| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 13, Number 1, February 2020, pages 44-51
Esophageal Carcinoma Cuniculatum Diagnosed on Mucosal Biopsies Using a Semiquantitative Histologic Schema: Report of Two Esophagectomy-Confirmed Cases
Xiuli Liua, e, Dennis Yangb, Xuefeng Zhangc, Olusola Oduntand
aDepartment of Pathology, Immunology, and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL, USA
bDepartment of Gastroenterology, Hepatology, and Nutrition, College of Medicine, University of Florida, Gainesville, FL, USA
cDepartment of Anatomic Pathology, Cleveland Clinic Foundation, Cleveland, OH, USA
dDepartment of Surgery, College of Medicine, University of Florida, Gainesville, FL, USA
eCorresponding Author: Xiuli Liu, Department of Pathology, Immunology, and Laboratory Medicine, P.O. Box 100275, Gainesville, FL 32610, USA
Manuscript submitted January 13, 2020, accepted January 23, 2020
Short title: Diagnosis of Esophageal Carcinoma Cuniculatum
doi: https://doi.org/10.14740/gr1262
| Abstract | ▴Top |
Esophageal carcinoma cuniculatum is a rare variant of squamous cell carcinoma characterized by a unique and common histologic pattern including hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic microabscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cyst/burrows observed in the resection specimens. Preoperative diagnosis can be extremely difficult. A semiquantitative histologic scoring system has been previously proposed for mucosal biopsies, which has been associated with improved diagnostic yield. However, this histologic schema for the diagnosis of carcinoma cuniculatum has not been applied prospectively. Herein, we describe two cases of esophageal carcinoma cuniculatum in patients presenting with progressive dysphagia and esophageal mass. Presurgical endoscopic mucosal biopsies showed features consistent with carcinoma cuniculatum, and a preoperative diagnosis was achieved by applying the aforementioned semiquantitative histologic schema. Both patients underwent neoadjuvant chemoradiation followed by esophagectomy. Both esophagectomy specimens showed residual adventitia-invading carcinoma cuniculatum, negative lymph nodes, marked tumor regression, and an exuberant histiocytic and giant response. To our best knowledge, these represent the first two cases of esophageal carcinoma cuniculatum diagnosed by applying this semiquantitative histologic schema to mucosal biopsies. Large studies are needed to further confirm these preliminary findings and validate this histologic scoring system.
Keywords: Carcinoma cuniculatum; Esophagus; Mucosal biopsy; Squamous cell carcinoma
| Introduction | ▴Top |
Carcinoma cuniculatum is a rare variant of squamous cell carcinoma first described in the plantar skin by Aird et al in 1954 [1]. Carcinoma cuniculatum has a distinctive morphology characterized by burrowing channels lined by extremely well-differentiated squamous epithelium [2]. We previously reported nine cases of esophageal carcinoma cuniculatum diagnosed on esophagectomy specimens in seven men and two women during a 20-year period [3]. A common histologic pattern including hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, intraepithelial neutrophilic microabscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cyst/burrows, was recognized in this unique variant of well-differentiated squamous cell carcinoma [3]. Similar morphology was also observed by other groups in surgically resected esophageal carcinoma cuniculatum [4, 5]. In addition, in resection specimen, exuberant inflammation has been noted and reported. For example, one recent case report described non-necrotizing granulomatous inflammation in the tumoral and peritumoral region, as well as the draining regional lymph nodes in one patient with chemoradiation-naive esophageal carcinoma cuniculatum [6].
Preoperative diagnosis of esophageal carcinoma cuniculatum is extremely difficult and in most cases, the preoperative biopsies are often interpreted as active esophagitis, Candida esophagitis, papilloma, or inconclusive. Most carcinoma cuniculatum of the esophagus reported in the literature were only diagnosed on esophagectomy [3-6], endoscopic mucosal resection (EMR) [7], or endoscopic submucosal dissection (ESD) specimens [8]. Chen et al retrospectively examined 35 preoperative esophageal mass biopsies obtained from 25 endoscopic procedures in 11 patients with a resection-proven diagnosis of carcinoma cuniculatum [7]. The authors used a semiquantitative histologic approach and evaluated the presence of hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic microabscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cyst/burrows with each feature accounting as one point. A tallied score of all present features was generated for each biopsy. Using a cutoff value of 7 for carcinoma cuniculatum in patients with an esophageal mass greatly improved the diagnostic accuracy with 100% specificity and 91% sensitivity at the patient level [7]. However this semiquantitative histologic schema for the diagnosis of carcinoma cuniculatum has not been validated or evaluated prospectively.
