| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 13, Number 3, June 2020, pages 107-113
Evaluation of Roles of MicroRNA-21 and MicroRNA-18a in Esophageal Squamous Cell Carcinoma and Comparison of Their Changes in Expression Post-Chemoradiotherapy
Mohd Talha Noora, b, Nivesh Seehraa, Jitendra Rajputa, Rajeev Sharmaa, Bhagwan Singh Thakura
aDepartment of Gastroenterology, Sri Aurobindo Medical College and Postgraduate Institute, Indore-Ujjain State Highway, Indore, Madhya Pradesh 453555, India
bCorresponding Author: Mohd Talha Noor, Department of Gastroenterology, Sri Aurobindo Medical College and Postgraduate Institute, Indore-Ujjain State Highway, Indore, Madhya Pradesh 453555, India
Manuscript submitted January 13, 2020, accepted April 28, 2020, published online June 18, 2020
Short title: Roles of MiRNAs in ESCC
doi: https://doi.org/10.14740/gr1261
| Abstract | ▴Top |
Background: A number of circulating microRNAs (miRNAs) have been reported to be highly expressed in several cancers; whether their expression is associated with clinicopathological factors and prognosis in patients of esophageal squamous cell carcinoma (ESCC) is still under investigation. Although studies have demonstrated their overexpression in tissues of ESCC, there are limited data for circulating miRNAs. Aim of this study was to evaluate the expressions of miRNA-21 and miRNA-18a in patients of ESCC and the effect of chemoradiotherapy (CRT) on expression of these miRNAs.
Methods: This was a case-control study conducted from September 2014 to December 2015 at Sri Aurobindo Medical College and Postgraduate Institute, Indore, India. We compared the expression of miRNA-21 and miRNA-18a in 30 ESCC patients and 30 healthy controls using TaqMan probe-based quantitative real-time polymerase chain reaction (qRT-PCR) and changes in the expression in 16 patients of ESCC, who completed CRT.
Results: Both miRNA-21 and miRNA-18a had significantly higher levels of expression in ESCC patients than healthy controls (95% confidence interval (CI): 5.73 - 34.79; P < 0.002 and 95% CI: 3,361.36 - 6,744.23; P < 0.001), respectively. Receiver operating characteristic (ROC) curve analysis showed that combination of serum miRNA-18a and miRNA-21 overexpression could efficiently distinguish patients of ESCC from healthy controls. The miRNA-21 expression positively correlated with tumor invasion (P < 0.004), lymphatic metastasis (P < 0.011), distant metastasis (P < 0.038), and tumor stage (P < 0.001); however, there was no such association observed with miRNA-18a. In the treatment phase (post-CRT), a significant reduction (P < 0.001) was observed in both miRNAs (73.4% in miRNA-18a and 81.02% in miRNA-21).
Conclusions: Both miRNA-21 and miRNA-18a were highly overexpressed in patients of ESCC and their expressions changed significantly with CRT. These miRNAs may be useful tools for the diagnosis and assessment of treatment response in ESCC patients. Further studies will be needed to validate these findings using large number of patients.
Keywords: Esophageal squamous cell carcinoma; MicroRNA; Chemoradiotherapy
| Introduction | ▴Top |
Esophageal squamous cell carcinoma (ESCC) is the ninth most common cancer in the world and the sixth leading cause of cancer-related death [1]. ESCC accounts for 90% of esophageal carcinomas in Asian countries [2] and remains one of the most aggressive carcinomas in the gastrointestinal tract. Recent improvements in surgical techniques and perioperative management have reduced surgery-related mortality, but even after curative surgery 5-year survival rate remains 20-40% only [3]. Therefore, ESCC must be detected at an early stage, and recurrent disease must be diagnosed when it is clinically occult, in order to improve the prognosis of patients with ESCC. Role of microRNA (miRNA) in identifying the patients of ESCC and monitoring the response to treatment modalities like chemotherapy, radiotherapy and chemoradiotherapy (CRT) is recently emerging.
