| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Review
Volume 17, Number 2, April 2024, pages 53-63
Clinical Overview of Sarcopenia, Frailty, and Malnutrition in Patients With Liver Cirrhosis
Alexander Kusnika, d, Amulya Penmetsab, Farooq Chaudharya, Keerthi Renjitha, Gopal Ramarajub, Marie Laryeab, Johane P. Allardc
aDepartment of Internal Medicine, Unity Hospital, Rochester, NY, USA
bDivision of Gastroenterology and Hepatology, University of Rochester Medical Center, Rochester, NY, USA
cDepartment of Medicine, Toronto General Hospital, University of Toronto, Toronto, Ontario, Canada
dCorresponding Author: Alexander Kusnik, Department of Internal Medicine, Unity Hospital, Rochester, NY 14626, USA
Manuscript submitted February 26, 2024, accepted April 3, 2024, published online April 30, 2024
Short title: Sarcopenia, Frailty, and Malnutrition
doi: https://doi.org/10.14740/gr1707
- Abstract
- Introduction
- Factors Contributing to Sarcopenia, Frailty, and Malnutrition in Patients With Cirrhosis
- Conclusion
- References
| Abstract | ▴Top |
Sarcopenia, frailty, and malnutrition in patients with liver cirrhosis are commonly observed and are associated with higher long-term mortality. Therefore, recognizing patients with increased nutritional risk and providing recommended interventions are essential in the long- and short-term management of cirrhosis, especially as alcoholic and non-alcoholic fatty liver disease continues to rise. Various assessment tools are available to gauge frailty and malnutrition but are infrequently used. Given the global burden of liver cirrhosis, periodic screening for malnutrition, sarcopenia, and frailty is desperately needed as it improves liver transplantation outcomes. Necessary steps include addressing knowledge gaps in professional healthcare workers and patients and using standardized assessment tools to counteract physical deconditioning as early as possible. One potential method for assessing sarcopenia involves using computed tomography to evaluate the skeletal muscle index. Regarding frailty, useful tools for longitudinal assessment include the liver frailty index and the Karnofsky performance status. Addressing educational requirements related to malnutrition involves seeking guidance from dieticians, who can provide counseling on achieving sufficient calorie and protein intake to combat the progression of malnutrition.
Keywords: Frailty; Sarcopenia; Liver cirrhosis; Malnutrition; Physical deconditioning
| Introduction | ▴Top |
The etiology of malnutrition, sarcopenia, and frailty in patients with underlying liver cirrhosis is multifactorial. Individuals with liver disease often experience sarcopenia, frailty, and malnutrition, significantly affecting their well-being and overall quality of life.
This review aims to provide a clinical framework for evaluating these conditions within routine clinical practice. Despite the paramount importance of this subject, proficiency in assessing and understanding the specified conditions is likely limited, particularly in institutions not specialized in hepatology and transplant hepatology. We present a clinical synopsis emphasizing critical aspects and the screening tools available for integration into managing patients with liver disease. These conditions overlap with cachexia, another wasting syndrome commonly occurring in individuals with chronic illnesses. In patients with cirrhosis, malnutrition is characterized by the depletion of skeletal muscle and adipose tissue mass. Although this combination is commonly referred to as cachexia, recent studies suggest that the primary nutritional consequence in cirrhosis is the loss of skeletal muscle mass [1], or sarcopenia, due to its more prominent occurrence [2]. Improving oral intake or providing nutritional support can usually correct malnutrition. Conversely, sarcopenia is commonly associated with aging, reduced mobility, or mild chronic inflammation that may not fully respond to nutritional interventions and exercise [3]. In contrast, cachexia is typically linked to catabolism associated with disseminated cancer or organ failure, such as liver or cardiac failure [4]. It is generally resistant to nutritional interventions or exercise. Frailty can be defined as a syndrome that involves a progressive decline in several physiological systems due to reduced physiologic reserve, resulting in vulnerability and adverse outcomes [1, 3].
Sarcopenia is more frequent in end-stage liver cirrhosis, particularly in patients listed for transplant. Therefore, recognizing patients at nutritional risk and providing adequate support is essential to management [5, 6]. However, a cohesive approach is required, and long-term solutions to counteract sarcopenia are desperately needed [7, 8]. Multiple studies indicate higher mortality and rates of complications among frail and sarcopenic cirrhotic patients, even after liver transplantation [9]. Furthermore, it is noteworthy that frailty and sarcopenia can reciprocally influence each other; compromised muscle contractile function can expedite the decline of muscle mass, and conversely, the loss of muscle mass can exacerbate impaired muscle function [9]. As alcoholic liver disease and non-alcoholic fatty liver disease continue to rise [10], sarcopenic obesity, the co-existence of low muscle mass and increased fat mass [10] is becoming more commonly observed [11]. The pathophysiology of sarcopenia and frailty in liver cirrhosis is complex and multifactorial, requiring a multidisciplinary approach that includes physicians, dieticians, pharmacists, and physiotherapists to overcome physical deconditioning and improve survival.
| Factors Contributing to Sarcopenia, Frailty, and Malnutrition in Patients With Cirrhosis | ▴Top |
Frailty, sarcopenia, and malnutrition are tightly connected and encompass many similar pathophysiological processes. Multiple studies showed the significance of socioeconomic factors on the burden of liver disease. Firstly, most patients with liver cirrhosis lack the knowledge to manage their condition and dietary intake effectively; unsurprisingly, low educational status is associated with increased severity of liver disease [12]. Secondly, many patients are unaware of the degree of their liver disease and desperately need proper education [13-15]. Thirdly, food insecurity, defined as a lack of consistent access to food for an active, healthy life, increases the risk of advanced liver fibrosis [16]. Particular dietary practices may also contribute to the problem. For example, ascites is one of the most common complications in liver cirrhosis [17]. As first-line therapy, it is treated with combined salt restriction and diuretic therapy per most clinical guidelines. However, recent studies [18], e.g., by Morando et al [13], indicated that patients who comply with the daily salt restriction significantly reduce caloric intake, leading to the worsening of pre-existing sarcopenia and frailty. Studies suggest that an overnight fast in a cirrhotic patient is comparable to prolonged starvation in healthy individuals. This relates to the rapid exhaustion of available glycogen and diminished gluconeogenesis in damaged hepatocytes. For this reason, hypoglycemia can be considered a poor prognostic sign in patients with decompensated liver cirrhosis [19]. Hospitalizations are often associated with procedures (e.g., for variceal banding) and inevitably require frequent fasting [20, 21], adding to the vicious cycle of inadequate nutrition.
