| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 11, Number 6, December 2018, pages 422-425
Isolated Gastric Metastasis of Endometrial Adenocarcinoma: First Case Report and Review of Pertinent Literature
Min Cuia, b, Xiaofei Zhangc, Noam Harpazc, d
aDepartment of Pathology, School of Medicine, Case Western Reserve University, Cleveland, OH 44106, USA
bUniversity Hospitals Cleveland Medical Center, Cleveland, OH 44106, USA
cDepartment of Pathology, Icahn School of Medicine, The Mount Sinai Hospital, New York, NY 10029, USA
dCorresponding Author: Noam Harpaz, Department of Pathology, Icahn School of Medicine, The Mount Sinai Hospital, 1 Gustave L. Levy Pl, New York, NY 10029, USA
Manuscript submitted July 8, 2018, accepted July 30, 2018
Short title: Gastric Metastasis of Endometrial Carcinoma
doi: https://doi.org/10.14740/gr1071w
| Abstract | ▴Top |
Upper gastrointestinal metastasis of endometrial carcinoma rarely occurs in the absence of locoregional disease and other distant metastases. We describe herein the unique case of an isolated gastric metastasis of a stage I endometrial adenocarcinoma. Because the metastatic tumor was initially misdiagnosed clinically and pathologically as a primary gastric carcinoma, we illustrate the histopathology and review the pertinent literature. A 42-year-old woman with Lynch syndrome underwent treatment of endometrial adenocarcinoma at an outside hospital comprising clinical and radiological staging including a positron emission tomography-computed tomography (PET-CT) scan followed by a total intra-abdominal hysterectomy, bilateral salpingoophorectomy and pelvic and paraaortic lymphadenectomy. The preoperative and pathology findings were consistent with a stage I tumor. Three months postoperatively, a PET-CT scan revealed a new 4.4 cm hypermetabolic lesion in the stomach. A biopsy of the lesion was interpreted pathologically as gastric adenocarcinoma with lymphoid stroma. Upon referral of the patient to our center for management, the biopsy was reviewed in consultation and the pathology materials from the hysterectomy procedure were retrieved for comparison. Based on the morphological and immunohistochemical similarities between the tumors the gastric tumor was diagnosed as metastatic endometrial adenocarcinoma. To our knowledge, this is the first documented case of an isolated gastric metastasis complicating stage I endometrial adenocarcinoma. Awareness of the potential for this occurrence and of the associated diagnostic pitfalls is crucial for accurate diagnosis and therapy.
Keywords: Endometrial carcinoma; Stomach; Metastasis; Lynch syndrome; PAX-8
| Introduction | ▴Top |
Upper gastrointestinal metastasis of endometrial carcinoma rarely occurs in the absence of locoregional disease and other distant metastases. Based on autopsy data, metastatic uterine and cervical tumors of all types account for only 4-5% of secondary gastric tumors, are associated with carcinomatosis, and are rarely diagnosed antemortem [1, 2]. Documented cases in which the gastrointestinal tract was involved by isolated metastases are exceedingly rare. Bosscher et al reported a case of endometrial carcinoma that metastasized to the small intestine [3]. Another study published in abstract form reported one case of endometrial carcinoma among 38 patients (3%) with metastatic tumors involving the upper gastrointestinal tract during a 17-year interval [4]. The complete clinical scenario is unavailable. We describe herein the unique case of an isolated gastric metastasis of a stage I endometrial adenocarcinoma. To our knowledge the only previous report of isolated gastric metastasis from the endometrium did not provide sufficient morphological or immunohistochemical documentation to exclude an alternative primary site [5].
| Case Report | ▴Top |
A 42-year-old woman with a family history of Lynch syndrome presented with menorrhea. She was diagnosed with stage I endometrial adenocarcinoma based on clinical and radiological evaluation which included a positron emission tomography-computed tomography (PET-CT) examination. A hysterectomy was performed which contained a FIGO grade 3 endometrioid adenocarcinoma featuring extensive lymphovascular invasion, with depth of invasion 1.1 cm of 2.5 cm myometrium. The accompanying lymph nodes (12 right pelvic, one right para-aortic, eight left pelvic and 10 left para-aortic) were negative for tumor.