The conventional serum tumor markers such as carcinoembryonic antigen (CEA) and squamous cell carcinoma antigen (SCCA), have been used for early detection and monitoring the tumor dynamics of ESCC [4]. However, these tumor markers lack sufficient sensitivity and specificity to facilitate early detection of cancer. Therefore, there is need of detecting specific novel biomarkers using a less invasive diagnostic assay for ESCC. Studies have shown role of miRNAs in carcinogenesis [5]. MiRNAs are small (typically about 22 nucleotides in size), endogenous, noncoding RNAs that regulate gene expression at the posttranscriptional level by repressing translation or decreasing miRNA stability [6]. MiRNAs have shown great potential as tissue-based markers for cancer recognition and altered miRNA expression has been reported in the tissues of various cancers [5]. Recently the role of circulating miRNA as clinical biomarker in ESCC has been emphasized. Circulating miRNAs are not only abundant in blood but also highly stable, which are important prerequisites as clinical biomarkers [7].
In this study, we evaluated the expressions of miRNA-21 and miRNA-18a in ESCC patients. As miRNA-21 is one of the most extensively researched miRNAs, but very few studies have seen its expression in serum/plasma of ESCC patients [8]. The other miRNA studied is miRNA-18a, although studies have shown its association with numerous malignancies, but to the best of our knowledge there is only one study by Hirajima et al [9], which showed its association in patients of ESCC, so we evaluated its expression in ESCC patients. In addition, we planned to study the effect of CRT on expressions of these miRNAs.
| Materials and Methods | ▴Top |
This was a hospital-based case control study, conducted over a period of 1.3 years from September 2014 to December 2015 at Sri Aurobindo Medical College and Postgraduate Institute, Indore. Institutional review board approval was taken prior to this study. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The study was divided into following parts: 1) Evaluation of expression of miRNA-21 and miRNA-18a in ESCC patients and healthy controls; 2) To look for association of various demographic and clinicopathological factors with expression of miRNA-21 and miRNA-18a in ESCC patients; 3) Evaluation of change in expression after CRT in ESCC patients.
Thirty patients of ESCC, diagnosed histologically using endoscopic biopsies, were enrolled in the study after taking written informed consent. Thirty age- and sex-matched healthy volunteers were also recruited as controls. Paired serum samples were also collected from ESCC patients who completed CRT, to look for change in expression of miRNAs. Patients with age less than 18 years, who did not give consent and with any other malignancy were excluded from our study.
Detailed clinical interview and physical examination were done at the time of enrollment. All patients underwent endoscopic evaluation using gastrointestinal video scope (Olympus, GIF Type Q150, Tokyo, Japan). Computed tomography (CT) scan of chest and abdomen was done for disease staging. Patients with T3, T4 stage, node positivity or metastatic disease on CT imaging were considered to have advanced disease. Patient’s tumor location was classified according to standard criteria of American Joint Committee on Cancer (AJCC) seventh edition, 2009 [10]. All cases of ESCC were graded histologically into well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated [11]. Dysphagia grading was done according to clinical classification [12].
Immediately after sample collection, the blood samples were centrifuged at 5,000 rpm for 10 min at 48 °C to spin down the blood cells; the supernatants containing the serum were transferred into fresh tubes and stored at -80 °C until further processing. Evaluation of serum miRNA-21 and miRNA-18a expression using quantitative real-time polymerase chain reaction (qRT-PCR) was done in all enrolled patients and controls, expressions (relative quantification (RQ)) were also seen in patients post-CRT which was assessed using 2-ΔΔCT method [13]. Total RNA was extracted from 500 µL of serum using a mirVana Isolation Kit (Ambion, USA), and finally eluted into 100 µL of preheated (95 °C) elution solution according to the manufacturer’s protocol.