Herein, in this case report, we describe two cases of carcinoma cuniculatum of the esophagus which were diagnosed based on the synthesis of clinical information, endoscopic finding, imaging study, and by applying the semiquantitative histologic schema proposed in our previous study [7].
| Case Reports | ▴Top |
Case 1
One 67-year-old man with a past medical history significant for diabetes mellitus, gastroesophageal reflux disease, and hypertension presented to our hospital for progressive dysphagia to solids and liquids for 4 - 5 month, and 40-lb weight loss. He denied any tobacco or alcohol use. His body mass index (BMI) was 23.2 kg/m2. Esophagogastroduodenoscopy (EGD) revealed a large villous, nodular, partially obstructive and ulcerated mass in the distal esophagus and gastroesophageal junction (GEJ) (Fig. 1a). Endoscopic ultrasound (EUS) revealed a large hypoechoic circumferential mass in the distal esophagus with invasion beyond the muscularis propria (layer 4) and the presence of five malignant appearing peri-tumoral lymph nodes (EUS staging of T3N2Mx) (Fig. 1b, c). Computed tomography (CT) of the chest revealed marked esophageal wall thickening in the mid to lower esophagus. Positron emission tomography/computed tomography (PET/CT) confirmed the presence of a hypermetabolic 4 cm × 6 cm mass in the distal esophagus with a standardized uptake value (SUV) of 8.0 (Fig. 1d).
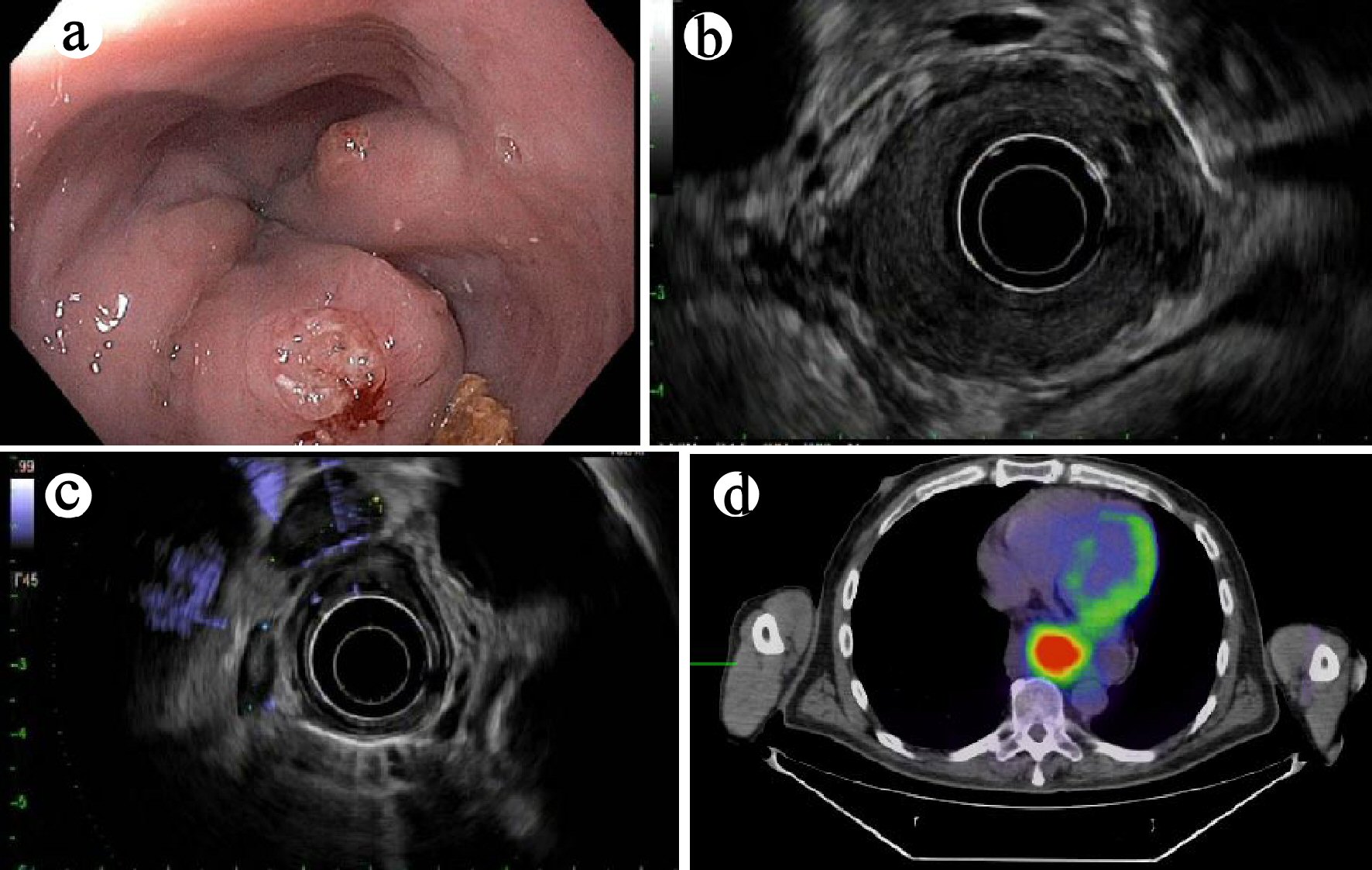 Click for large image | Figure 1. (a) Endoscopic finding of an esophageal mass on esophagogastroduodenoscopy (case 1). (b) Mural destruction by the mass on endoscopic ultrasound (EUS) examination. (c) Enlarged hypoechoic nodes, suspicious for metastases on EUS examination. (d) Positron emission tomography (PET) revealed a hypermetabolic mass in the distal esophagus (axial view). |
Two sets of mucosal biopsies obtained from the distal esophageal/GEJ mass on two separate endoscopic procedures were evaluated for hyperkeratosis (either parakeratosis and/or orthokeratosis), acanthosis (overall thickening or hyperplasia of squamous epithelium), dyskeratosis (defined as the presence of individual apoptotic keratinocytes), abnormal/deep keratinization (paradoxical keratinization), keratin-filled furrows/cysts, koilocyte-like cells, intraepithelial neutrophils (defined as the presence of neutrophils in the squamous epithelium), intraepithelial neutrophilic microabscesses (defined as the presence of six or more neutrophils with associated epithelial injury), and atypia. Histologic review of these two sets of mucosal biopsies revealed similar histologic features (Fig. 2). The biopsies consisted of multiple fragments of squamous epithelium demonstrating hyperkeratosis, acanthosis, neutrophilic inflammation, neutrophilic microabscess, dyskeratosis, focal atypia, koilocyte-like cells, and furrows. Luminal keratinous debris with neutrophilic inflammation and Candida organisms was present (pictures not shown). A score of 9 was tallied for all these features present using the previously proposed histologic scoring system by Chen et al [7]. Based on the clinical, endoscopic, radiographic and histological presentation, a diagnosis of carcinoma cuniculatum of the esophagus was reached at the multidisciplinary tumor board meeting. The patient underwent neoadjuvant chemotherapy (five cycles of paclitaxel/carboplatin) and radiation (50.4 Gy) followed by esophagectomy.