Immunohistochemical staining for DNA mismatch repair proteins of the hysterectomy specimen revealed retained expression of MLH1 and PMS2 accompanied by aberrant cytoplasmic expression and loss of nuclear expression of MSH2 and MSH6 in the tumor cells. Postoperatively, the patient underwent a genetic evaluation which revealed heterozygous MSH2 mutation c1906G>C (p.Ala636pro).
Three months postoperatively, a follow-up PET-CT examination revealed a new 4.4-cm hypermetabolic lesion in the gastric wall with mucosal and mural involvement. No other lesions were detected. An endoscopic biopsy of the gastric lesion was performed and diagnosed as “undifferentiated malignant neoplasm most consistent with gastric carcinoma with lymphoid stroma (lymphoepithelial-like carcinoma/medullary carcinoma)”. An endoscopic ultrasound examination was consistent with uT3N1Mx gastric carcinoma.
The patient was referred to our oncology service and her biopsy slides were reviewed in consultation. Based on the referral diagnosis of gastric carcinoma, the plan was to administer neoadjuvant chemotherapy followed by surgical resection of the gastric lesion.
As shown in Figure 1a, H&E-stained sections of the gastric lesion revealed a poorly differentiated carcinoma characterized by dyscohesive tumor cells with a solid growth pattern, prominent necrosis, high nuclear-to-cytoplasmic ratios, large, round to oval nuclei, prominent nucleoli, frequent mitoses and variable amounts of cytoplasm. No obvious gland formation was identified. Scattered intratumoral lymphocytes were noted and confirmed by immunohistochemical staining for leukocyte common antigen (LCA). Referred immunohistochemical stains of the tumor cells were focally positive for AE1/3, diffusely positive for CAM-2 (Fig. 1b) and negative for S100, LCA and CDX-2 (Fig. 1c). Additional immunohistochemical stains performed in our laboratory were diffusely positive for PAX-8 (Fig. 1d) and focally positive for estrogen and progesterone receptor proteins. Because of doubts regarding the referral diagnosis, the biopsy slides were compared with slides from the hysterectomy specimen. This disclosed a moderately differentiated endometrioid adenocarcinoma accompanied by a portion of poorly differentiated carcinoma with tumor necrosis and extensive lymphovascular invasion (Fig. 2). Based on the morphological similarity of the latter component to the biopsy tissue and the immunohistochemical findings, the gastric neoplasm was diagnosed as metastatic endometrial carcinoma.