The reverse transcription reaction was carried out with a TaqMan MiRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) in 15 µL containing 5 µL of RNA extract, 0.15 µL of 100 mMdNTPs, 1 µL of MultiScribe reverse transcriptase (50 U/µL), 1.5 µL of 10 × reverse transcription buffer, 0.19 µL of RNAse inhibitor (20 U/µL), 1 µL of gene-specific primers (has-miR-18a; assay ID: 002422, RNU6B; assay ID: 001973 and has-miR-21; assay ID: 000397, RNU6B; assay ID: 001973), and 4.16 µL of nuclease-free water. For the synthesis of complimentary DNA (cDNA), reaction mixtures were incubated at 16 °C for 30 min, at 42 °C for 30 min, and at 85 °C for 5 min and then held at 4 °C. Next, 1.33 µL of cDNA solution was amplified using 10 µL of TaqMan 2 × universal PCR master mix with No AmpErase UNG (Applied Biosystems), 1 µL of gene-specific primer/probe, and 7.67 µL of nuclease-free water in a final volume of 20 µL. Real-time PCR was run on a 7500 Fast Dx Real-time PCR system (Applied Biosystems, Foster City, CA). The cycle threshold (Ct) values were calculated with SDS 1.4 software (Applied Biosystems, Foster City, CA).
The miRNA RQ was calculated using the Ct values and evaluated by the 2-ΔΔCT method; where ΔCt was calculated by subtracting the Ct values of RNU6B from the Ct values of the chosen miRNA, and ΔΔCT was then calculated by subtracting mean ΔCt of the reference sample from ΔCt of tested samples [13].
Chemotherapy comprised two courses of protracted infusion of 5-fluorouracil (5-FU) 400 mg/m2 on days 1 - 5, 8 - 12, 36 - 40, and 43 - 47 with cisplatin (CDDP) 40 mg/m2 on days 1, 8, 36, and 43. Concurrent radiotherapy involved 60 Gy irradiation (30 fractions) for 8 weeks with a 2-week break [14].
We evaluated the clinical response in patients who received CRT by assessing endoscopic regression which was defined as no obvious growth seen on follow-up endoscopy [15]. Also, radiological regression was taken into account according to response evaluation criteria in solid tumors (RECIST) version 1.1 [16], in which maximum dimension of tumor deposits, i.e., unidimensional measurement and sum of these measurement was used to evaluate response assessment. In radiological regression, complete response was defined as disappearance of all disease. Partial response was defined as decrease in more than 30% in sum of maximum tumor dimension. Stable disease was defined as decrease in less than 30% but more than 20% in sum of maximum tumor dimension. Progressive disease was defined as increase in 20% in sum of maximum tumor dimension.
Data analysis was performed using MedCalc software trial version (version 15.6, MedCalc software bvba, Ostend, Belgium). Significance of difference of median of miRNA-21 and miRNA-18a between two groups was determined using Mann-Whitney U-test. The descriptive statistics were used to present clinicopathological features of patients and controls. A probability value of < 0.05 was considered significant. Receiver operating characteristic (ROC) curve analysis was applied to evaluate the overexpression of serum miRNA-21 and miRNA-18a in ESCC patients.
| Results | ▴Top |
Demographic and clinical characterizations of study population are summarized in Tables 1, 2.
 Click to view | Table 1. Demographic Characteristics of Patients and Expression Profile of MiRNA-21 and MiRNA-18a |
 Click to view | Table 2. Clinicopathological Characteristics of Patients and Expression Profile of MiRNA-21 and MiRNA-18a |
Association of clinicopathological factors with expression of miRNA-21 and miRNA-18a
Changes in expression of miRNA-21 and miRNA-18a with age, sex, risk factors, grade of dysphagia, histological grade and advanced disease were evaluated. Expression levels of miRNA-21 correlated with tumor invasion (P = 0.004), lymphatic metastasis (P = 0.011), distant metastasis (P = 0.038) and tumor stage (P = 0.001); however, there was no such association observed with miRNA-18a. There was no significant association of miRNA-21 and miRNA-18a expression with any other factors (Tables 1, 2).