Finally, system issues such as a lack of recognition of sarcopenia, malnutrition, or frailty by healthcare professionals [22], lack of screening and assessment processes, and inadequate multidisciplinary nutritional interventions contribute further to nutritional deterioration [23].
At a physiologic level, hypermetabolism, malabsorption, and a chronic inflammatory state are the main drivers for worsening sarcopenia, malnutrition, and frailty. Hypermetabolism in liver cirrhosis describes the presence of increased resting energy expenditure (REE). REE is the quantity of energy required for the body to perform vital organ functions without additional activities. Multiple studies indicated that more than one-third of patients with liver cirrhosis are in a state of hypermetabolism [24]. The observed increase in REE is poorly understood, but contributory factors include increased concentrations of circulating catecholamines [24], pro-inflammatory cytokines like tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and IL-33 [25, 26]. Increased circulating catecholamines increase sympathetic nervous system activation and muscle breakdown [27]. In addition, increased circulating pro-inflammatory cytokines are involved in the pathophysiology of anorexia, resulting in an insufficient dietary intake [28].
Malabsorption is frequently observed in patients with liver cirrhosis. The origin of malabsorption is of multifactorial origin. Pace et al [29] showed in a post-mortem study that alcoholic liver cirrhosis and chronic pancreatitis often occur concurrently and, therefore, might exacerbate inadequate nutrition and malabsorption resulting in a deficiency of fat-soluble vitamins [30, 31]. Additionally, guideline-recommended use of diuretics can result in deficiencies of zinc [32] and magnesium if not substituted adequately [9]. Other commonly observed micronutrient deficiencies include selenium [33] and iron [34]. Liver cirrhosis also causes impaired urea synthesis [35], resulting in hyperammonemia, directly affecting the liver-muscle axis [25, 36]. These alterations lead to a dysregulated molecular signaling cascade that evolves into sarcopenia. Moreover, it is firmly established that modifications in the endocrine system within the context of liver disease impact the liver-muscle axis. This includes a decline in testosterone levels, partly influenced by heightened aromatase activity [37], and the altered response of muscles to growth hormone (GH) [38]. Sinclair et al [39] examined the impact of testosterone replacement on patients with liver cirrhosis and sarcopenia. Their study involving 101 males with low testosterone levels demonstrated that testosterone replacement enhances muscle mass, decreases fat mass, and improves glucose metabolism.
However, the replacement of testosterone should be avoided in individuals with a history of hepatocellular carcinoma, malignancy, or previous thrombosis and in female patients, thus limiting its usage in these patient cohorts. The changed muscle reaction to GH in individuals with liver cirrhosis is predominantly ascribed to fluctuations in GH secretion and sensitivity. GHs prompt the generation of insulin-like growth factor 1 (IGF-1), which functions as a muscle-building agent [40]. A recent study conducted by Kumari et al [40], utilizing an open-label, randomized controlled trial design, investigated the impacts of prolonged GH treatment in individuals with decompensated cirrhosis. This study comprised two groups, each of 38 patients, randomly assigned in a 1:1 ratio. GH was administered subcutaneously based on the levels of IGF-1. The findings of the study demonstrated a correlation with enhanced nutritional status; however, there was no observed influence on survival rates after 12 months [41].
Sarcopenia in liver cirrhosis
Sarcopenia in patients with cirrhosis entails “a progressive and generalized skeletal muscle disorder associated with an increased likelihood of adverse outcomes including falls, fractures, disability, and mortality” [40, 42] (Table 1). A possible approach to evaluating sarcopenia includes assessing the skeletal muscle index (SMI) via computed tomography [43]. The SMI is defined as a muscle cross-sectional in cm2 at a vertebral level normalized by the square of the height in m2. Regarding the vertebral level, multiple studies indicate a highly prognostic value of the SMI at the third lumbar vertebrae (L3-SMI) [44, 45], but also the level at T12 showed a correlation with mortality [46]. Muscle mass measurement can be obtained from pre-existing imaging, often available as part of screening for hepatocellular carcinoma or surgical planning [47]. However, obtaining routine imaging to assess sarcopenia is not recommended [40, 48]. This technique defines sarcopenia as an SMI < 50 cm2/m2 in males and < 39 cm2/m2 in females. Other practical methods to clinically assess sarcopenia at the bedside are combining anthropometric measurements like mid-arm muscle circumference [49, 50] or subjective global assessment [51] to determine muscle wasting [52] by using handgrip strength to determine muscle weakness [53, 54]. Combining these techniques will provide a clinical estimation of the presence and degree of sarcopenia. If available, bioelectrical impedance analysis with phase angle measurement is also another tool to assess body composition and determine muscle mass [55]. The technique of bioimpedance analysis (BIA) involves measuring the electrical impedance of body tissues to evaluate body composition. It is a valuable tool for determining the severity of malnutrition in people with liver cirrhosis. It can provide information about changes in both skeletal muscle mass and body fat mass [56]. Phase angle is a value derived from BIA that represents the ratio of reactance to electrical current as it passes through the body’s tissues. BIA has some limitations, including susceptibility to errors from fluid retention, edema, and ascites [57]. Additionally, it may not be readily available in all healthcare settings. The assessment of sarcopenia is vital as multiple studies indicate the independent association between waitlist and post-liver transplant morbidity [58]. Masuda et al [59] demonstrated in a study that sarcopenia was predictive of postoperative sepsis (hazard ratio = 5.31, P = 0.009) and mortality in post-living donor liver transplantation. Other studies, including a meta-analysis published by van Vugt et al [60], confirmed similar outcomes. These conclusions lend credence to the idea of adding sarcopenia to our current scoring systems, such as the model for end-stage liver disease (MELD) score, to create a more accurate assessment of the health of our patients [61].