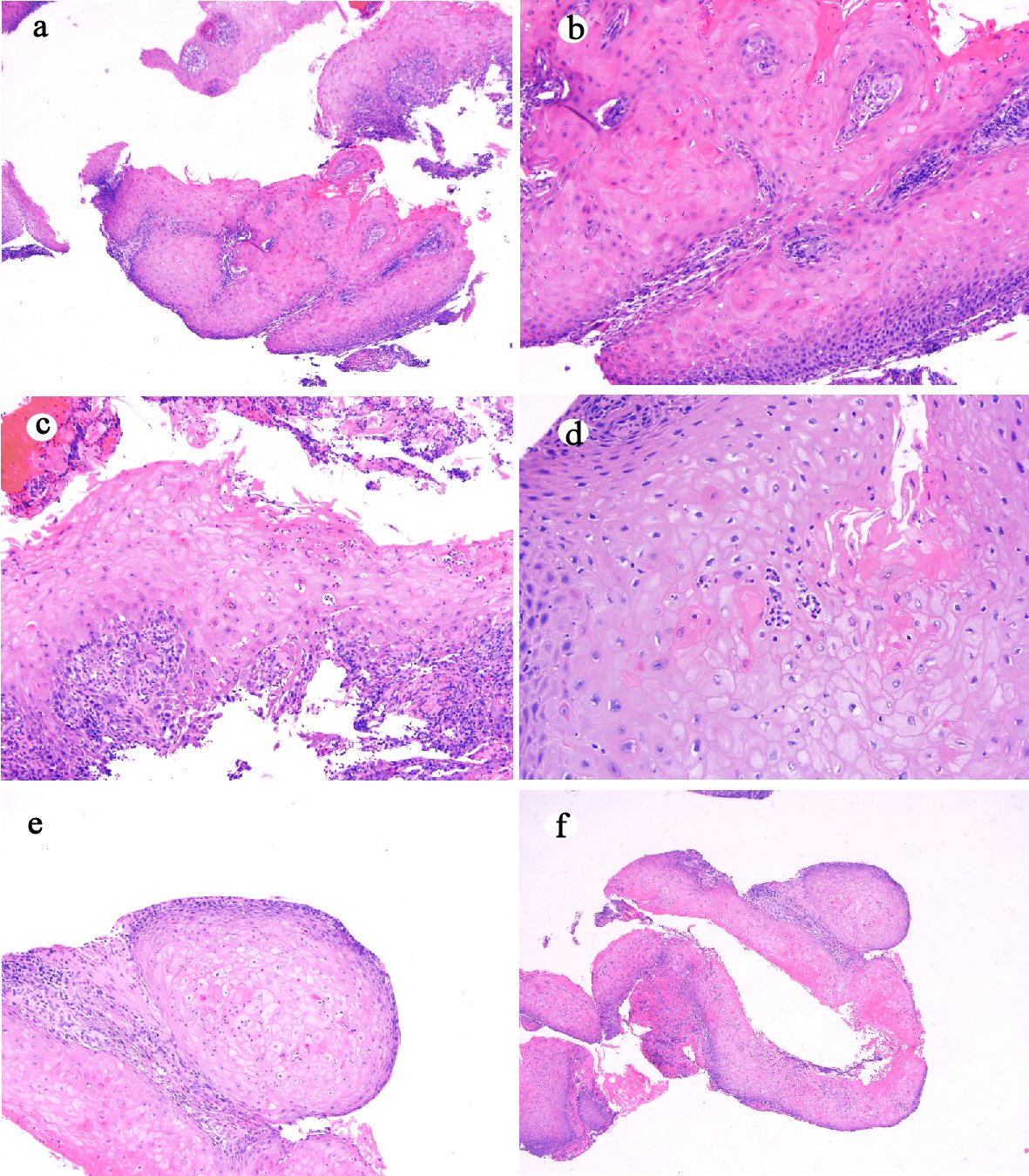 Click for large image | Figure 2. Histologic features of mucosal biopsy from the mass (case 1). Squamous epithelium shows hyperkeratosis and acanthosis ((a) hematoxylin and eosin stain, original magnification × 40), focal atypia ((b) hematoxylin and eosin stain, original magnification × 200), intraepithelial neutrophilic inflammation and microabscesses and dyskeratosis ((c) hematoxylin and eosin stain, original magnification × 200), koilocyte-like cells ((d) hematoxylin and eosin stain, original magnification × 200), deep keratinization ((e) hematoxylin and eosin stain, original magnification × 100), and furrow ((f) hematoxylin and eosin stain, original magnification × 20). |
The esophagectomy specimen consisted of an esophagus with attached stomach. The portion of the esophagus measured 13.5 cm in length and 3.5 cm in open circumference. The portion of stomach measured 7.5 × 7.5 × 3.0 cm. A 4.3 cm × 1.0 cm, indurated tan-white near circumferential lesion was identified at the GEJ. The 1.3 cm distal esophagus was fibrotic and appeared to extend through the esophageal adventitia. Multiple lymph nodes measuring from 0.6 to 3.0 cm were retrieved from the periesophagogastric tissue. The lesion at the GEJ and the fibrotic area in the distal esophagus were entirely submitted