Expression of miRNA-21 and miRNA-18a in ESCC patients and healthy controls
MiRNA-21 was found to be highly overexpressed (mean RQ = 5,079.48 ± 844.967) in patients with ESCC when compared to healthy controls (mean RQ = 26.68 ± 34.46) (P < 0.0001; Fig. 1a). Similarly, miRNA-18a was also significantly overexpressed (mean RQ = 22.25 ± 7.25) in ESCC patients than healthy controls (mean RQ = 1.98 ± 1.45) (P < 0.0001; Fig. 1b). ROC curve for miRNA-based diagnosis of ESCC was plotted with ROC curve expression profile obtained from microarray which showed area under curve value of 0.997 and 0.900 for miRNA-21 and miRNA-18a, respectively (Table 3, Fig. 2).
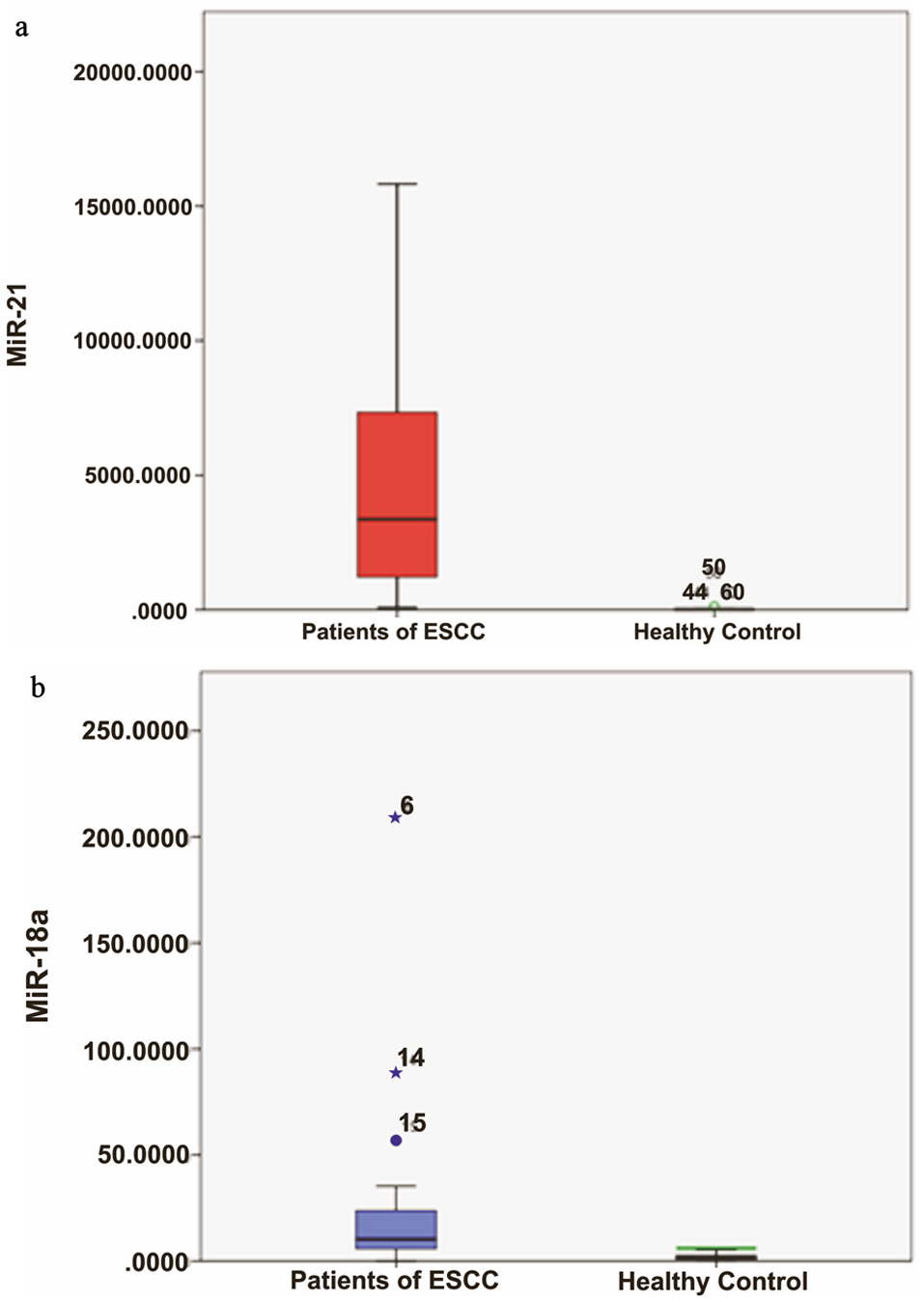 Click for large image | Figure 1. (a) Relative quantification (RQ) of miRNA-21 in ESCC patients (P) and controls (C). (b) RQ of miRNA-18a in ESCC P and C. ESCC: esophageal squamous cell carcinoma. |
 Click to view | Table 3. AUC of Expression Profile of MiRNA-21 and MiRNA-18a Using ROC Curve |
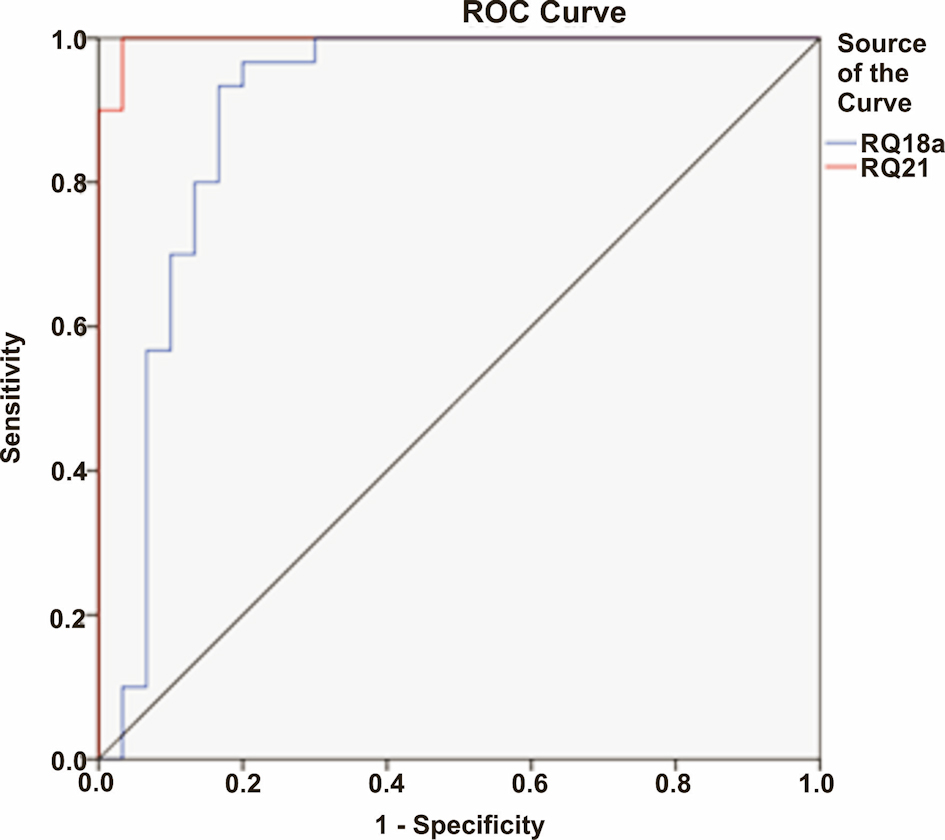 Click for large image | Figure 2. The receiver operating characteristic (ROC) curve expression profile obtained from microarray for miRNA-21 and miRNA-18a, respectively. |
Change in expression of miRNA-21 and miRNA-18a after CRT
Twenty patients underwent CRT, 16 patients due to their advanced nature of disease, i.e., T3, T4 stage or node positivity or with metastatic disease, two patients who were unfit for surgery (because of poor performance status) and two patients who were unwilling for surgery. Four patients did not complete CRT (two patients did not tolerate CRT and two patients were lost to follow-up) were excluded from the study. Sixteen patients who completed CRT responded well to therapy, 12 patients with endoscopic regression and 14 patients with radiological regression of tumor. Expressions of miRNA-21 and miRNA-18a were also seen after completion of CRT in these patients. Patients who completed CRT had significant reduction of miRNA-21 (P < 0.0001; Fig. 3a) and miRNA-18a (P < 0.0001; Fig. 3b) expressions. The patients who had complete response or partial response to CRT (radiological response according to RECIST version as described earlier) had significantly higher percentage reduction (88.58±4.94%) of miRNA-21 expression as compared to patients who had stable disease or progressive disease (58.37 ± 16.22) (P = 0.001). However, no such significant association was seen with miRNA-18a (P = 0.446).
