| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 14, Number 2, April 2021, pages 104-111
Gastric Antral Vascular Ectasia: Trends of Hospitalizations, Biodemographic Characteristics, and Outcomes With Watermelon Stomach
Asim Kichlooa , Dhanshree Solankib
, Jagmeet Singhc
, Dushyant Singh Dahiyaa, g
, Darshan Lalc
, Khwaja Fahad Haqd
, Michael Aljadaha
, Darshan Gandhie
, Shantanu Solankic
, Hafiz Muzaffar Akbar Khanf
aDepartment of Internal Medicine, Central Michigan University College of Medicine, Saginaw, MI, USA
bDepartment of Internal Medicine, Rutgers University, New Brunswick, NJ, USA
cDepartment of Internal Medicine, Geisinger Commonwealth School of Medicine, Scranton, PA, USA
dDivision of Gastroenterology, Henry Ford Hospital, Detroit, MI, USA
eDepartment of Radiology, Hartford Healthcare, Hartford, CT, USA
fDivision of Gastroenterology, Guthrie Robert Packer Hospital, Sayre, PA, USA
gCorresponding Author: Dushyant Singh Dahiya, Department of Internal Medicine, Central Michigan University College of Medicine, 1000 Houghton Avenue, Saginaw, MI 48602, USA
Manuscript submitted March 20, 2021, accepted April 8, 2021, published online April 21, 2021
Short title: Trends in Hospitalizations for GAVE
doi: https://doi.org/10.14740/gr1380
| Abstract | ▴Top |
Background: Gastric antral vascular ectasia (GAVE) syndrome is a rare but significant cause of acute or chronic gastrointestinal (GI) bleeding, particularly in the elderly. The primary objective of this study was to determine the biodemographic characteristics, adverse outcomes, and the impact of GAVE hospitalizations on the US healthcare system.
Methods: This retrospective database cross-sectional study used the National Inpatient Sample (NIS) from 2001 to 2011 to identify all adult hospitalizations with a primary discharge diagnosis of GAVE, with and without hemorrhage, using the International Classification of Diseases, Ninth Revision (ICD-9) codes. Individuals less than 17 years of age were excluded from the study. The outcomes included biodemographic characteristics, comorbidity measures, and inpatient mortality and the burden of the disease on the US healthcare system in terms of healthcare cost and utilization.
Results: We noted an increase in the total hospitalizations for GAVE from 25,423 in 2001 to 44,787 in 2011. Furthermore, GAVE hospitalizations with hemorrhage rose from 19,168 in 2001 to 27,679 in 2011 while GAVE hospitalization without hemorrhage increased from 6,255 in 2001 to 17,108 in 2011. We also noted a female predominance, the proportional trend of which did not show significant difference from 2001 to 2011. For GAVE hospitalizations, the inpatient mortality decreased from 2.20% in 2001 to 1.73% in 2011. However, the cost of hospitalization increased from $11,590 in 2001 to $12,930 in 2011. After adjusting for possible confounders, we observed that the presence of hemorrhage in GAVE hospitalizations was associated with an increased risk of mortality (odds ratio (OR): 1.27; 95% confidence interval (CI): 1.1 - 1.46; P = 0.001).
Conclusions: For the study period, the total number of GAVE hospitalizations increased with an increase noted in the proportion of GAVE hospitalizations without bleeding, reflecting an improvement in diagnostic and therapeutic techniques. Although inpatient mortality for GAVE slightly decreased, we noted a significant increase in the cost of care likely secondary to increased use of advanced and expensive interventions.