 Click to view | Table 1. A Summary of the Definitions for Sarcopenia, Frailty, and Malnutrition, as Well as the Screening Tools Available for Assessing These Conditions |
Patients with liver cirrhosis are often obese, particularly in the context of nonalcoholic fatty liver disease (NAFLD) [62], and a proportion of these patients may have sarcopenia [63]. Sarcopenic obesity relates to a condition of decreased muscle mass accompanied by increased fat mass. As a standardized definition of sarcopenia is not entirely established, certain definitions also include reduced muscle strength or physical performance [42]. Current definitions entail the concurrence of a body mass index (BMI) ≥ 30 kg/m2 and a low-age adjusted SMI [64]. Multiple studies indicated that sarcopenic obesity is an independent risk factor correlating with mortality and risk of decompensation [64].
However, simple weight loss can not be universally recommended to each patient with sarcopenic obesity as it might lead to worsening frailty and sarcopenia [65, 66]. Caution is warranted in patients with decompensated liver cirrhosis, and weight loss should be accompanied by adequate protein intake and regular support from a multidisciplinary team. Current guidelines recommend a combination of aerobic and resistance exercises based on the frequency, intensity, time, and type (FITT) principles [40]. No data are available concerning the duration and frequency of activity. It is rational to follow the Centers for Disease Control guidelines for at least 150 - 300 min of moderate-intensity (or 75 - 150 min of vigorous-intensity, or an equivalent combination of both) aerobic activity spread throughout the week. Muscle-strengthening activities involving all major muscle groups are recommended at least twice weekly. Regular exercise has shown many positive effects in frail and cirrhotic patients. For example, Zenith et al showed an improved cardiopulmonary function after only 8 weeks of weight exercise compared with controls (95% confidence interval (CI): 2.9 - 7.8; P = 0.001) [67], and Roman et al indicated in a small sample size risk a decreased risk of falls as seen by an improved Timed Up&Go (TUG) test compared to baseline (9.6 ± 0.4 s vs. 9.1 ± 0.4 s, difference -0.50 s, 95% CI: -0.12 to -0.87, P = 0.02) [68]. It should be highlighted that the studies mentioned above only included small sample sizes.
Regarding more invasive measures, studies showed that transjugular intrahepatic portosystemic stent shunt (TIPS) placement might offer simultaneous advantages in improving nutritional parameters and body composition, likely related to the decrease in ascites [69]. Still, a TIPS should be only placed for standard indications and not for the sole purpose of sarcopenia and/or frailty. To further underscore the complexities in diagnosing sarcopenia, we created an algorithm, as shown in Figure 1, that delineates each of the steps mentioned in this section.
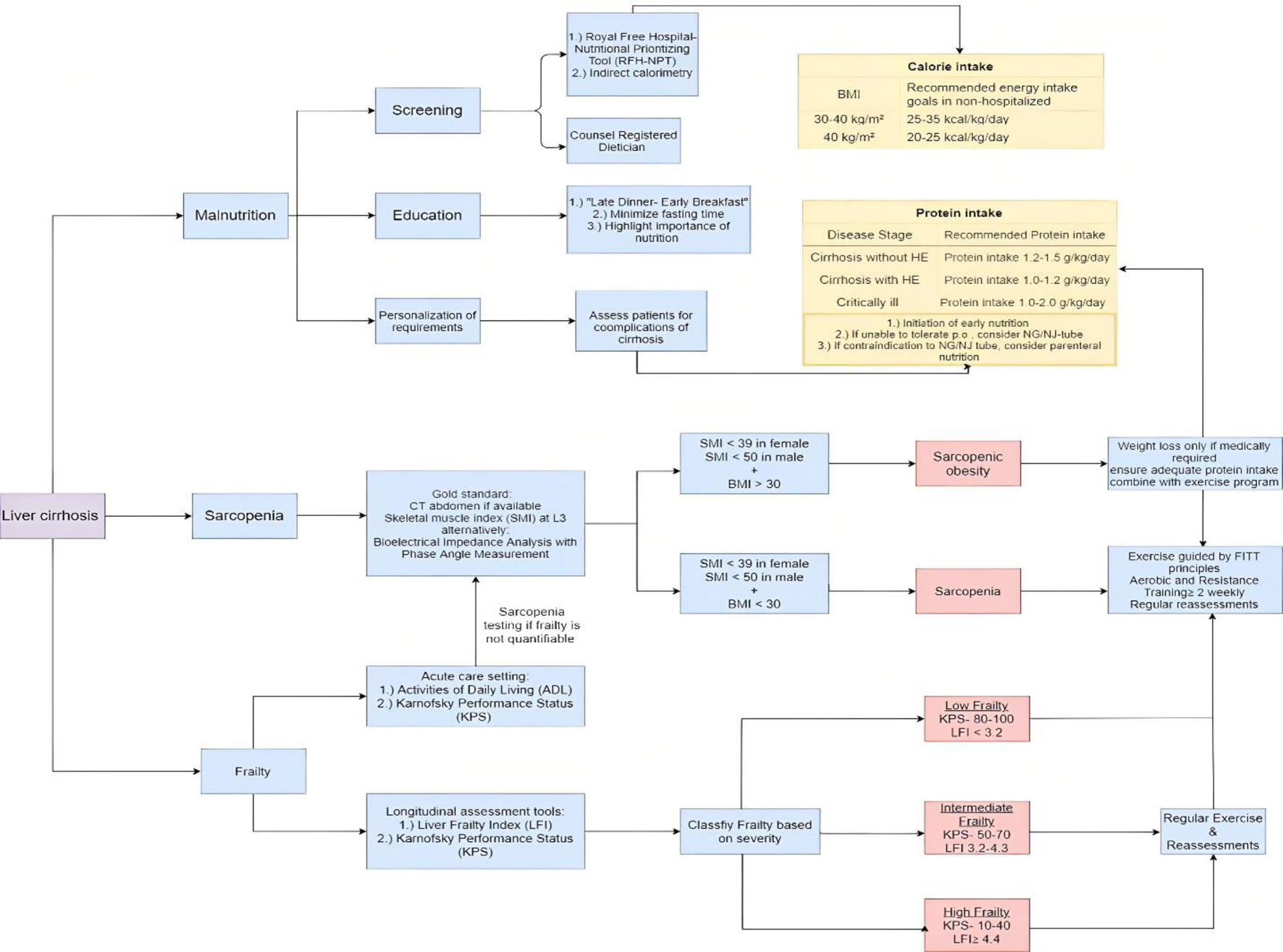 Click for large image | Figure 1. Algorithm in the assessment of sarcopenia, frailty, and malnutrition. This algorithm highlights the essential steps in assessing frailty, sarcopenia, and malnutrition in patients with liver cirrhosis. This flowchart illustrates only a selection of available assessment tools. We recommend regularly using the same tools to increase the likelihood of noticing subtle changes. Regular assessments are based on the patient’s clinical status and should be conducted at least annually. In case of decompensation or clinical deterioration, periodic reviews should be performed every 3 to 6 months in ambulatory clinics. For nutritional purposes, use dry weight-based body mass index and subtract weight depending on the amount of volume overload. |
Frailty
