tenderness over the left costophrenic angle. The rest of the systemic examination was not significant.
Laboratory results reflected an active inflammatory process (white cell count of 15,000/mm3 and C-reactive protein of 122 mg/dL). The urine analysis revealed microscopic hematuria. Following fluid resuscitation, a contrast-enhanced computed tomography (CT) of the abdomen demonstrated active inflammatory changes with mural enhancement at the distal ileum, nearly 20 cm from the ileocecal valve, with a localized perforation and small gas locules and fluid collection (Fig. 1). In addition, a large but non-occlusive thrombus was visualized at the distal descending thoracic aorta (Fig. 2a, b) and a further smaller thrombus was noted at the level of the left renal artery with a wedge-shaped defect in the left kidney consistent with a left renal infarct (Fig. 3a, b). Notably, these aortic thrombi appeared new compared to the most recent imaging. No evidence of atherosclerotic aortic disease was noted. A mesenteric CT angiogram revealed a patent celiac axis and superior mesenteric artery (SMA) in the setting of the perforated ileum, with no evidence of acute or chronic mesenteric ischemia. Bedside vascular examination revealed bilaterally palpable pulsation at the dorsalis pedis (DP), tibialis posterior (TP), popliteal, and femoral arteries with well-perfused warm distal lower extremities. An electrocardiogram showed sinus rhythm with no ischemic changes.
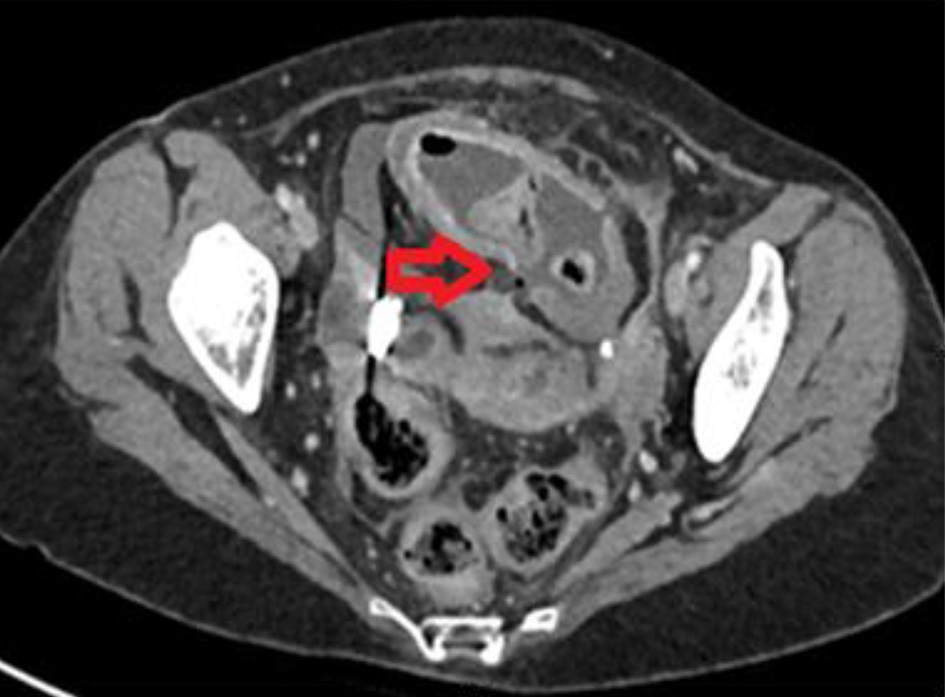 Click for large image | Figure 1. Axial image of contrast-enhanced computed tomography of abdomen with an active flare-up of Crohn’s disease showing a localized perforation at the distal ileum (horizontal red arrow). |
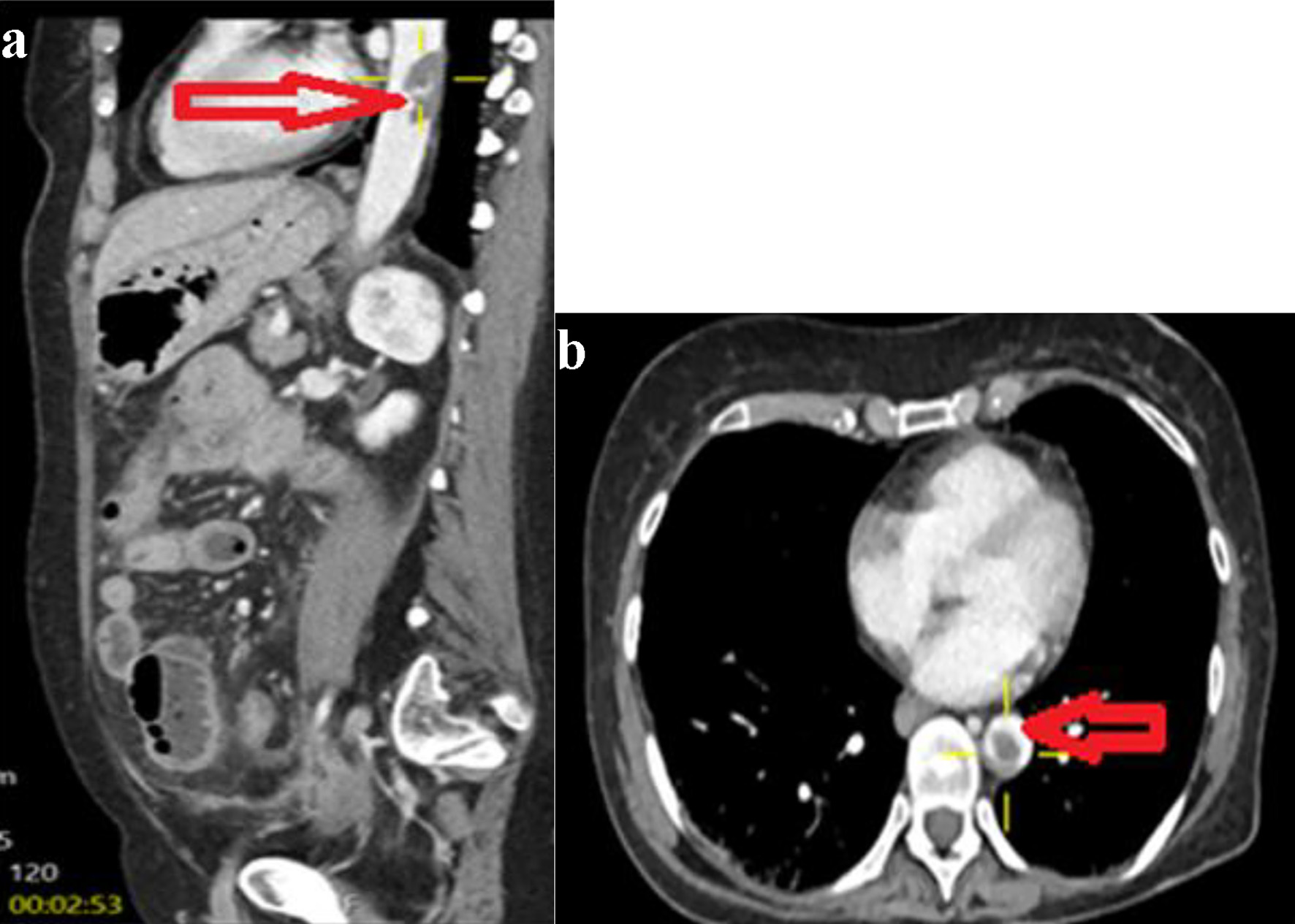 Click for large image | Figure 2. Sagittal (a) and axial (b) image of contrast-enhanced computed tomography of abdomen showing a large thrombus at the distal descending aorta (horizontal arrow). |
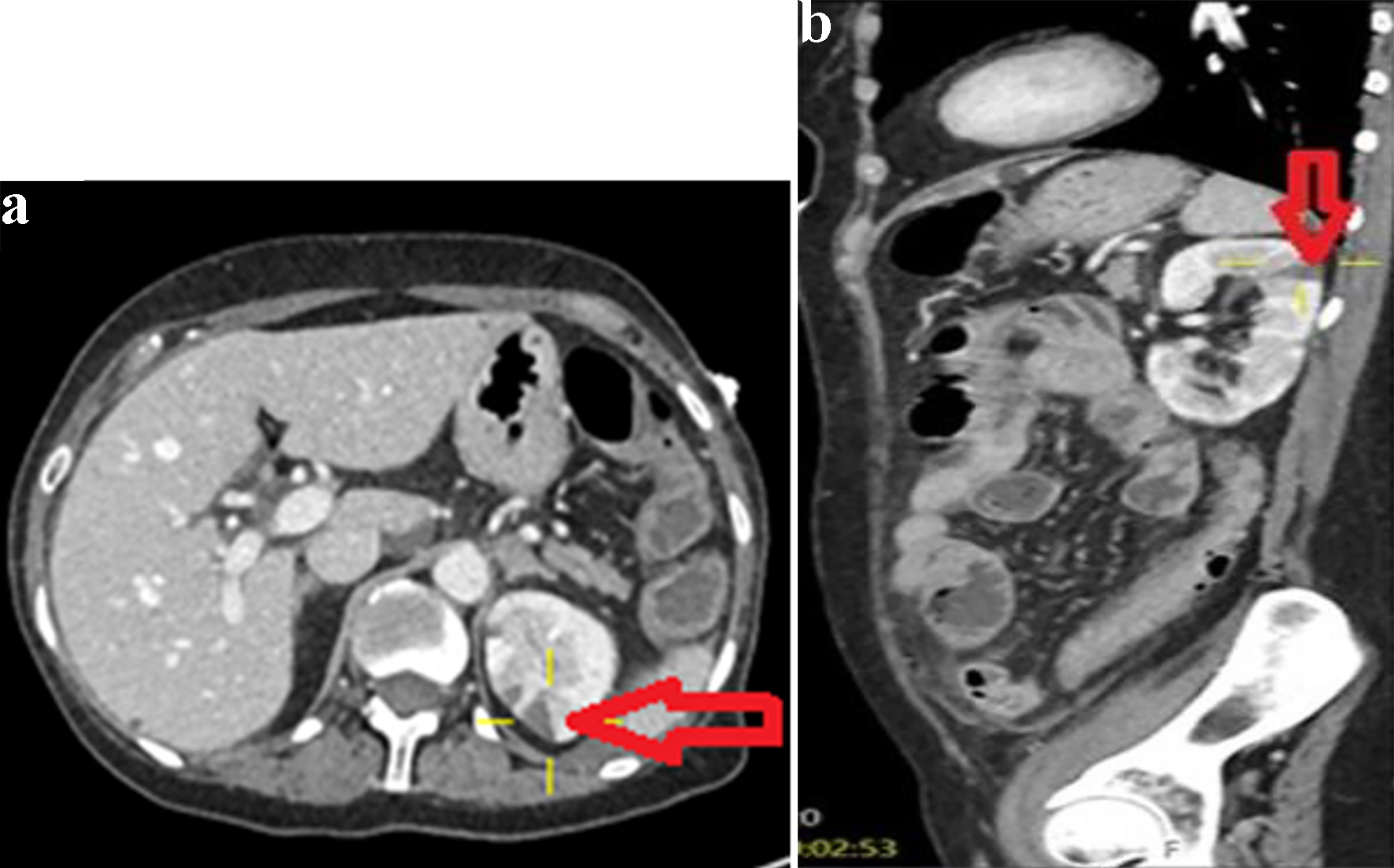 Click for large image | Figure 3. Axial image (a) and sagittal image (b) of contrast-enhanced CT abdomen showing a left renal infarct (arrows). |
Vascular surgery consultation advised therapeutic anticoagulation with heparin infusion for aortic thrombosis and renal infarction. Gastroenterology consultation recommended an operative intervention, and the patient underwent an emergent exploratory laparotomy. Operative findings included a perforation at the distal ileum with a focal abscess and adhesive friable small bowel loops around the perforation site. A careful examination revealed no evidence of proximal small bowel or colonic pathology considering the extensive aortic thrombosis visualized on the preoperative imaging. A right hemicolectomy with an end ileostomy was performed due to a substantial risk of anastomotic leak. Histopathology of the resected bowel segment confirmed an active CD flare-up.
Hematology services were consulted to evaluate for an underlying etiology of aortic thromboembolism. A detailed vasculitic panel (including p-ANCA, c-ANCA, anti-dsDNA, ANF, and complement levels C3 and C4) was negative. Additionally, a thrombophilia screening was also unrevealing (that included lupus anticoagulant, anti-cardiolipin antibodies, antithrombin III, factor V Leiden, protein C, protein S, and lipoprotein (a)). Transesophageal echocardiography (TEE) with bubble studies showed normal right and left ventricular function with no intracardiac thrombi or masses, and there was no evidence of intracardiac shunts. Continuous cardiac monitoring with an event recorder depicted only very occasional (< 1%) premature atrial contractions (PACs).