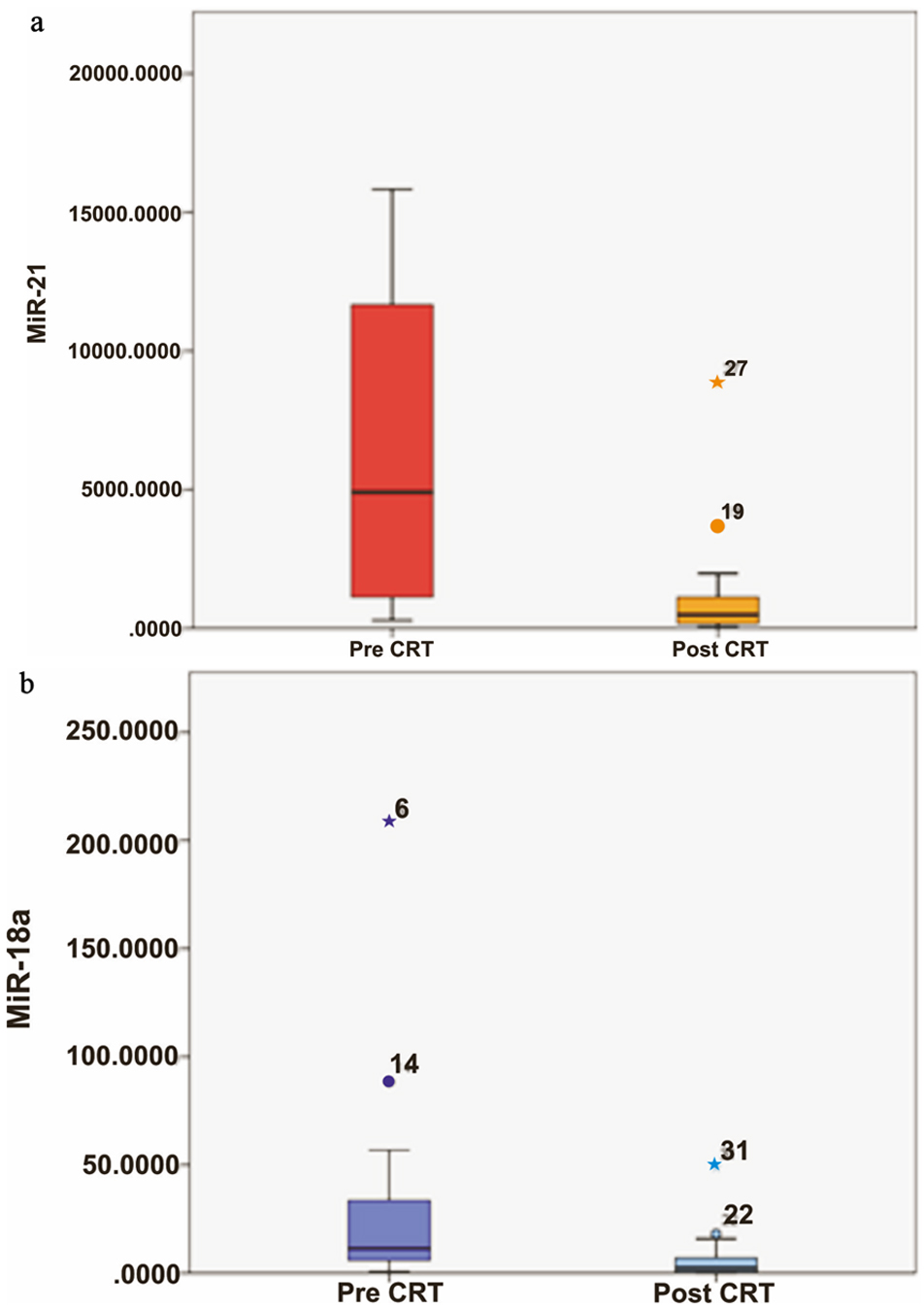 Click for large image | Figure 3. (a) Relative quantification (RQ) of miRNA-21 in ESCC patients in treatment-naive patient (pre-CRT) and after completion of their CRT (post-CRT). (b) RQ of miRNA-18a in ESCC patients in pre-CRT and post-CRT. ESCC: esophageal squamous cell carcinoma; CRT: chemoradiotherapy. |
| Discussion | ▴Top |
In present study we enrolled 30 patients of ESCC, evaluated expression of circulating miRNA-21 and miRNA-18a, and also looked for the effect of CRT on expression of these miRNAs in 16 patients who completed CRT. We found that miRNA-21 and miRNA-18a were highly overexpressed in patients of ESCC as compared to healthy controls. There was no significant association of these miRNAs expression with age, sex, risk factors, degree of dysphagia and histological grading. MiRNA-21 expression was significantly associated with tumor invasion, node positivity, metastatic disease and advanced stage of tumor, however, no such association was seen with miRNA-18a. Expression of miRNA-21 and miRNA-18a significantly reduced after completion of CRT.
We studied diagnostic role of circulating miRNA-21 and miRNA-18a in ESCC patients, as there has been increasing interest in circulating miRNAs as cancer biomarkers, due to their high stability, their putative capability to be more informative than messenger RNA. Numerous studies showed miRNA-21 overexpression similar to our study in ESCC patients (Mitchell et al [17], Kurashige et al [18], and Tanaka et al [19]). The role of circulating miRNA-18a in plasma of patients with ESCC was depicted in a study conducted by Hirajima et al [9]. Because of the paucity of data available on expression of miRNA-18a in ESCC, we included this marker to study its role in diagnosis as well as prognosis.