for histology. Microscopically, the sections from the GEJ lesion and the distal esophagus showed ulceration, residual bland squamous epithelium-lined cysts, marked mural fibrosis, inflammation and multifocal giant cell and histiocytic response to keratinous material, dyskeratotic and residual squamous carcinoma cells (Fig. 3a-d). Fifteen lymph nodes were negative for metastases. All margins were negative. The final histopathological diagnosis was residual carcinoma cuniculatum infiltrating through the muscularis propria into the adventitia and with marked treatment effect. The final pathology stage was ypT3N0Mx.
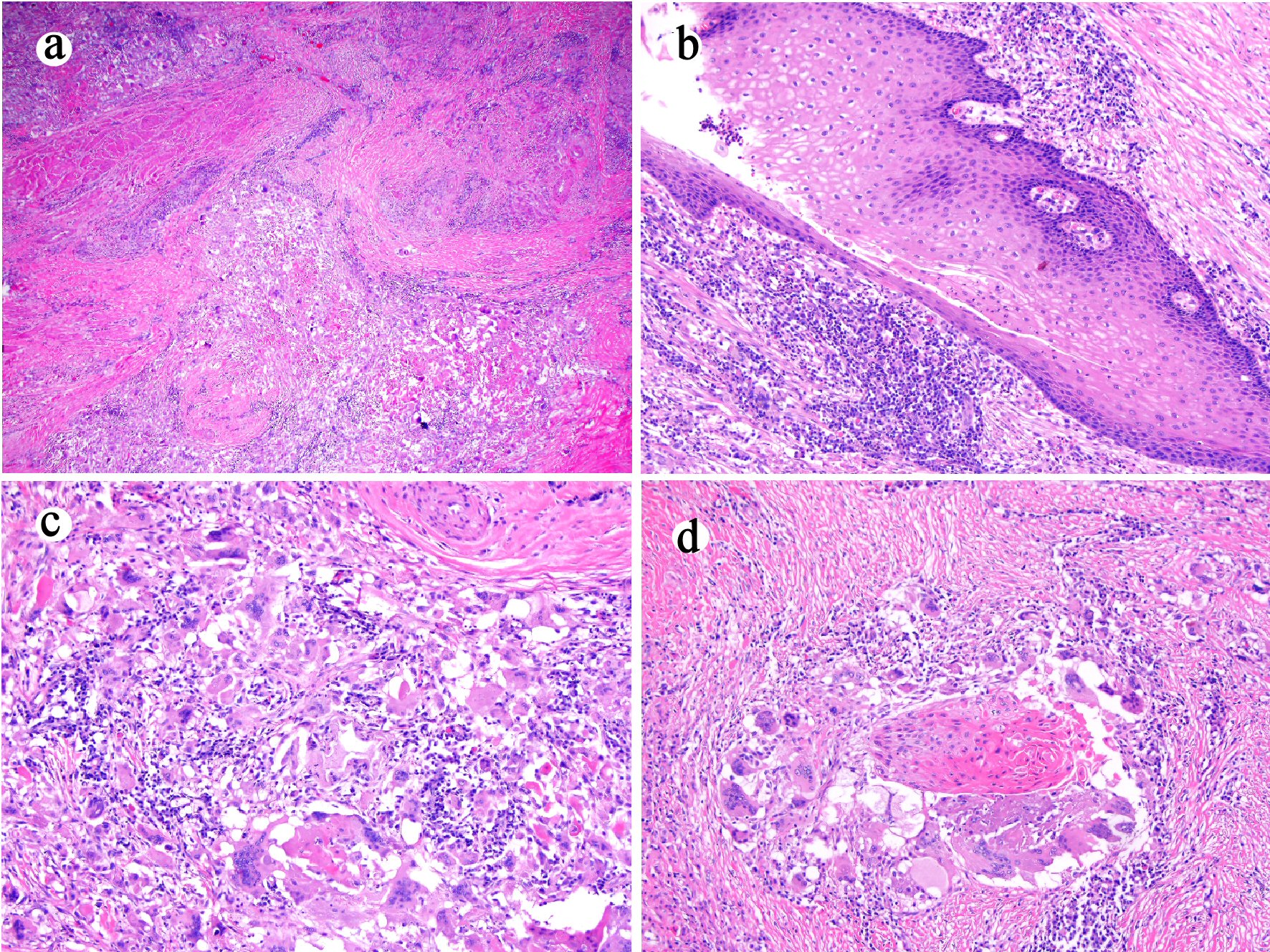 Click for large image | Figure 3. Esophagectomy (case 1) shows mural fibrosis and inflammation ((a) hematoxylin and eosin stain, original magnification × 20), few residual cyst lined with well-differentiated bland squamous epithelium ((b) hematoxylin and eosin stain, original magnification × 100), and histiocytic and giant cell response to keratinous material ((c) hematoxylin and eosin stain, original magnification × 200) and residual squamous carcinomatous cells ((d) hematoxylin and eosin stain, original magnification × 200). |
The patient had an uneventful recovery and was discharged on postoperative day 8. The patient was well 1 month after the esophagectomy.