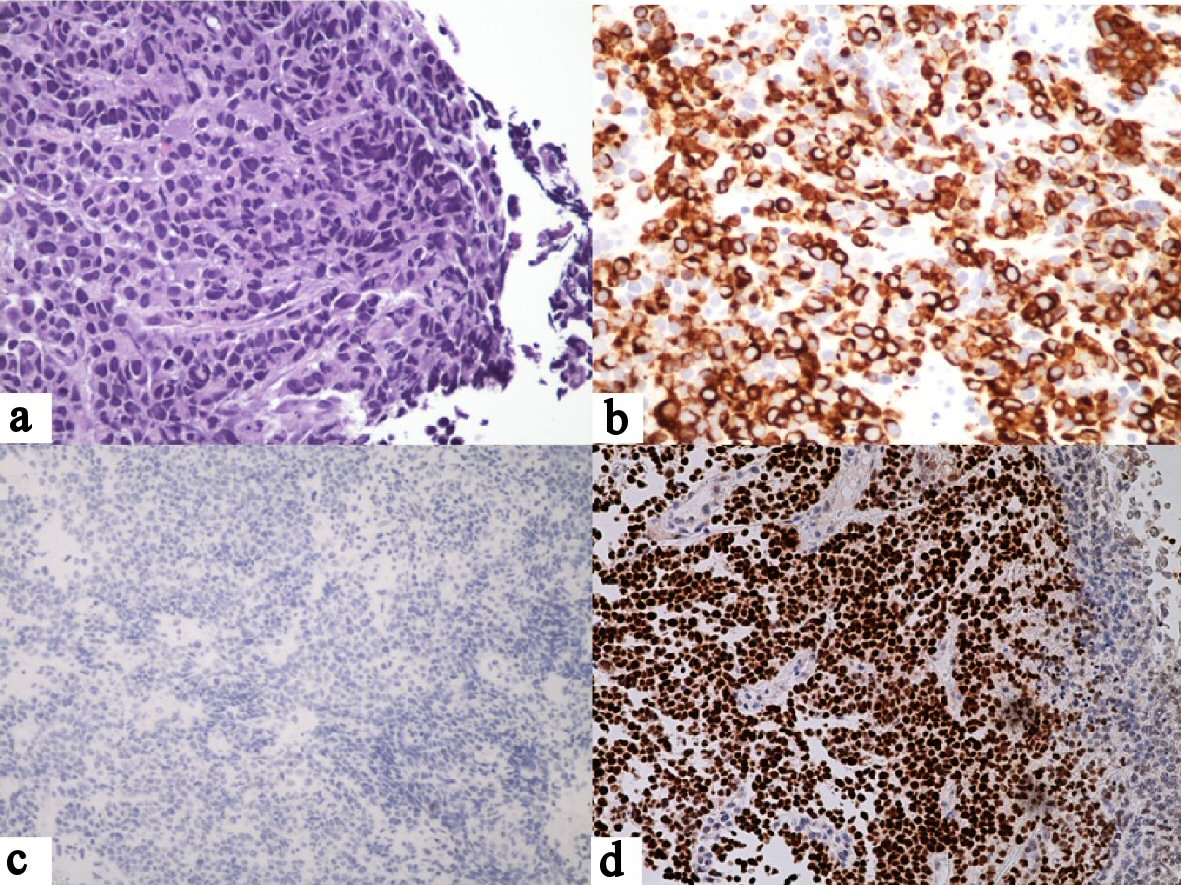 Click for large image | Figure 1. Gastric biopsy. (a) Large tumor cells with solid growth pattern, large, pleomorphic nuclei and high nuclear-to-cytoplasmic ratios. There are scattered intratumoral lymphocytes but no significant peritumoral lymphocytosis (H&E, × 40). (b) Positive tumor staining for CAM5.2 (× 20). (c) Negative tumor staining for CDX-2 (× 20). (d) Positive tumor staining for PAX-8 (× 20). |
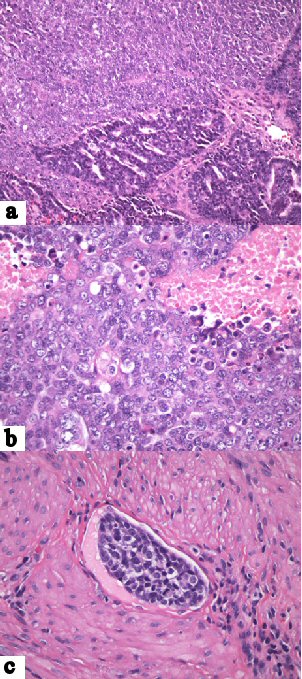 Click for large image | Figure 2. Endometrial carcinoma. (a) Sharply demarcated regions of moderately and poorly differentiated carcinoma (H&E, × 20). (b) Poorly differentiated tumor component featuring solid growth pattern, large, pleomorphic nuclei, high nuclear-to-cytoplasmic ratios and necrosis (× 40). (c) Lymphovascular invasion by poorly differentiated component (× 40). |
The patient underwent chemotherapy with 5-fluorourcil, leucovorin, oxaliplatin and docetaxel (FLOT) starting 1 month after the biopsy. A partial gastrectomy performed 2 months later showed no residual carcinoma.
| Discussion | ▴Top |
Gastric carcinoma with lymphoid stroma, also referred to as medullary carcinoma and lymphoepithelioma-like carcinoma, is a rare entity that was first reported in 1976 [6]. It is characterized microscopically by pushing tumor borders, cohesive tumor cells with a syncytial growth pattern, indistinct tumor cell borders, intratumoral lymphocytes and non-desmoplastic peritumoral stroma containing abundant lymphocytes and plasma cells, and either microsatellite instability or evidence of intratumoral Epstein-Barr virus antigens [7-9].
Lynch syndrome patients are at increased risk of developing gastric carcinoma. One study has reported a standardized incidence ratio of 3.4 and mean age of diagnosis of 56 years [10]. These cancers are more commonly intestinal type than diffuse type [11, 12]. There is little data regarding the prevalence of medullary carcinoma of stomach in patients with Lynch syndrome, presumably due to its rarity. In a recently published study, three of five patients with gastric medullary carcinoma were determined to have Lynch Syndrome [8].