The frailty assessment is complex as significant overlaps exist with other entities such as disability, sarcopenia, and malnutrition. Therefore, it is necessary to distinguish those entities from each other and not use the different terminology interchangeably. Frailty should be considered a syndrome comprised of a cumulative decline in multiple physiological systems secondary to decreased physiologic reserve resulting in vulnerability and adverse outcomes. Regarding liver cirrhosis, most studies have focused on physical frailty, the phenotypical impairment of contractile muscle function [40]. Physical inactivity and reduced tolerance to physical activity in patients with liver cirrhosis correlate with mortality and survival post-transplant [70, 71] and culminate in sarcopenia and, subsequently, frailty [72, 73]. Multiple studies report beneficial effects from exercise programs aimed at counteracting decreased physical activity and simultaneously frailty [67, 74]. However, it is vital that clinicians adequately communicate these beneficial effects to the patients to improve compliance and potentially outcome [15]. Multiple subjective and objective tools for the assessment of frailty are available. The current AASLD toolbox provides an overview of functional tests for assessing frailty, including the 6-min walking test, short physical performance battery (including gait speed, chair stands, and standing balance) (modified) - Fried frailty instrument, and others ranging from subjective to objective. Current guidance [40] highlights a substantial predictive value of using the activities of daily living (ADL) and Karnofsky performance status (KPS) evaluations in the acute care setting.
Valuable tools for long-term assessments also include the KPS and the liver frailty index (LFI). In clinical practice, we use the 6-min walking tests and the LFI to assess frailty in patients being considered for liver transplants. The LFI entails four components (gender, grip strength, chair stands, and balance). The chair stands performance assesses the frequency of repeated rises from sitting to standing and back within 30 s. A poor performance is associated with an increased risk of falls, and early identification of this potential risk factor can be beneficial [75]. The hand grip strength (HGS) is assessed in kilograms via a dynamometer of the dominant hand. Three attempts are approximated, and a lower HGS (e.g., men < 30 kg, women < 15 kg) is correlating with a higher risk of mortality [76]. Finally, balance assessment includes holding a position (feet placed side-to-side, semi-tandem, and tandem) for 10 s.
The KPS is a subjective assessment tool that categorizes the functional performance of individuals by using a scale of 0 - 100 in increments of 10; the lower the score, the higher the degree of frailty [77]. Many studies highlight the significance of frailty in patients with cirrhosis independent of the etiological origin of cirrhosis. Furthermore, it is essential to not view frailty as a static measure but rather as an ongoing process in which the available tools can aid in preventing the progression of frailty. In this context, it appears evident that frailty has prognostic implications regarding waitlist mortality [78], development of ascites, and encephalopathy (Fig. 2) [79]. Lai et al indicated in a study with over 1,000 patients with cirrhosis that worsening of the LFI over 3 months is associated with a more than two-fold increase in waitlist mortality [80] (95% CI: 1.35 - 3.09). Similarly, Thuluvath et al [81] showed that patients with a lack of improvement in KPS and a low baseline KPS have a worse outcome post-liver transplant with regards to graft (1.31 - 1.46, P < 0.01) and patient survival (1.35 - 1.52, P < 0.01). Both the LFI and KPS can be used for periodic evaluations and to classify patients into three categories depending on the severity of frailty [82]. Growing evidence indicates that the more objective LFI could be prioritized as a standard assessment tool as it provides a more accurate estimation of mortality, especially in patients listed for a liver transplant. One potential downside is that the LFI requires specialized equipment (e.g., adjustable handle), which might not be readily available compared to the KPS [83, 84]. But as recent studies [85] highlighted that reduced handgrip strength is a valuable tool to stratify the risk of development of overt hepatic encephalopathy, these specialized tools might become more widely available in the future as they might have implications on a higher number of patients. Furthermore, the assessment of handgrip was found to be associated with decreased cognitive performance in females as it correlates with inhibitory control testing used to assess minimal hepatic encephalopathy (0.60, P < 0.001) [75].