The patient was discharged on an oral anticoagulant for 6 months. A repeat CT aortogram 2 months post-operatively revealed an interval decrease of both thrombi sizes with no new lesions.
| Discussion | ▴Top |
Aortic thrombosis represents an exceedingly rare entity associated with IBD with only a limited number of cases being reported in the reviewed literature [3, 5-19]. This event may be associated with worse outcomes compared to venous thromboembolism (VTE) [1-4]. The management involves a multidisciplinary team of gastroenterologists and IBD specialists, hematologists, and often colorectal and vascular surgeons [3].
Pathophysiology of arterial thromboembolism among patients with IBD remains poorly understood [2-4]. Many theories were postulated, and a multifactorial complex process of interaction between a number of factors was assumed [1, 3, 4]. The chronic inflammatory status and autoimmune activity constitute major triggers of the downstream release of certain procoagulant cytokines (tissue-necrosis factor and interleukin 6 (IL-6)) that trigger endothelial cells injury and initiate thrombotic cascades by enhancing expression of CD40 ligand on platelets surface [1-7]. This hypercoagulable state is further augmented by a range of prothrombotic conditions that encompass disease-associated complications (such as dehydration, reactive thrombocytosis, and vitamins deficiencies that result in elevated homocysteine levels), treatment-related sequelae (such as steroid use, recurrent surgeries with post-operative states, and prolonged immobilization), and exposure to environmental risks (like active smoking and combined oral contraceptive (COCP) use) [1-4].
The review of the medical literature identified a total of 21 cases of IBD-associated aortic mural thrombosis [3, 5-19]. Table 1 briefly summarizes the patient demographics, clinical presentation, thrombosis site, management, and outcome.
 Click to view | Table 1. Summary of Cases of IBD-Associated Aortic Mural Thrombosis |
The majority of cases were associated with an active IBD status [5, 7-10], in accordance with the literature that reported a markedly reduced level of natural anticoagulants and an upregulated expression of various procoagulants cytokines during disease activity [1, 3]. Furthermore, complicated Crohn’s disease (with abscess perforation or enteric fistulae) was strongly associated with systemic endotoxemia that further activates coagulation cascades [20]. Nevertheless, there was only one case that was described with quiescent disease [14].
Most aortic thrombi related to IBD involved infrarenal aorta [7, 8, 10-12, 14, 17], followed by the aortoiliac segment [3, 9, 14, 15]. Interestingly, aortic arch thrombosis was found in four cases, either as a solitary lesion [13, 16] or in association with concurrent aortic thrombi [18, 19].
Aortic mural thrombosis results in a spectrum of devastating sequelae such as small bowel infarction [5], colonic ischemia [7] and splenic infarction [6] due to mesenteric emboli, renal infarction complicating renal arteries emboli [5, 12], and acute ischemia of the upper extremities [19] and lower extremities [10, 11, 16, 17].
Therapeutic anticoagulation was employed in most patients in conjunction with medical treatment of IBD flare-up and surgical or endovascular interventions for complications (such as acute extremity ischemia or bowel infarction) that were tailored to each individualized case [5-19].
The Canadian Association of Gastroenterology guidelines for IBD-related venous thromboembolism recommend therapeutic anticoagulation for at least 3 months post-IBD exacerbation [21], but there is no clear consensus yet on the standard treatment duration for the associated arterial disease including aortic events [1, 3].
The outcome of aortic thrombosis associated with IBD included a complete thrombus resolution [3, 10, 15, 17], extremity amputation [9, 19], and death from multisystemic failure due to bowel ischemia [5, 7].
Conclusion
Aortic mural thrombosis is an exceedingly rare extraintestinal complication associated with the flare-up of CD. This condition deserves careful attention as it may result in devastating consequences such as mesenteric infarction or extremities ischemia. Early recognition and timely management are essential to improving the outcome of aortic thromboembolic events.
Acknowledgments
The authors would like to acknowledge the Department of Radiology at Saint Francis Presence Hospital for providing valuable input to this case presentation.
Financial Disclosure
The authors confirm that there is no funding for this case report.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Informed Consent
Informed written consent was obtained from the patient to write and publish their case as a case report with all accompanying clinical and radiological images. No ethical clearance is deemed required for case report writing as per our local Research Board.