Keywords: Gastric antral vascular ectasia; Hemorrhage; Outcome; Mortality; Predictors of mortality; Nationwide inpatient sample; Comorbidities; Cost of care
| Introduction | ▴Top |
Gastric antral vascular ectasia (GAVE) syndrome was first described by Rider et al in 1953 [1]. It was further investigated 25 years later by van Vliet et al in a case series of three patients. In those patients, van Vliet et al reported the presence of a spotty red pre-pyloric antrum [2]. Based on the endoscopic view of the gastric mucosa, GAVE is known by names such as “watermelon stomach” which is characterized by the presence of red spots organized in stripes radially moving away from pylorus and “honeycomb stomach” as these red spots are arranged in a diffused fashion [3, 4]. Although these red spots, also known as angioectasias, are most frequently noted in the gastric antrum, they can be found anywhere in the gastrointestinal (GI) tract [5]. As per current literature, GAVE accounts for almost 4% of all non-variceal GI bleeding and 6% of upper GI bleeds in patients with liver cirrhosis [6]. However, there is significant gap in knowledge about the disease entity as there have been no population-based studies in the USA. Therefore, we used the National Inpatient Sample (NIS) database to analyze biodemographic characteristics, hospitalization rates, and comorbidities associated with GAVE hospitalizations. We also determine inpatient mortality, predictors of inpatient mortality and attempt to understand the economic impact of the disease on the US healthcare system in terms of healthcare costs and resource utilization. Furthermore, we strongly advocate for the need for additional large prospective multi-center study to further investigate GAVE, particularly in an elder demographic.
| Materials and Methods | ▴Top |
Source of data
We analyzed the NIS database from 2001 to 2011 to identify hospitalizations with a primary discharge diagnosis of GAVE, with and without hemorrhage, using the International Classification of Diseases, Ninth Revision (ICD-9) codes. NIS, designed by the Agency for Healthcare Research and Quality (AHRQ), is the largest all-payer inpatient database in the USA. NIS is designed to provide a 20% classified sample of community health care systems in the USA which correlates to national estimates. The data are compiled yearly and contain discharge information from over 1,200 hospitals located across the states [7]. The internal validity of the database is guaranteed by the annual data quality assessments, while comparisons with data sources like the American Hospital Association (AHA) Annual Survey of Hospitals, National Hospital Discharge Survey from the National Center for Health Statistics, and Medicare Provider and Analysis Review (MedPAR) inpatient data from the Centers for Medicare and Medicaid Services guarantee the external validity [8].