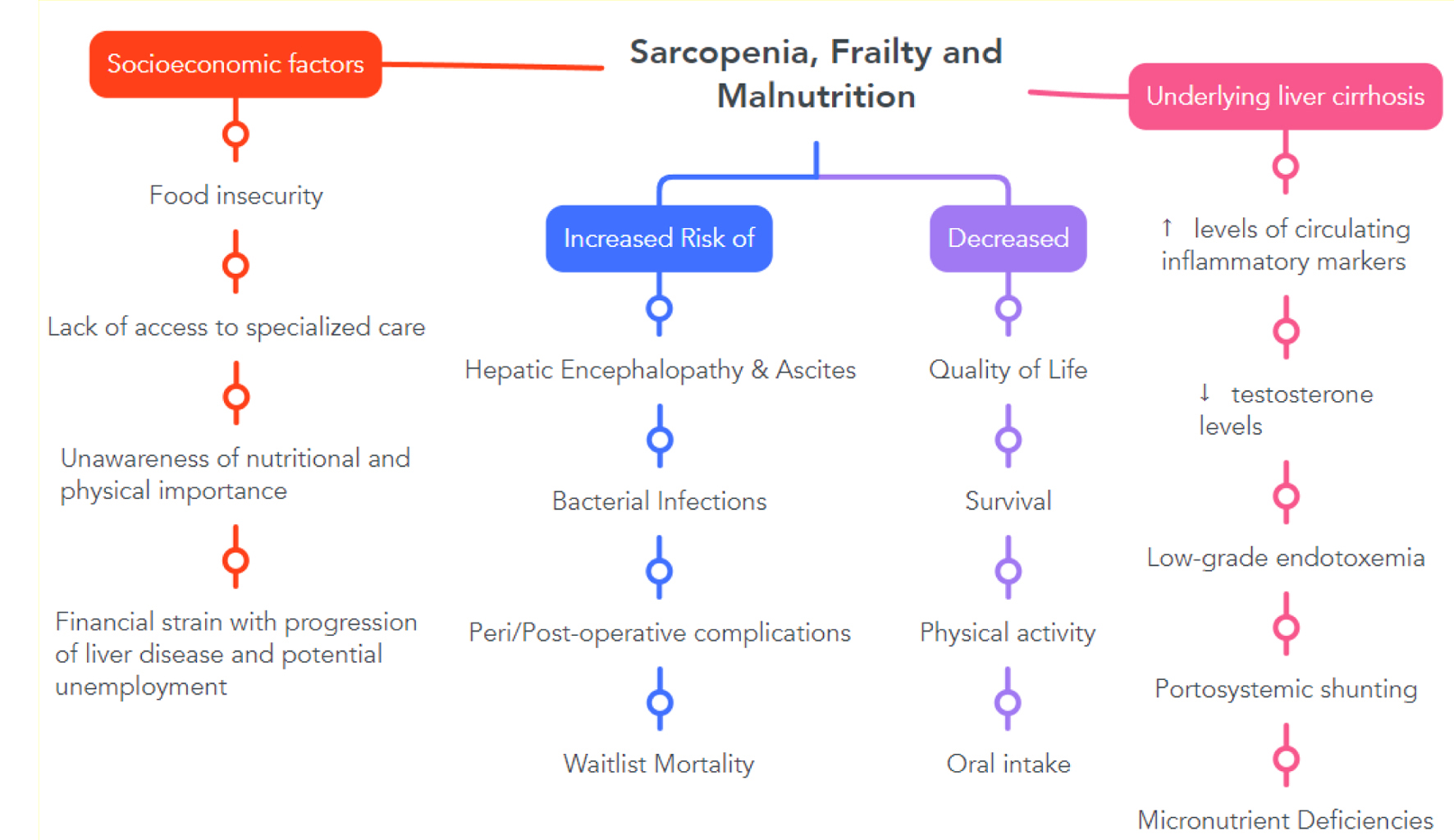 Click for large image | Figure 2. Implications of malnutrition, sarcopenia, and frailty in the context of cirrhosis from multiple viewpoints. |
Home-based exercises will likely become more integral to managing frailty as technologies such as wearables and smartphones are readily available [86] and objectify activity levels that patients and physicians often overestimate [87]. For example, a pilot study (STRIVE) by Lai et al [88] included 83 patients with cirrhosis randomized 2:1 to receive a 30-min strength training video plus a health coach for 12 weeks against the regular standard of care. Despite a poor adherence of only 14%, positive results were seen, with a median improvement in LFI from 3.8 to 3.6 (ΔLFI -0.1) and self-reported quality of life. Overall, determining frailty involves a series of steps, further summarized in the algorithm below (Fig. 1).
Malnutrition
In general, malnutrition is defined as a “state of nutrition in which a deficiency or excess (or imbalance) of energy, protein, and other nutrients causes measurable adverse effects on tissue/body form (body shape, size, and composition) and function, and clinical outcome” [89] (Table 1).
Multiple studies showed that most patients with liver cirrhosis lack the knowledge to manage their disease and dietary intake properly and effectively, indicating the continuous need to provide regular patient education [13, 14, 90]. Reuter et al also noted that educating physicians and nutritionists is necessary to improve malnutrition in patients with liver cirrhosis [23]. A meaningful assessment of the patient’s current nutritional status can not be based solely on weight and height; it also must include the dynamically changing energy and nutrient requirements, body composition, and organ functions. Therefore, all hospitalized patients with cirrhosis require consultation by a dietician to address potential obstacles and the need for oral nutritional supplements (ONS) [13]. A handy screening tool for malnutrition is the “The Royal Free Hospital-Nutritional Prioritizing Tool” (RFH-NPT), developed and validated as a screening tool for cirrhotic patients [57, 91]. Borhofen et al performed a study assessing the different nutritional screening tests in 84 patients with liver cirrhosis. The results indicated that the RFH-NPT, assessed initially and after a median of 500 days, correlates with complications such as ascites, hepatorenal syndrome, and hepatic encephalopathy and is an independent predictor of transplant-free survival [91] (Fig. 2).
As previously mentioned, many patients with liver cirrhosis tend to be in a constant state of hypermetabolism. Indirect calorimetry is the gold standard for measuring REE [40]. Alternatively, using handheld calorimeters is possible and accurately estimates REE [92]. The total 24 h energy demand is about 1.3 times the measured REE [57, 93]. Furthermore, the patient’s dry weight-based BMI must be considered, and energy goals must be adjusted accordingly. In cases of significant volume overloaded states, post-paracentesis, body weight or subtraction of the weight percentage (according to the severity of ascites) based on clinical judgment is indicated. In these cases, ascites can be classified as mild, moderate, and severe, with a subsequent subtraction of 5%, 10%, or 15% based on severity. An additional 5% should be added with bilateral pedal edema [94].
Regarding general nutrition guidelines for cirrhotic patients, 25 - 35 kcal/kg/day are recommended for patients with a BMI between 30 and 40 kg/m2, and 20 - 25 kcal/kg/day in patients with a BMI more than 40 kg/m2 [40]. In addition, non-obese individuals are recommended to have a minimum energy intake of 35 kcal/kg/day.