Case 2
A 62-year-old man presented with chest pain and dysphagia of 5-month duration and a weight loss of 30 lbs. The patient has a smoking history of 40 pack-years. He denied alcohol use. Chest CT revealed distal esophageal thickening. PET/CT revealed a hypermetabolically active lesion in the distal esophagus. EGD revealed a large 5 cm, partially obstructive and ulcerated friable mass in the distal esophagus extending 1 cm into the gastric cardia. The lesion could not be traversed with the echoendoscope. Limited EUS revealed extension of the mass beyond the muscularis propria (layer 4) with a 6 mm hypoechoic peritumoral lymph node (EUS staging of T3N1Mx).
Three sets of mucosal biopsies were obtained from the mass on three separate endoscopic procedures during a 1-month period. All biopsies revealed similar histologic features (Fig. 4). The biopsies consisted of multiple fragments of squamous epithelium demonstrating hyperkeratosis, acanthosis, neutrophilic inflammation, neutrophilic microabscess, dyskeratosis, focal atypia, koilocyte-like cells, and furrows/cysts with a tallied score of 9 using the previously proposed histologic schema [7]. Luminal keratinous debris with neutrophilic inflammation and Candida organisms was present as well (pictures not shown). Similar to case 1, a diagnosis of carcinoma cuniculatum was reached based on the aggregate clinical, endoscopic, radiographic and histologic features. The patient underwent neoadjuvant chemotherapy (5-fluorouracil and cisplatin) and radiation. EGD at the completion of chemoradiation revealed a 1 cm friable, nodular, ulcerated, flat mass at GEJ and the tumor was downstaged to T2NxMx by EUS. The patient underwent successful esophagectomy 2 months following completion of neoadjuvant therapy.
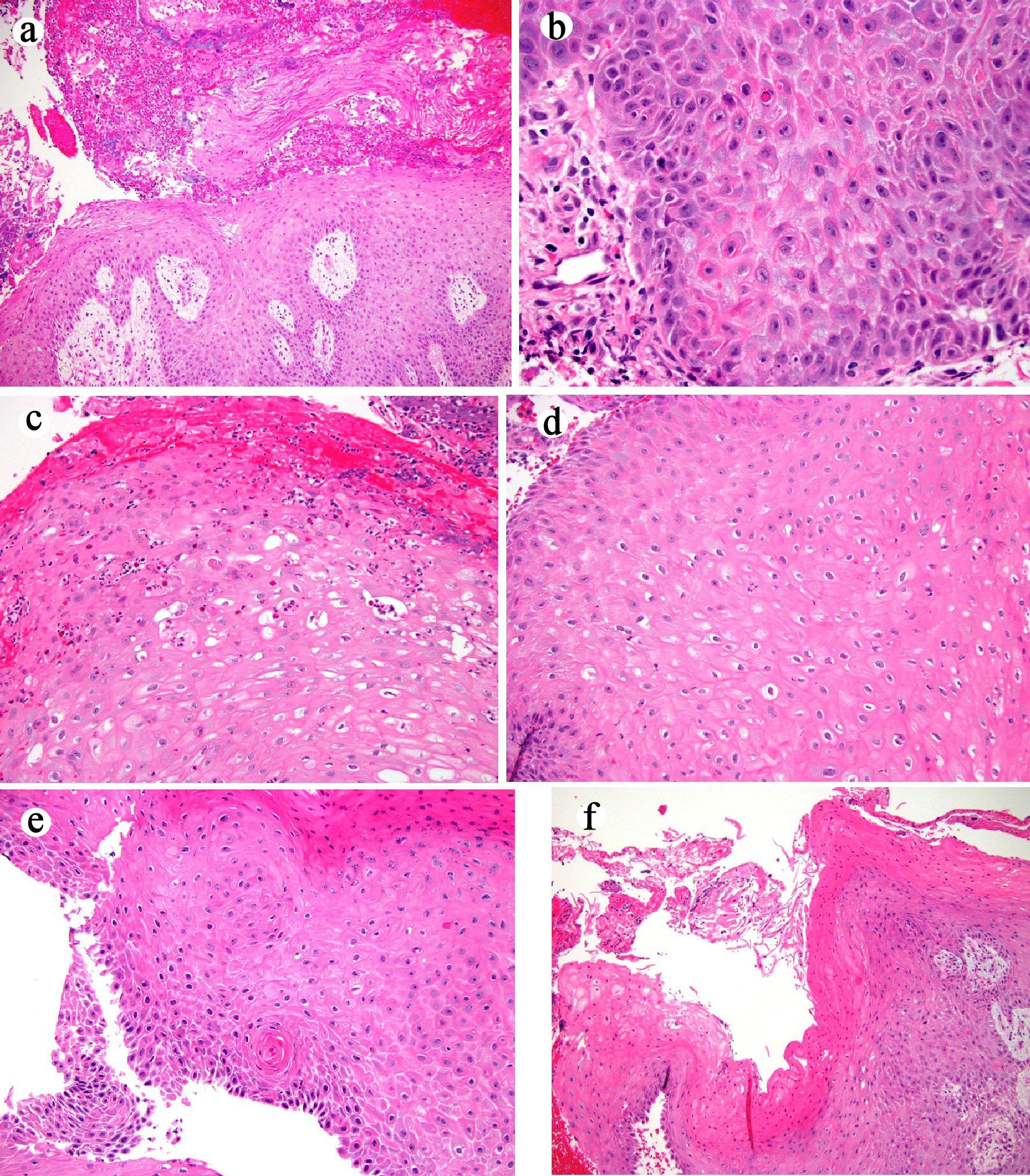 Click for large image | Figure 4. Histologic features of mucosal biopsy from the mass (case 2). Squamous epithelium shows hyperkeratosis and acanthosis ((a) hematoxylin and eosin stain, original magnification × 40), focal atypia ((b) hematoxylin and eosin stain, original magnification × 400), intraepithelial neutrophilic inflammation and microabscesses and dyskeratosis ((c) hematoxylin and eosin stain, original magnification × 200), koilocyte-like cells ((d) hematoxylin and eosin stain, original magnification × 200), deep keratinization ((e) hematoxylin and eosin stain, original magnification × 200), and furrow ((f) hematoxylin and eosin stain, original magnification × 40). |
The esophagectomy specimen consisted of an esophagus with attached stomach. The portion of the esophagus measured 13 cm in length. The portion of stomach measured 6.5 × 3.5 × 0.3 cm. A 2.1 × 1.2 cm, ulcerated lesion was identified at the GEJ. Multiple lymph nodes (ranging from 0.1 to 0.8 cm) were retrieved from the periesophageal tissue. The lesion at the GEJ was entirely submitted for histology. Microscopically, the sections from the lesion and the distal esophagus showed marked surface ulceration, mural fibrosis, and multifocal giant cell and histiocytic response to keratinous material and terminally differentiated squamous cell carcinoma cells (Fig. 5). Twenty-eight lymph nodes were negative for metastases. All margins were negative. The final histopathological diagnosis of the esophagectomy specimen was residual carcinoma cuniculatum infiltrating through the muscularis propria into the adventitia and with marked treatment effect. The pathology stage was ypT3N0Mx.