Study design
Our study was exempt from Institutional Review Board (IRB) as it did not have any identifiable patient data. This cross-sectional retrospective study queried the NIS database from 2001 to 2011 to identify GAVE hospitalizations, with and without hemorrhage, using the ICD-9 codes (Fig. 1). Individuals less than 17 years of age were excluded from the study. We determined the total number of hospitalizations, biodemographic characteristics, comorbidity measures, adverse outcomes, and the burden of the disease on the US healthcare system. The NIS data were merged with cost-to-charge ratio (CCR) files to calculate estimated cost of hospitalizations [9].
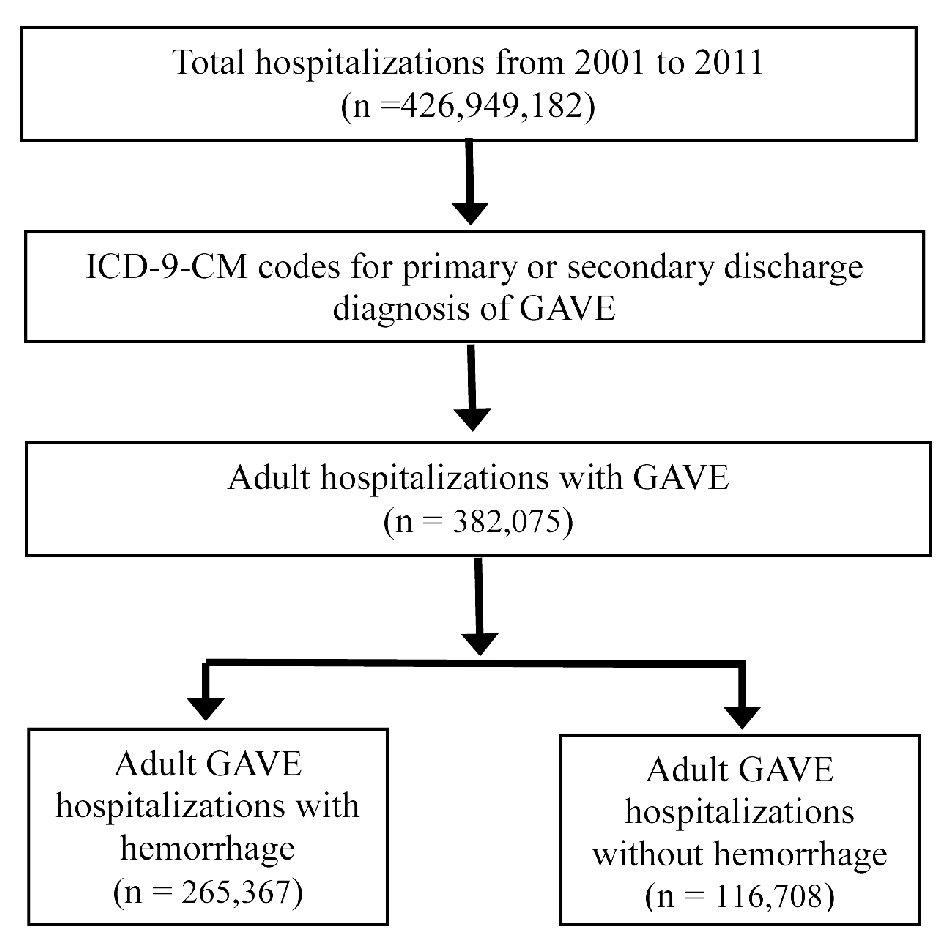 Click for large image | Figure 1. Sequential derivation of the study population for GAVE hospitalizations from 2001 to 2011. GAVE: gastric antral vascular ectasia; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification. |
Variable and statistical analysis
We used the SAS 9.4 (SAS Institute Inc., Cary, NC, USA) software for statistical analyses. The analysis was performed using the hospital-level discharge weights provided by NIS to obtain national estimates as NIS represents a 20% stratified random sample of US hospitals. GAVE hospitalizations per million were calculated by dividing the number of these hospitalizations per year by the US census population from 2001 to 2011. GAVE hospitalizations were also calculated in subgroups of age (18 - 34, 35 - 49, 50 - 64, 65 - 79, and ≥ 80 years old), gender, race (White, Black, Hispanic, Asian or Pacific Islander, Native American, and other), insurance status (Medicare, Medicaid, private insurance, self-pay/other), hospital location (Northeast, Midwest, South, West), and teaching status of the hospital. We used the Cochran-Armitage trend test to calculate the trends for categorical variables and Wilcoxon rank sum test to assess continuous variables. These methods have been used in previous NIS-based studies [8, 10, 11]. We used the multivariate logistic regression model to determine predictors of mortality. A P value less than 0.05 was considered statistically significant.
| Results | ▴Top |
Demographics
We noted a significant increase in total number of GAVE hospitalizations for the study period (Fig. 2a) from 25,423 in 2001 to 44,787 in 2011 (Table 1). GAVE hospitalizations with hemorrhage rose from 19,168 in 2001 to 27,679 in 2011, while GAVE hospitalization without hemorrhage increased from 6,255 in 2001 to 17,108 in 2011 (Table 1). Furthermore, we noted a significant female (Fig. 2b) and White predominance throughout the study period (P < 0.001). The 65 - 79 age group had the highest number of GAVE hospitalizations for the study period (Table 1).