A daily protein intake of 1.2 - 1.5 g protein/kg ideal body weight should be ensured in patients with liver cirrhosis [95]. Further nutritional recommendations include avoiding prolonged fasting by having a late dinner and early breakfast (“late dinner, early breakfast”) and adding an oral dietary supplement or snack in the evening, which helps to replenish body protein stores [96-98]. In terms of additional supplementation with branched-chain amino acids (BCAA: leucine, isoleucine, and valine), Gluud et al [99] indicated in a meta-analysis including 16 randomized clinical trials and 827 participants with various degrees of hepatic encephalopathy that there is no benefit of supplementing BCAA with regards to mortality or quality of life. However, Shirabe et al [100] indicated in a retrospective study involving 236 patients undergoing living donor liver transplantation that preoperative BCAA supplementation might reduce the incidence of post-transplant bacteremia and sepsis.
Patients with liver cirrhosis are at increased risk for clinical decompensation and the development of acute on chronic liver failure. Therefore, adequate nutrition must be ensured during periods of increased stress like hepatic encephalopathy or ascites. Hepatic encephalopathy is associated with altered mental status and somnolence; protein and calorie intake are often inadequate. Consensus guidelines recommend consideration of early enteral nutrition, e.g., via nasogastric/nasojejunal tube (NG/NJ tube), if the patient is not at increased risk for potential complications such as aspiration pneumonia. In clinical practice, patients eligible for transplants should be closely followed regarding fulfilling caloric needs. If the patient cannot meet the caloric needs, the placement of NG/NJ tubes is strongly recommended.
Esophageal varices are not a contraindication for inserting an NG/NJ tube. A risk of bleeding was described in one retrospective study [101] but is likely outweighed by the beneficial effect of early initiation of nutrition. In the case of insufficient nutrition or contraindication to an enteral tube (e.g., absent cough and/or gag reflex), parenteral nutrition with concomitant supplementation of vitamins, electrolytes, and trace elements as a second-line option is recommended. The estimated protein target is approximately 1.2 - 2.0 g/kg ideal body weight per day during the state of critical illness. However, in patients with hyperacute hepatic encephalopathy, hyperammonemia, and a high risk of cerebral edema, protein support can be suspended for the first 24 - 48 h [40, 57]. The algorithm mentioned above (Fig. 1) also summarizes the steps in defining/diagnosing malnutrition.
| Conclusion | ▴Top |
Improving screening strategies for malnutrition is crucial given the global burden of liver cirrhosis. This involves addressing knowledge gaps among healthcare professionals and difficulties in diagnosing frailty, sarcopenia, and malnutrition in these patients. Primary gastroenterologists and primary care physicians should initiate counseling on adequate protein intake and prevention of malnutrition-related complications during early cirrhosis management, prior to referral to a transplant center. This includes optimizing standards for assessing liver cirrhosis through regular nutritional consults, indirect calorimetry, and longitudinal frailty assessments. Patient education plays a vital role in improving diet and physical activity.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for this manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare
Informed Consent
Not applicable.
Author Contributions
AK, JA, and AP contributed significantly to the conception, design, acquisition, analysis, and interpretation of the study. They were also involved in the initial drafting of the manuscript. JA, GR, FC, KR, and ML conducted a thorough critical review of the manuscript, focusing on its important intellectual content. AK, JA, AP, GR, ML, FC, and KR have provided their approval for the final version of the manuscript to be published. Furthermore, they have collectively agreed to be accountable for all aspects of the work, ensuring that any questions related to the accuracy or integrity of the content are appropriately investigated and resolved.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255-263.
doi pubmed - Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16(1):95-131.
doi pubmed pmc - Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75(Suppl 1):S147-S162.
doi pubmed pmc - Yoshida T, Delafontaine P. Mechanisms of cachexia in chronic disease states. Am J Med Sci. 2015;350(4):250-256.
doi pubmed pmc - Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6(7):e102.
doi pubmed pmc - Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126-135.
doi pubmed pmc - Aamann L, Dam G, Borre M, Drljevic-Nielsen A, Overgaard K, Andersen H, Vilstrup H, et al. Resistance training increases muscle strength and muscle size in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2020;18(5):1179-1187.e1176.
doi pubmed - Tandon P, Dunn MA, Duarte-Rojo A. Resistance training reduces risk of sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2020;18(5):1036-1039.
doi pubmed pmc - Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dorup I. Decreased muscle strength and contents of Mg and Na,K-pumps in chronic alcoholics occur independently of liver cirrhosis. J Intern Med. 2003;253(3):359-366.
doi pubmed - Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
doi pubmed - Welch N, Dasarathy J, Runkana A, Penumatsa R, Bellar A, Reen J, Rotroff D, et al. Continued muscle loss increases mortality in cirrhosis: Impact of aetiology of liver disease. Liver Int. 2020;40(5):1178-1188.
doi pubmed pmc - Stroffolini T, Sagnelli E, Sagnelli C, Morisco F, Babudieri S, Furlan C, Pirisi M, et al. The association between education level and chronic liver disease of any etiology. Eur J Intern Med. 2020;75:55-59.
doi pubmed - Morando F, Rosi S, Gola E, Nardi M, Piano S, Fasolato S, Stanco M, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35(5):1508-1515.
doi pubmed - Beg S, Curtis S, Shariff M. Patient education and its effect on self-management in cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2016;28(5):582-587.
doi pubmed - Chascsa DM, Lai JC, Dunn MA, Montano-Loza AJ, Kappus MR, Dasarathy S, Carey EJ. Patient and caregiver attitudes and practices of exercise in candidates listed for liver transplantation. Dig Dis Sci. 2018;63(12):3290-3296.
doi pubmed pmc - Tamargo JA, Sherman KE, Campa A, Martinez SS, Li T, Hernandez J, Teeman C, et al. Food insecurity is associated with magnetic resonance-determined nonalcoholic fatty liver and liver fibrosis in low-income, middle-aged adults with and without HIV. Am J Clin Nutr. 2021;113(3):593-601.
doi pubmed pmc - Garbuzenko DV, Arefyev NO. Current approaches to the management of patients with cirrhotic ascites. World J Gastroenterol. 2019;25(28):3738-3752.