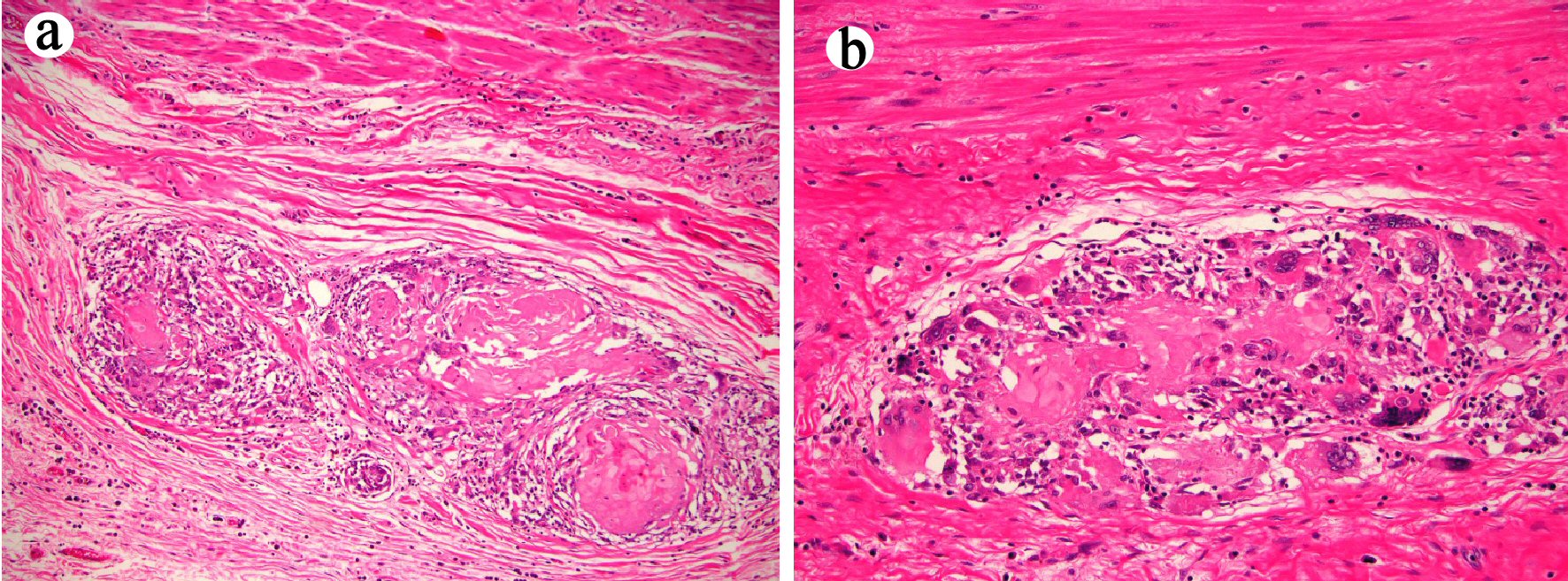 Click for large image | Figure 5. Esophagectomy (case 2) shows histiocytic and giant cell response to keratinous material and residual terminally differentiated squamous carcinomatous cells ((a) and (b) hematoxylin and eosin stain, original magnification × 100 and × 200, respectively). |
The patient had an uneventful recovery from his esophagectomy. Twelve months after the esophagectomy, the patient developed primary squamous cell carcinoma of the right lung and underwent lobectomy. No recurrence or metastases of his esophageal or pulmonary squamous cell carcinoma were detected during his follow-up. EGD performed at 32 months after the esophagectomy revealed benign-appearing esophageal and gastric mucosa. Biopsy confirmed the absence of dysplasia or carcinoma. He was admitted to hospice care due to mixed obstructive-restrictive lung disease, and expired 36 months after the esophagectomy.
The clinical, endoscopic, radiographic, histopathologic findings in mucosal biopsy and esophagectomy specimens and clinical follow-up in these two cases are summarized in Tables 1 and 2.
 Click to view | Table 1. Clinical, Radiologic, and Endoscopic Findings in These Two Patients With Esophageal Carcinoma Cuniculatum |
 Click to view | Table 2. Histopathologic Findings and Clinical Follow-Up in These Two Patients With Esophageal Carcinoma Cuniculatum |
| Discussion | ▴Top |
Carcinoma cuniculatum, a rare variant of squamous cell carcinoma, has been increasingly recognized by clinicians and pathologists, and about two dozen of cases have been reported in the literature primarily in esophagectomy specimens [3-5]. A few cases have also been reported in endoscopically resected (EMR and ESD) specimens [7, 8]. Preoperative diagnosis on mucosal biopsies obtained by EGD is extremely challenging due to its bland cytology and presence of inflammation [3, 6, 7]. Hence, it can often be misdiagnosed as active esophagitis, Candida esophagitis, papilloma, or is inconclusive [3, 4, 8].
With the increased number of cases being reported in the literature, researchers proposed a semiquantitative histological scoring system based on features observed in resected specimens and biopsies prior to the resection [3, 7]. This approach includes assessing for the presence of hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic microabscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cyst/burrows in the biopsy. For each feature present in the biopsy, one point is given