In our case the diagnosis of carcinoma with lymphoid stroma was questioned on the basis of the dyscohesiveness of the tumor cells and sparse intratumoral mononuclear cells with no prominent lymphocytes and plasma cells infiltrates which did not meet the required diagnostic criteria.
The diagnosis of metastatic endometrial carcinoma was suggested by immunohistochemical tumor expression of PAX-8. PAX-8 is a transcriptional factor for organogenesis of Mullerian system, thyroid gland and kidney. Its value in differentiating endometrial from gastric carcinoma has been established in the literature. One comprehensive immunohistochemical study of over 1,100 normal and neoplastic tissues reported PAX-8 expression in 113 of 134 endometrial cancers (84%) and its absence in six of six gastric cancers (100%) [13]. Another study of 1,357 tumors from various organs reported PAX-8 expression in 152 of 155 endometrial carcinomas (98%) and its absence in 32 of 32 gastric carcinomas (100%) [14].
Conclusions
To our knowledge, this is the first documented case of an isolated gastric metastasis from endometrial adenocarcinoma and is all the more unusual in that the primary tumor was clinically and pathologically stage I. Awareness of this potential occurrence and the associated diagnostic pitfalls is crucial for accurate diagnosis and therapy.
Funding
None.
Conflict of Interest
We do not have any financial or non-financial potential conflict of interest.
Author Contributions
Cui M: conception, acquisition of data, drafting the article; Zhang X: acquisition of data; Harpaz N: critical revisions, final approval.
| References | ▴Top |
- Oda, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33(6):507-510.
doi pubmed - Moldovan B, Banu E, Pocreata D, Buiga R, Rogoz S, Pripisi L, Cimpeanu L, et al. Gastric metastasis of cervix uteri carcinoma, rare cause of lower gastric stenosis. Chirurgia (Bucur). 2012;107(6):816-820.
- Bosscher J, Barnhill D, O'Connor D, Park R. Clinical stage IB endometrial adenocarcinoma with an isolated small bowel metastasis. Gynecol Oncol. 1994;52(1):99-101.
doi pubmed - Patel V, Nasri E, Kresak J, et al. Metastatic malignancy to the upper gastrointestinal tract encountered on endoscopic biopsy: a clinicopathologic study. Mod Pathol. 2017;30:157-210 [775].
- Clavero-Fernandez E, Souto-Ruzo J, Alonso-Aguirre P, Suarez-Fuentetaja R, Alvarez-Martinez M. Gastric metastasis originating from an adenocarcinoma of the endometrium. Rev Esp Enferm Dig. 2015;107(7):436-437.
pubmed - Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38(1):232-243.
doi - Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16(7):641-651.
doi pubmed - Gonzalez RS, Cates JMM, Revetta F, McMahon LA, Washington K. Gastric carcinomas with lymphoid stroma: categorization and comparison with solid-type colonic carcinomas. Am J Clin Pathol. 2017;148(6):477-484.
doi pubmed - Chetty R. Gastrointestinal cancers accompanied by a dense lymphoid component: an overview with special reference to gastric and colonic medullary and lymphoepithelioma-like carcinomas. J Clin Pathol. 2012;65(12):1062-1065.
doi pubmed - Aarnio M, Salovaara R, Aaltonen LA, Mecklin JP, Jarvinen HJ. Features of gastric cancer in hereditary non-polyposis colorectal cancer syndrome. Int J Cancer. 1997;74(5):551-555.
doi - Gylling A, Abdel-Rahman WM, Juhola M, Nuorva K, Hautala E, Jarvinen HJ, Mecklin JP, et al. Is gastric cancer part of the tumour spectrum of hereditary non-polyposis colorectal cancer? A molecular genetic study. Gut. 2007;56(7):926-933.
doi pubmed - Park YJ, Shin KH, Park JG. Risk of gastric cancer in hereditary nonpolyposis colorectal cancer in Korea. Clin Cancer Res. 2000;6(8):2994-2998.
pubmed - Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19(4):293-299.
doi pubmed - Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, Chirieac LR, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35(6):816-826.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