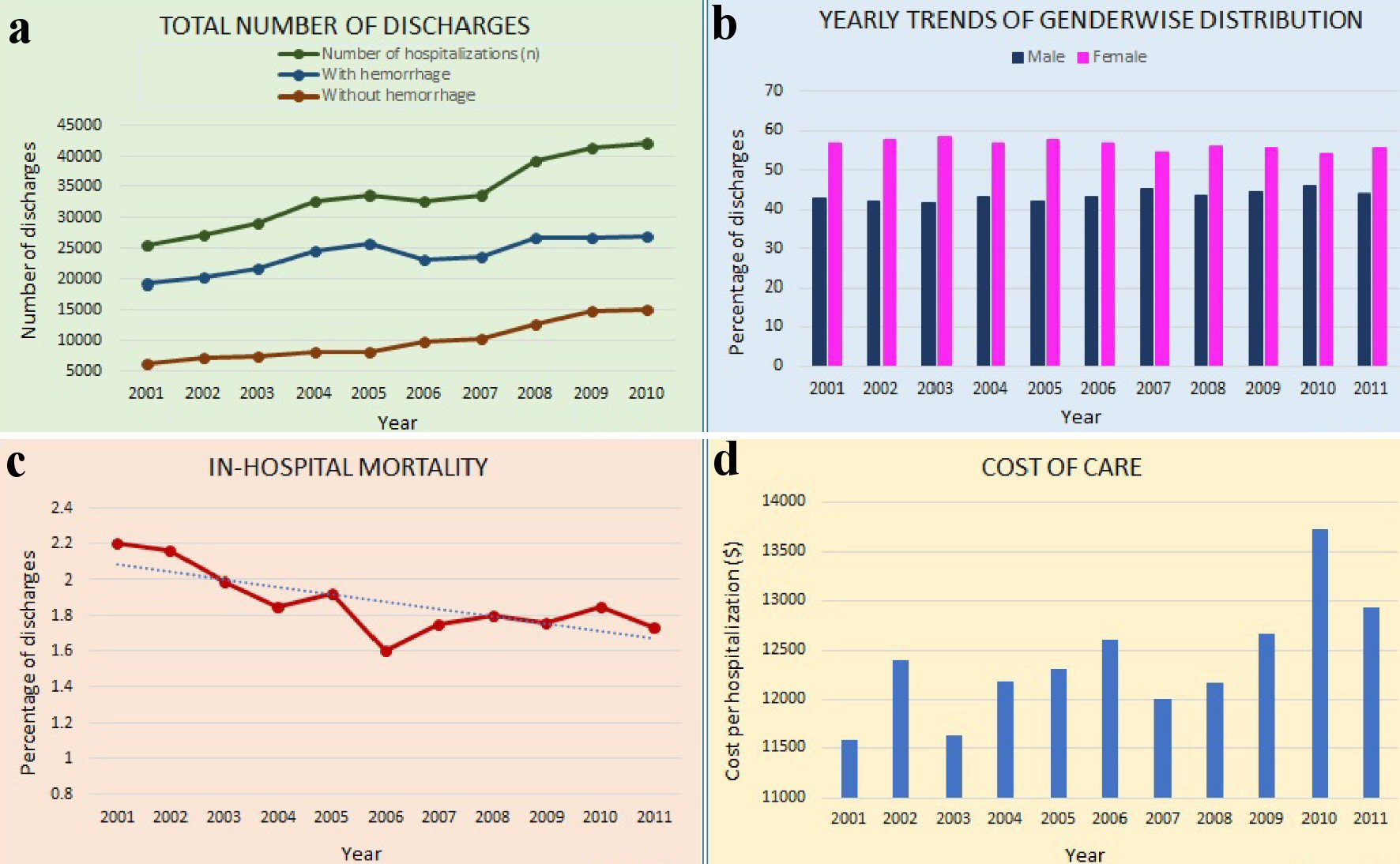 Click for large image | Figure 2. Description of characteristics of GAVE hospitalizations. (a) Total number of discharges with GAVE. (b) Gender distribution for GAVE hospitalizations. (c) Inpatient mortality for GAVE hospitalizations. (d) Cost of care for GAVE hospitalizations. GAVE: gastric antral vascular ectasia. |
 Click to view | Table 1. Baseline Characteristics of Gastric Antral Vascular Ectasia (GAVE) Hospitalizations From 2001 to 2011 |
AHRQ comorbidities
As per our analysis for 2011, the two most common diagnosis associated with GAVE hospitalizations included hypertension and renal failure (Table 2). For GAVE hospitalizations, the proportion of patients with hypertension increased significantly from 39.6% in 2002 to 69.6% in 2011 (P < 0.0001, Table 2) and the proportion of patients with renal failure increased from 24.31% in 2002 to 50.87% in 2011 (Table 2). Additionally, we also noted an increase for other comorbid conditions such as liver disease, diabetes mellitus, obesity, and peripheral vascular disease (Table 2).