and the sum of all features present is generated for a given case. Using a cutoff value of 7 for carcinoma cuniculatum in patients with an esophageal mass has been associated with improved diagnostic yield for carcinoma cuniculatum with 100% specificity and 91% sensitivity at the patient level [7]. However this semiquantitative approach has not been used prospectively to diagnose carcinoma cuniculatum to validate its clinical use, although one case with a score of 5 - 6 was reported on mucosal biopsies in an esophagectomy-proven carcinoma cuniculatum [6].
In this report, we described two patients with progressive dysphagia and obstructive esophageal mass invading the esophageal wall as noted on cross-sectional imaging and EUS. In both cases, multiple mucosal biopsies obtained on repeated endoscopic procedures demonstrated similar features, including hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic microabscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cyst/burrows, generating a score of 9 using the semiquantitative histological schema proposed previously by Chen et al in 2013 [7]. Based on the synthesis of all clinical, endoscopic, radiographic, and most importantly, the histologic information on mucosal biopsies, these two patients were diagnosed as having carcinoma cuniculatum and underwent neoadjuvant chemoradiation therapy and shortly followed by esophagectomy. In both cases, a residual carcinoma cuniculatum infiltrating into the adventitia was identified. Extensive mural fibrosis and inflammation were also present in the tumor bed within the esophagectomy specimens. Of note, a unique histiocytic and giant cell (granulomatous) response to the keratinous material and/or carcinoma cells, either dyskeratotic/apoptotic or terminally differentiated was noted in both cases. Peritumoral and extra-tumoral (Crohn’s-like) lymphoid aggregates with non-necrotizing granulomas in the tumor, peritumoral esophagus and stomach, and draining regional lymph nodes has been previously reported in one case of chemoradiation-naive carcinoma cuniculatum [6]. The granulomatous inflammation in our two cases were exuberant and most foci of granulomatous inflammation contained terminally differentiated or dyskeratotic squamous carcinoma cells. The mechanism leading to this phenomenon is likely the death of chemoradiation-sensitive cells in the basal layer of the cysts/furrows leading to exposure of luminal keratinous material which incites a strong histiocytic and giant cell response.