Our observation that miRNA-21 expression correlated with tumor invasion, lymphatic invasion, metastatic disease and advanced stage of tumor were agreed to the previous studies (Kurashige et al [18], Tanaka et al [19], and Akagi et al [20]). All these observations suggest miRNA-21 overexpression predicts tumor staging and tumor dynamics. In contrast to miRNA-21, we did not find any significant association of clinicopathological factors with miRNA-18a expression similar to the study by Hirajima et al [9].
We also looked expression profile of miRNA-21 and miRNA-18a in ESCC patients who completed CRT and found significant reduction in their expression. These findings were consistent with study by Kurashige et al, who also reported decline in expression of serum miRNA-21 with chemotherapy [18]. MiRNA-21 expression reduction was significant in patients who had tumor reduction after CRT than patients who had progressive disease or non-responders to CRT, thus change in miRNA-21 expression with CRT can predict tumor responsiveness.
There were few limitations in our study. Firstly, the number of patients enrolled in study was small as this was single institution-based study. Secondly, we did not use qRT-PCR due to limited research funds, so we assessed expression of miRNA by RQ method.
Although endoscopy and biopsy are still the gold standard method for detecting ESCC, their drawback being invasive procedure, insensitivity to detect small lesion, sampling error and the need of repeated endoscopy to monitor the response of treatment, has limited their efficacy. However, to date there are no population-based screening test recommended for ESCC. The option in future might be annual screening of circulating miRNA in asymptomatic populations in high risk areas of ESCC, and those with positive test result can be subjected to endoscopic biopsy for final diagnosis.