 Click to view | Table 2. Comorbidities Associated With Gastric Antral Vascular Ectasia (GAVE) Hospitalizations From 2002 to 2011 |
All-cause inpatient mortality
We noted a decrease in inpatient mortality rates for GAVE hospitalizations from 2.20% in 2001 to 1.73% in 2011 (P < 0.0001, Fig. 2c).
Cost of care
The cost of management for GAVE hospitalizations increased significantly (Fig. 2d) from $11,590 in 2001 to $12,930 in 2011 after adjusting for inflation (Table 1).
Predictors of mortality
After adjusting for possible confounders such as age, sex, race and Elixhauser comorbidity index for GAVE hospitalizations, we observed that the presence of hemorrhage was associated with increased risk of mortality (OR 1.27; 95% CI: 1.1 - 1.46; p = 0.001) (Table 3). Furthermore, every one-point increase in the Elixhauser co-morbidity index significantly increased the risk of inpatient mortality (odds ratio (OR): 1.18; 95% confidence interval (CI): 1.14 - 1.22; P < 0.0001). Additionally, Hispanics admitted to the hospital with GAVE had a high risk of mortality (OR: 1.25; 95% CI: 1.01 - 1.55; P = 0.04, Table 3). However, increasing age, hospital bed size, teaching status of the hospital, and type of insurance showed no significant association with risk of mortality.
 Click to view | Table 3. Predictors of Mortality for Gastric Antral Vascular Ectasia (GAVE) Hospitalizations From 2001 to 2011 |
| Discussion | ▴Top |
GAVE is an uncommon but often severe cause of acute or chronic GI bleeding. It is usually seen in elderly patients and comprises of almost 4% of all non-variceal GI bleeding in the general population. The pathogenesis of this acquired disease is currently not well understood. However, it appears to be multifactorial given dissimilar endoscopic appearances such as watermelon and honeycomb patterns [5]. Studies have implicated the role of mechanical stress from gastric peristalsis, abnormal antral motor response to food particles, catecholamines, and autoantibodies in the pathogenic process [12-14]. Histologically, the condition is characterized by the presence of vascular ectasia of mucosal capillaries, intravascular fibrin thrombosis, fibrohyalinosis, and spindle cell proliferation [5, 15]. From a clinical perspective, there is significant paucity of data on GAVE in an inpatient setting. Hence, this study was designed to identify the epidemiology, associated comorbidities, and adverse outcomes of GAVE hospitalizations while detailing the disease burden on the US healthcare system.
In our study, we noted a significant increase in total number of GAVE hospitalizations with and without hemorrhage. The number of hospitalizations increased from 25,423 in 2001 to 44,787 in 2011 accounting for a 76% increase in a span of just 11 years. This may be because of increased awareness and widespread availability of newer diagnostic tools and techniques across the USA. Additionally, patients with GAVE are known to have higher recurrence of bleeding after endoscopic intervention, and blood loss in these patients may continue for a chronic duration. Literature reports that up to 62% of patients with GAVE may remain transfusion dependent [16]. Hence, we believe that it is imperative to identify patient demographics and establish risk factors for GAVE hospitalizations to design a robust management plan to reduce morbidity and mortality.
It has been well established that GAVE is more commonly seen in women compared to men [17]. As per literature, the most common presenting sign for GAVE in women is anemia secondary to chronic gastric bleeding [18, 19]. In our study population, a female predominance was noted which was in line with current literature, but there is no established pathophysiologic cause for this female predominance [18, 19]. However, it is worth noting that studies investigating the use of antineoplastic drugs in women with gastric adenocarcinoma and gastric stromal tumors have reported the presence of GAVE [20, 21]. This suggests that there may be a neoplastic or drug-induced pathophysiology for GAVE in these women [20, 21].
In literature, a retrospective cohort study by Smith et al on 135 patients revealed a significant correlation of GAVE with liver disease, diabetes mellitus, body mass index (BMI), and vascular disease [16]. Similarly in our study, we noted increasing proportion of patients with comorbidities such as hypertension, renal failure, cirrhosis, diabetes mellitus, obesity, and peripheral vascular disease in these hospitalizations (Table 2). Comorbidities that affect vascular remodeling may have a key role to play in the pathogenesis of GAVE.