doi pubmed pmc - Sharma P, Gupta C, Kumar A, Arora A, Anikhindi SA, Singla V, Bansal N, et al. Nutritional assessment and factors affecting dietary intake in patients with cirrhosis: A single-center observational study. Nutrition. 2021;84:111099.
doi pubmed - Krahenbuhl L, Lang C, Ludes S, Seiler C, Schafer M, Zimmermann A, Krahenbuhl S. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int. 2003;23(2):101-109.
doi pubmed - Vidot H, Teevan K, Carey S, Strasser S, Shackel N. A prospective audit of preprocedural fasting practices on a transplant ward: when fasting becomes starving. J Clin Nurs. 2016;25(5-6):829-835.
doi pubmed - Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020;51(1):64-77.
doi pubmed - Gundling F, Seidl H, Pehl C, Schmidt T, Schepp W. How close do gastroenterologists follow specific guidelines for nutrition recommendations in liver cirrhosis? A survey of current practice. Eur J Gastroenterol Hepatol. 2009;21(7):756-761.
doi pubmed - Reuter B, Shaw J, Hanson J, Tate V, Acharya C, Bajaj JS. Nutritional assessment in inpatients with cirrhosis can be improved after training and is associated with lower readmissions. Liver Transpl. 2019;25(12):1790-1799.
doi pubmed pmc - Muller MJ, Bottcher J, Selberg O, Weselmann S, Boker KH, Schwarze M, von zur Muhlen A, et al. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69(6):1194-1201.
doi pubmed - Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54(10):845-859.
doi pubmed pmc - She S, Wu X, Zheng D, Pei X, Ma J, Sun Y, Zhou J, et al. PSMP/MSMP promotes hepatic fibrosis through CCR2 and represents a novel therapeutic target. J Hepatol. 2020;72(3):506-518.
doi pubmed - Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232-1244.
doi pubmed pmc - Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1(1):9-21.
doi pubmed pmc - Pace A, de Weerth A, Berna M, Hillbricht K, Tsokos M, Blaker M, Pueschel K, et al. Pancreas and liver injury are associated in individuals with increased alcohol consumption. Clin Gastroenterol Hepatol. 2009;7(11):1241-1246.
doi pubmed - Ferre N, Camps J, Prats E, Girona J, Gomez F, Heras M, Simo JM, et al. Impaired vitamin E status in patients with parenchymal liver cirrhosis: relationships with lipoprotein compositional alterations, nutritional factors, and oxidative susceptibility of plasma. Metabolism. 2002;51(5):609-615.
doi pubmed - Kubesch A, Quenstedt L, Saleh M, Ruschenbaum S, Schwarzkopf K, Martinez Y, Welsch C, et al. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PLoS One. 2018;13(11):e0207162.
doi pubmed pmc - Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G625-633.
doi pubmed pmc - Fu X, Zhong Z, Hu F, Zhang Y, Li C, Yan P, Feng L, et al. The protective effects of selenium-enriched Spirulina platensis on chronic alcohol-induced liver injury in mice. Food Funct. 2018;9(6):3155-3165.
doi pubmed - Mehta KJ, Farnaud SJ, Sharp PA. Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol. 2019;25(5):521-538.
doi pubmed pmc - Shangraw RE, Jahoor F. Effect of liver disease and transplantation on urea synthesis in humans: relationship to acid-base status. Am J Physiol. 1999;276(5):G1145-1152.
doi pubmed - Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, Kalhan SC. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54(5):915-921.
doi pubmed pmc - Dasarathy S, Mullen KD, Dodig M, Donofrio B, McCullough AJ. Inhibition of aromatase improves nutritional status following portacaval anastomosis in male rats. J Hepatol. 2006;45(2):214-220.
doi pubmed - Moller S, Becker U, Gronbaek M, Juul A, Winkler K, Skakkebaek NE. Short-term effect of recombinant human growth hormone in patients with alcoholic cirrhosis. J Hepatol. 1994;21(5):710-717.
doi pubmed - Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65(5):906-913.
doi pubmed - Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(3):1611-1644.
doi pubmed pmc - Kumari S, De A, Kalra N, Singh V. Growth hormone therapy in decompensated cirrhosis: an open-label, randomized control trial. Am J Gastroenterol. 2024;119(1):116-126.
doi pubmed - Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423.
doi pubmed pmc - Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, Dunn MA, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23(5):625-633.
doi pubmed pmc - Bhanji RA, Narayanan P, Moynagh MR, Takahashi N, Angirekula M, Kennedy CC, Mara KC, et al. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl. 2019;25(1):14-24.
doi pubmed pmc - Kappus MR, Wegermann K, Bozdogan E, Patel YA, Janas G, Shropshire E, Parish A, et al. Use of skeletal muscle index as a predictor of wait-list mortality in patients with end-stage liver disease. Liver Transpl. 2020;26(9):1090-1099.
doi pubmed - Tapper EB, Zhang P, Garg R, Nault T, Leary K, Krishnamurthy V, Su GL. Body composition predicts mortality and decompensation in compensated cirrhosis patients: A prospective cohort study. JHEP Rep. 2020;2(1):100061.
doi pubmed pmc - Paris MT, Tandon P, Heyland DK, Furberg H, Premji T, Low G, Mourtzakis M. Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin Nutr. 2020;39(10):3049-3055.
doi pubmed pmc - Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70(5):1816-1829.
doi pubmed pmc - Figueiredo FA, Dickson ER, Pasha TM, Porayko MK, Therneau TM, Malinchoc M, DiCecco SR, et al. Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transpl. 2000;6(5):575-581.
doi pubmed - Dhaliwal A, Armstrong MJ. Sarcopenia in cirrhosis: a practical overview. Clin Med (Lond). 2020;20(5):489-492.
doi pubmed pmc - Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21(2):113-117.
doi pubmed - Kalafateli M, Konstantakis C, Thomopoulos K, Triantos C. Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol. 2015;21(24):7357-7361.
doi pubmed pmc - Ciocirlan M, Cazan AR, Barbu M, Manuc M, Diculescu M, Ciocirlan M. Subjective global assessment and handgrip strength as predictive factors in patients with liver cirrhosis. Gastroenterol Res Pract. 2017;2017:8348390.