There is tendency for esophageal carcinoma to spread rapidly due to lack of serosa and consequently most of the patients have advanced disease at presentation. Henceforth, the development to search for novel biomarker which can detect at an earlier stage remains an emerging challenge. In addition, there is need to search for tumor-specific miRNA for early detection of esophageal carcinoma and prediction of tumor invasion, node positivity, metastatic disease, advanced stage and tumor responsiveness to CRT. As the resources are limited in developing country, there is minimal role of invasive endoscopy at an earlier stage in lieu of economic burden. Therefore, there is need to screen the high-risk population for ESCC at the earlier stage with non-invasive biomarker like miRNA.
Furthermore, tumor burden could be correlated with the upregulation of circulating miRNAs. MiRNA expression may be used in future to monitor response to therapy or early detection of tumor recurrence after treatment. These miRNAs can be studied for their possible roles as putative therapeutic targets. MiRNAs are evolving area of research, and the research is still in its infancy. More research in this field will give us knowledge in the management of this aggressive malignancy.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All the authors have no conflict of interest to declare.
Informed Consent
Informed consent was taken from all patients prior to their inclusion in the study.
Author Contributions
MTN and NS hypothesized the study. MTN and NS designed the study, performed the data analysis and interpretation. MTN and BST did the literature review and supervised the study. MTN and NS collected and processed the data MTN, BST, JR, and RS wrote the manuscript.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
miRNA: microRNA; ESCC: esophageal squamous cell carcinoma; CRT: chemoradiotherapy; qRT-PCR: quantitative real-time polymerase chain reaction
| References | ▴Top |
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
doi pubmed - Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121(8):1643-1658.
doi pubmed - Ruol A, Castoro C, Portale G, Cavallin F, Sileni VC, Cagol M, Alfieri R, et al. Trends in management and prognosis for esophageal cancer surgery: twenty-five years of experience at a single institution. Arch Surg. 2009;144(3):247-254; discussion 254.
doi pubmed - Kosugi S, Nishimaki T, Kanda T, Nakagawa S, Ohashi M, Hatakeyama K. Clinical significance of serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen levels in esophageal cancer patients. World J Surg. 2004;28(7):680-685.
doi pubmed - Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857-866.
doi pubmed - Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281-297.
doi - Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997-1006.
doi pubmed - Nouraee N, Van Roosbroeck K, Vasei M, Semnani S, Samaei NM, Naghshvar F, Omidi AA, et al. Expression, tissue distribution and function of miR-21 in esophageal squamous cell carcinoma. PLoS One. 2013;8(9):e73009.
doi pubmed - Hirajima S, Komatsu S, Ichikawa D, Takeshita H, Konishi H, Shiozaki A, Morimura R, et al. Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2013;108(9):1822-1829.
doi pubmed - American Joint Commission for cancer staging and end results reporting eophageal cancer. Manual for Staging of cancer. 1977:65-70.
- Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. Lyon, France: IARC Press. 2010.
- Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31(5):1213-1236.
doi - Li L, Guo Z, Wang J, Mao Y, Gao Q. Serum miR-18a: a potential marker for hepatitis B virus-related hepatocellular carcinoma screening. Dig Dis Sci. 2012;57(11):2910-2916.
doi pubmed - Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, Takiuchi H, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81(3):684-690.
doi pubmed - Lim JT, Truong PT, Berthelet E, Pai H, Joe H, Wai E, Larsson S, et al. Endoscopic response predicts for survival and organ preservation after primary chemoradiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57(5):1328-1335.
doi - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513-10518.
doi pubmed - Kurashige J, Kamohara H, Watanabe M, Tanaka Y, Kinoshita K, Saito S, Hiyoshi Y, et al. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2012;106(2):188-192.
doi pubmed - Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119(6):1159-1167.
doi pubmed - Akagi I, Miyashita M, Ishibashi O, Mishima T, Kikuchi K, Makino H, Nomura T, et al. Relationship between altered expression levels of MIR21, MIR143, MIR145, and MIR205 and clinicopathologic features of esophageal squamous cell carcinoma. Dis Esophagus. 2011;24(7):523-530.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