Moreover, studies have reported an all-cause inpatient mortality of around 1.4% for GAVE patients. In our study, the mortality rate for GAVE hospitalizations in 2011 was noted to be 1.73%, which was a downtrend from the 2.20% from 2001. This may be because of wider availability of diagnostic tools and improvement in therapeutic techniques over the years. Additionally, the lower rates of morality may in part be due to an increasing prevalence of GAVE without hemorrhage compared to GAVE with hemorrhage, which is known to be major contributor to the mortality rate. Furthermore, literature has reported higher rates of mortality for GAVE patients refractory to traditional therapies undergoing surgical intervention. A 30-day mortality of 50% and a perioperative mortality of 7.4% have been reported in patients undergoing surgery [6]. However, there is paucity of large-scale study describing mortality rates in patients undergoing endoscopic or pharmacologic interventions.
Although GAVE is a relatively rare cause of upper GI bleeding, it can cause significant and severe bleeding leading to adverse outcomes such as mortality, especially in elderly patients with multiple comorbidities [22]. In this study, we attempted to identify the predictors of inpatient morality for GAVE hospitalizations. After adjusting for confounders, we report that the presence of hemorrhage and every one-point increase in Elixhauser comorbidity index was associated with significantly increased risk of inpatient mortality for GAVE hospitalizations. Furthermore, literature reports a higher prevalence of GAVE in Caucasians followed by the African American population [23]. However, there exists a significant gap in knowledge in terms of the racial distribution and subset of the population at the highest risk of mortality from GAVE. In our study, we found that the Hispanic population had a high risk of inpatient mortality, but we did not find statistical significance in the mortality rates for other races. Additionally, increasing age, hospital bed size, teaching status of the hospital, and type of insurance showed no significant association with the risk of mortality.
From a treatment perspective, endoscopic interventions including argon plasma coagulation (APC), endoscopic band ligation, neodymium-doped: yttrium-aluminum-garnet (Nd: YAG) laser treatment, cryotherapy, and radiofrequency have all been demonstrated in the literature [24-26]. The data supporting endoscopic resolution of GAVE are limited. In a study conducted by Garg et al in 2017, endoscopic resolution was reported in only 40% of GAVE patients undergoing APC [27]. Moreover, recurrence of bleeding has been reported in about 35-80% of patients treated with APC [25, 28]. Furthermore, antrectomy has shown benefit in refractory cases, but carries a high mortality risk [29]. Other experimental therapeutic options include hormonal therapy such as estrogen-progesterone therapy, tranexamic acid, methylprednisolone, thalidomide, and cyclophosphamide [26, 30-35].
Like any study, this study is not without limitations. The selection of the study samples relies on a retrospective database which stores data using specific codes known as the ICD-9 codes. The errors associated with coding process could have confounded some of our findings. Additionally, this study included data for the 2001 to 2011 study period. Therefore, there may be limitations in the generalizability of the data to the more recent time period. Furthermore, this was a retrospective study; hence, all biases seen in database retrospective studies are applicable to our study. However, despite these limitations, the large sample size which closely reflects the US population and the analytical study design yielded higher power for our study results. Additionally, this is also one of the first nationwide study looking at the burden and outcomes of GAVE in the US population.
Conclusions
For the study period, the total number of GAVE hospitalizations increased significantly with a higher increase in the proportion noted for GAVE hospitalizations without bleeding. This was most likely due to widespread availability of diagnostic tools and improvement in therapeutic techniques. We also noted that the risk of mortality increases significantly with presence of hemorrhage for GAVE hospitalizations. Although inpatient mortality for GAVE hospitalizations decreased slightly over the study period, there was a significant increase in the cost of care for these patients likely due to increased use of advanced and expensive interventions.
Acknowledgments
None to declare.
Financial Disclosure
The authors have received no funding with respect to research, authorship, and/or publication of this article.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conception and design: Shantanu Solanki, Asim Kichloo, and Khwaja Fahad Haq. Administrative support: Dhanshree Solanki, Darshan Lal, and Jagmeet Singh. Provision of study materials or patients: Shantanu Solanki. Collection and assembly of data: Shantanu Solanki. Data analysis and interpretation: Shantanu Solanki, Asim Kichloo, and Dushyant Singh Dahiya. Manuscript writing: all authors. Final approval of manuscript: all authors.