Authors Contributions
ES and AA contributed to the conceptualizing and writing the first manuscript. MA and AB have performed the critical review and editing of the final draft. All authors were involved in the clinical management of the reported patients. All authors agreed to the final draft submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Pezold M, Pergamo M, Rockman C, Lugo J. Presentation and management of arterial thromboembolisms during active inflammatory bowel disease: case series and literature review. Ann Vasc Surg. 2020;67:532-541.e3.
doi pubmed - Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53(4):542-548.
doi pubmed - Novacek G, Haumer M, Schima W, Muller C, Miehsler W, Polterauer P, Vogelsang H. Aortic mural thrombi in patients with inflammatory bowel disease: report of two cases and review of the literature. Inflamm Bowel Dis. 2004;10(4):430-435.
doi pubmed - Di Fabio F, Obrand D, Satin R, Gordon PH. Intra-abdominal venous and arterial thromboembolism in inflammatory bowel disease. Dis Colon Rectum. 2009;52(2):336-342.
doi pubmed - Khan AB, Bhat N, Hassan A, Al Saied G. Aortic thrombosis complicating Crohn disease: an unusual complication. Can J Surg. 2009;52(5):E182-184.
- Kok HK, Maguire S, Corr A, Sadlier M, Patchett S, Harewood G. Intra-aortic mural thrombosis and splenic infarction in association with ulcerative colitis. Ir J Med Sci. 2012;181(3):377-379.
doi pubmed - Talbot RW, Heppell J, Dozois RR, Beart RW, Jr. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61(2):140-145.
doi - Perler BA, Kadir S, Williams GM. Aortic mural thrombus in young women: premature arteriosclerosis or separate clinical entity? Surgery. 1991;110(5):912-916.
- Novotny DA, Rubin RJ, Slezak FA, Porter JA. Arterial thromboembolic complications of inflammatory bowel disease. Report of three cases. Dis Colon Rectum. 1992;35(2):193-196.
doi pubmed - Hahn TL, Dalsing MC, Sawchuk AP, Cikrit DF, Lalka SG. Primary aortic mural thrombus: presentation and treatment. Ann Vasc Surg. 1999;13(1):52-59.
doi pubmed - Lehmann JM, Shnaker A, Silverberg D, Dayan K, Witz M. Arterial thromboembolism from a distal aortic thrombus in a patient with Crohn's disease. Isr Med Assoc J. 2001;3(3):226-227.
- Szychta P, Reix T, Sevestre MA, Brazier F, Pietri J. Aortic thrombosis and ulcerative colitis. Ann Vasc Surg. 2001;15(3):402-404.
doi pubmed - Grothues F, Welte T, Grote HJ, Roessner A, Klein HU. Floating aortic thrombus in systemic aspergillosis and detection by transesophageal echocardiography. Crit Care Med. 2002;30(10):2355-2358.
doi pubmed - Delay C, Schwein A, Lejay A, Gaertner S, Aleil B, Thaveau F, Georg Y, et al. Aortitis and aortic occlusion in Crohn disease. Ann Vasc Surg. 2015;29(2):365.e5-9.
doi pubmed - Singh K, Marco SA, Wang ML, Milone L, Deitch JS. Aortoiliac thrombi in inflammatory bowel disease. Ann Vasc Surg. 2012;26(8):1128.e11-14.
doi pubmed - Elder K, Hawi R, Al Solaiman F. Inflammatory bowel disease-related thoracic aortic thrombosis. South Med J. 2010;103(2):172-174.
doi pubmed - Leblanc S, Lloret Linares C, Cacheux W, Mouthon L, Chaussade S. Aortic thrombosis in young women with Crohn's disease receiving adalimumab: report of two cases. Inflamm Bowel Dis. 2011;17(3):862-863.
doi pubmed - Sinapi I, Hammer F, Hainaut P. Aortic mural thrombi associated with ulcerative colitis. Acta Clin Belg. 2010;65(1):54-55.
doi pubmed - Stordiau GE, Alecha JS, Goni S, Zozaya JM, Centeno R, Tricas JM. Arterial thromboembolism from aortic floating thrombus in a patient with Crohn's Disease. J Crohns Colitis. 2011;5(2):169-170.
doi pubmed - Cybulsky MI, Chan MW, Movat HZ. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Pathology Reviews. 1989;1989:103-116.
doi - Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, Geerts W, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835-848.e6.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