Tumor regression in the esophagectomy specimen in patients who receive neoadjuvant chemoradiation is prognostic [9]. Tumor regression in carcinoma cuniculatum is difficult to assess as distinguishing dyskeratotic/apoptotic cells from terminally differentiated but viable cells is difficult. In addition, the biology of these few viable and terminally differentiated (keratinized) cells is not clear. Given the excellent prognosis in patients with carcinoma cuniculatum treated with esophagectomy alone, the tumor regression score may not be prognostically relevant. But this needs additional study of a reasonably sized cohort of patients with carcinoma cuniculatum subjected to neoadjuvant treatment.
In the esophagectomy specimen from these two cases, 15 and 28 lymph nodes were retrieved and all of them were negative. This finding is consistent with previous reports that these enlarged, presumed “metastasis positive” nodes detected by imaging studies are actually reactive lymph nodes [3-6].
Etiology of carcinoma cuniculatum of the esophagus remains unclear. Potential risk factors include reflux disease, smoking, immunosuppression, and achalasia [3]. One patient in this report had a history of gastroesophageal reflux disease, and one patient had a significant history of smoking. In the latter patient, heavy smoking may have had contributed to the development of both esophageal carcinoma cuniculatum and lung squamous cell carcinoma. This also calls for a high vigilance for synchronous or metachronous squamous cell carcinoma in the aerodigestive tract and respiratory system in patients with esophageal carcinoma cuniculatum and a significant history of smoking.
In summary, we reported the use of the previously proposed semiquantitative histologic schema in diagnosing carcinoma cuniculatum on mucosal biopsy specimens in two patients who presented with progressive dysphagia and esophageal mass. Both patients underwent neoadjuvant chemoradiation therapy and subsequently underwent esophagectomy. Histologic examination of the esophagectomy specimens from both patients confirmed the diagnosis of a deeply infiltrative carcinoma cuniculatum with marked treatment effect. The exposure of keratinous material after chemoradiation incited an exuberant intratumoral histiocytic and giant cell response. Large studies are needed to further validate the clinical use of this semiquantitative histology schema to preoperatively diagnose esophageal carcinoma cuniculatum.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare for all authors.
Informed Consent
Obtained and recorded by OO.
Author Contributions
XL: study design and draft the manuscript; DY: endoscopic interpretation and critical review the manuscript; XZ: histologic interpretation and critical review the manuscript; OO: study design and critical review the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Aird I, Johnson HD, Lennox B, Stansfeld AG. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42(173):245-250.
doi pubmed - Barreto JE, Velasquez EF, Ayala E, et al. Carcinoma cuniculatum: a distinct variant of penile squamous cell carcinoma. Am J Surg Pathol. 2007;31:71-75.
doi pubmed - Landau M, Goldblum JR, DeRoche T, Dumot J, Downs-Kelly E, Rice TW, Xiao SY, et al. Esophageal carcinoma cuniculatum: report of 9 cases. Am J Surg Pathol. 2012;36(1):8-17.
doi pubmed - De Petris G, Lewin M, Shoji T. Carcinoma cuniculatum of the esophagus. Ann Diagn Pathol. 2005;9(3):134-138.
doi pubmed - Goh GH, Venkateswaran K, Leow PC, Loh KS, Thamboo TP, Petersson F. Carcinoma cuniculatum of the esophagus and tongue: report of two cases, including TP53 mutational analysis. Head Neck Pathol. 2014;8(3):261-268.
doi pubmed - Dick TM, El Hag M, Mallery JS, Amin K. Esophageal carcinoma cuniculatum associated with non-necrotizing granulomatous inflammation and lymphadenopathy: clinicopathologic features and diagnostic challenges. Am J Case Rep. 2018;19:790-795.
doi pubmed - Chen D, Goldblum JR, Landau M, Rice TW, Pai RK, Xiao SY, Liu X. Semiquantitative histologic evaluation improves diagnosis of esophageal carcinoma cuniculatum on biopsy. Mod Pathol. 2013;26(6):806-815.
doi pubmed - Coman RM, Collinsworth A, Draganov PV. Endoscopic submucosal dissection in a rare case of carcinoma cuniculatum of the esophagus initially misdiagnosed as benign squamous papilloma. Endoscopy. 2014;46(Suppl 1 UCTN):E531-532.
doi pubmed - Puetz K, Bollschweiler E, Semrau R, Monig SP, Holscher AH, Drebber U. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer - which response grading system best impacts prognostic discrimination? Histopathology. 2019;74(5):731-743.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