Data Availability
We used and/or analyzed the NIS database 2016 and 2017, available online at http: //www.hcup- us.ahrq.gov. The NIS is a large publicly available all-payer inpatient care database in the USA, containing data on more than 7 million hospital stays yearly. Its large sample size is ideal for developing national and regional estimates and enables analyses of rare conditions, uncommon treatments, and special populations.
Abbreviations
GAVE: gastric antral vascular ectasia; GI: gastrointestinal; NIS: National Inpatient Sample; AHRQ: Agency for Healthcare Research and Quality; AHA: American Hospital Association; MedPAR: Medicare Provider and Analysis Review; IRB: Institutional Review Board; CCR: cost-to-charge ratio; BMI: body mass index; APC: argon plasma coagulation; Nd: YAG: neodymium-doped, yttrium-aluminum-garnet
| References | ▴Top |
- Rider JA, Klotz AP, Kirsner JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24(1):118-123.
doi - van Vliet AC, ten Kate FJ, Dees J, van Blankenstein M. Abnormal blood vessels of the prepyloric antrum in cirrhosis of the liver as a cause of chronic gastrointestinal bleeding. Endoscopy. 1978;10(2):89-94.
doi pubmed - Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87(5):1165-1170.
doi - Chawla SK, Ramani K, Lo Presti P. The honeycomb stomach: coalesced gastric angiodysplasia. Gastrointest Endosc. 1990;36(5):516-518.
doi - Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5(1):6-13.
doi pubmed - Swanson E, Mahgoub A, MacDonald R, Shaukat A. Medical and endoscopic therapies for angiodysplasia and gastric antral vascular ectasia: a systematic review. Clin Gastroenterol Hepatol. 2014;12(4):571-582.
doi pubmed - Overview of the National (Nationwide) Inpatient Sample (NIS). Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed March 30, 2020.
- Solanki S, Chakinala RC, Haq KF, Khan MA, Kifayat A, Linder K, Khan Z, et al. Inpatient burden of gastric cancer in the United States. Ann Transl Med. 2019;7(23):772.
doi pubmed - Kaur BP, Lahewala S, Arora S, Agnihotri K, Panaich SS, Secord E, Levine D. Asthma: hospitalization trends and predictors of in-hospital mortality and hospitalization costs in the USA (2001-2010). Int Arch Allergy Immunol. 2015;168(2):71-78.
doi pubmed - Solanki S, Haq KF, Chakinala RC, Khan Z, Aronow WS, Ali Khan M, Siddiqui MT, et al. Inpatient burden of esophageal varices in the United States: analysis of trends in demographics, cost of care, and outcomes. Ann Transl Med. 2019;7(18):480.
doi pubmed - Solanki SD, Haq KF, Farkas Z, et al. Temporal trends and demographic variations in hospitalizations with angiodysplasia of the intestine: A U.S. population based study. Electron J Gen Med. 2018;15:em80.
doi - Charneau J, Petit R, Cales P, Dauver A, Boyer J. Antral motility in patients with cirrhosis with or without gastric antral vascular ectasia. Gut. 1995;37(4):488-492.
doi pubmed - Gostout CJ, Viggiano TR, Ahlquist DA, Wang KK, Larson MV, Balm R. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15(3):256-263.
doi pubmed - Quintero E, Pique JM, Bombi JA, Bordas JM, Sentis J, Elena M, Bosch J, et al. Gastric mucosal vascular ectasias causing bleeding in cirrhosis. A distinct entity associated with hypergastrinemia and low serum levels of pepsinogen I. Gastroenterology. 1987;93(5):1054-1061.
doi - Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77(2):131-137.
doi pubmed - Smith E, Tekola B, Patrie J, Cornella S, Caldwell S. Clinical characterization of gastric antral vascular ectasia: a potential manifestation of the metabolic syndrome. Am J Med. 2016;129(12):1329 e1319-1329 e1323.
doi pubmed - Sebastian S, O'Morain CA, Buckley MJ. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18(2):157-165.
doi pubmed - Toyota M, Hinoda Y, Nakagawa N, Arimura Y, Tokuchi S, Takaoka A, Kitagawa S, et al. Gastric antral vascular ectasia causing severe anemia. J Gastroenterol. 1996;31(5):710-713.
doi pubmed - Unnikrishnan R, Vijayakumar P, Senan S, et al. Gastric antral vascular ectasia: an uncommon cause of anemia in the elderly. Dubai Med J. 2019;2:99-101.
doi - Fukuda K, Kurita N, Sakamoto T, Nishikii H, Okoshi Y, Sugano M, Chiba S. Post-transplant gastric antral vascular ectasia after intra-venous busulfan regimen. Int J Hematol. 2013;98(1):135-138.
doi pubmed - Saad Aldin E, Mourad F, Tfayli A. Gastric antral vascular ectasia in a patient with GIST after treatment with imatinib: case report and literature review. Jpn J Clin Oncol. 2012;42(5):447-450.
doi pubmed - Alkhormi AM, Memon MY, Alqarawi A. Gastric antral vascular ectasia: a case report and literature review. J Transl Int Med. 2018;6(1):47-51.
doi pubmed - Matin T, Naseemuddin M, Shoreibah M, Li P, Kyanam Kabir Baig K, Wilcox CM, Peter S. Case series on multimodal endoscopic therapy for gastric antral vascular ectasia, a tertiary center experience. World J Gastrointest Endosc. 2018;10(1):30-36.
doi pubmed - Dias de Castro F, Boal Carvalho P, Curdia Goncalves T, Magalhaes J, Moreira MJ, Marinho C, Cotter J. Treating gastric antral vascular ectasia - when argon therapy is not enough. GE Port J Gastroenterol. 2016;23(5):249-253.
doi pubmed - Zepeda-Gomez S. Endoscopic treatment for gastric antral vascular ectasia: current options. GE Port J Gastroenterol. 2017;24(4):176-182.
doi pubmed - Hsu WH, Wang YK, Hsieh MS, Kuo FC, Wu MC, Shih HY, Wu IC, et al. Insights into the management of gastric antral vascular ectasia (watermelon stomach). Therap Adv Gastroenterol. 2018;11:1756283X17747471.
doi pubmed - Garg S, Aslam B, Nickl N. Endoscopic resolution and recurrence of gastric antral vascular ectasia after serial treatment with argon plasma coagulation. World J Gastrointest Endosc. 2017;9(6):263-266.
doi pubmed - Chiu YC, Lu LS, Wu KL, Tam W, Hu ML, Tai WC, Chiu KW, et al. Comparison of argon plasma coagulation in management of upper gastrointestinal angiodysplasia and gastric antral vascular ectasia hemorrhage. BMC Gastroenterol. 2012;12:67.
doi pubmed - Novitsky YW, Kercher KW, Czerniach DR, Litwin DE. Watermelon stomach: pathophysiology, diagnosis, and management. J Gastrointest Surg. 2003;7(5):652-661.
doi - Lorenzi AR, Johnson AH, Davies G, Gough A. Gastric antral vascular ectasia in systemic sclerosis: complete resolution with methylprednisolone and cyclophosphamide. Ann Rheum Dis. 2001;60(8):796-798.
doi pubmed - Manning RJ. Estrogen/progesterone treatment of diffuse antral vascular ectasia. Am J Gastroenterol. 1995;90(1):154-156.
- Nardone G, Rocco A, Balzano T, Budillon G. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13(11):1429-1436.
doi pubmed - Park RH, Danesh BJ, Upadhyay R, Howatson AG, Lee FD, Russell RI. Gastric antral vascular ectasia (watermelon stomach) - therapeutic options. Postgrad Med J. 1990;66(779):720-723.
doi pubmed - Tran A, Villeneuve JP, Bilodeau M, Willems B, Marleau D, Fenyves D, Parent R, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94(10):2909-2911.
doi pubmed - van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet. 1990;335(8695):953-955.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.