doi pubmed pmc - Nishikawa H, Yoh K, Enomoto H, Ikeda N, Aizawa N, Koriyama T, Nishimura T, et al. Anthropometric measurements and frailty in patients with liver diseases. Diagnostics (Basel). 2020;10(6):433.
doi pubmed pmc - Kikuchi N, Uojima H, Hidaka H, Iwasaki S, Wada N, Kubota K, Nakazawa T, et al. Evaluation of skeletal muscle mass in patients with chronic liver disease shows different results based on bioelectric impedance analysis and computed tomography. Ann Nutr Metab. 2022;78(6):336-344.
doi pubmed - Nishikawa H, Enomoto H, Iwata Y, Nishimura T, Iijima H, Nishiguchi S. Clinical utility of bioimpedance analysis in liver cirrhosis. J Hepatobiliary Pancreat Sci. 2017;24(7):409-416.
doi pubmed - Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485-521.
doi pubmed pmc - DiMartini A, Cruz RJ, Jr., Dew MA, Myaskovsky L, Goodpaster B, Fox K, Kim KH, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19(11):1172-1180.
doi pubmed pmc - Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, Uchiyama H, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20(4):401-407.
doi pubmed - van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, JN IJ. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277-2292.
doi pubmed - van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68(4):707-714.
doi pubmed - Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679-689.
doi pubmed pmc - Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Sarcopenic obesity in liver cirrhosis: possible mechanism and clinical impact. Int J Mol Sci. 2021;22(4):1917.
doi pubmed pmc - Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, Hattori A, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55(8):863-870.
doi pubmed - Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, Calleja JL, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65(4):1293-1305.
doi pubmed - Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38(10):1706-1717.
doi pubmed - Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1920-1926.e1922.
doi pubmed - Roman E, Garcia-Galceran C, Torrades T, Herrera S, Marin A, Donate M, Alvarado-Tapias E, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One. 2016;11(3):e0151652.
doi pubmed pmc - Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96(8):2442-2447.
doi pubmed - Hayashi F, Matsumoto Y, Momoki C, Yuikawa M, Okada G, Hamakawa E, Kawamura E, et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res. 2013;43(12):1264-1275.
doi pubmed - Dharancy S, Lemyze M, Boleslawski E, Neviere R, Declerck N, Canva V, Wallaert B, et al. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86(8):1077-1083.
doi pubmed - Sieber CC. Frailty - From concept to clinical practice. Exp Gerontol. 2017;87(Pt B):160-167.
doi pubmed - Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23(5):899-905.
doi pubmed pmc - Roman E, Torrades MT, Nadal MJ, Cardenas G, Nieto JC, Vidal S, Bascunana H, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59(8):1966-1975.
doi pubmed - Tapper EB, Derstine B, Baki J, Su GL. Bedside measures of frailty and cognitive function correlate with sarcopenia in patients with cirrhosis. Dig Dis Sci. 2019;64(12):3652-3659.
doi pubmed - Hanai T, Shiraki M, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: A sex-stratified analysis. Hepatol Res. 2019;49(12):1414-1426.
doi pubmed - Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187-193.
doi pubmed - Xu CQ, Mohamad Y, Kappus MR, Boyarsky B, Ganger DR, Volk ML, Rahimi RS, et al. The relationship between frailty and cirrhosis etiology: from the Functional Assessment in Liver Transplantation (FrAILT) Study. Liver Int. 2021;41(10):2467-2473.
doi pubmed pmc - Lai JC, Covinsky KE, McCulloch CE, Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol. 2018;113(2):235-242.
doi pubmed pmc - Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, Ganger DR, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol. 2020;73(3):575-581.
doi pubmed pmc - Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69(4):818-825.
doi pubmed - Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564-574.
doi pubmed pmc - McNally BB, Carey EJ. Objective versus subjective assessment of functional status in candidates for liver transplantation. Transplant Proc. 2018;50(10):3508-3512.
doi pubmed - Kardashian A, Ge J, McCulloch CE, Kappus MR, Dunn MA, Duarte-Rojo A, Volk ML, et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology. 2021;73(3):1132-1139.
doi pubmed pmc - Miwa T, Hanai T, Nishimura K, Maeda T, Ogiso Y, Imai K, Suetsugu A, et al. Handgrip strength stratifies the risk of covert and overt hepatic encephalopathy in patients with cirrhosis. JPEN J Parenter Enteral Nutr. 2022;46(4):858-866.
doi pubmed - Kusnik A, Itzel T, Dooley S, Dropmann A, Stallkamp J, Ganslandt T, Ebert M, et al. Digital gastroenterology. J Gastrointestin Liver Dis. 2020;29(4):493-496.
doi pubmed - Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl. 2016;22(10):1324-1332.
doi pubmed - Lai JC, Dodge JL, Kappus MR, Wong R, Mohamad Y, Segev DL, McAdams-DeMarco M. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: the strength training intervention (STRIVE). Am J Gastroenterol. 2021;116(4):717-722.
doi pubmed pmc - Lochs H, Allison SP, Meier R, Pirlich M, Kondrup J, Schneider S, van den Berghe G, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin Nutr. 2006;25(2):180-186.
doi pubmed - Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. Am J Gastroenterol. 2013;108(3):302-305.
doi pubmed pmc - Borhofen SM, Gerner C, Lehmann J, Fimmers R, Gortzen J, Hey B, Geiser F, et al. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61(6):1735-1743.
doi pubmed - Glass C, Hipskind P, Tsien C, Malin SK, Kasumov T, Shah SN, Kirwan JP, et al. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol (1985). 2013;114(5):559-565.
doi pubmed pmc - Nielsen K, Kondrup J, Martinsen L, Dossing H, Larsson B, Stilling B, Jensen MG. Long-term oral refeeding of patients with cirrhosis of the liver. Br J Nutr. 1995;74(4):557-567.
doi pubmed - European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172-193.
doi pubmed pmc - Cordoba J, Lopez-Hellin J, Planas M, Sabin P, Sanpedro F, Castro F, Esteban R, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43.
doi pubmed - Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, McIlroy K, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557-566.
doi pubmed - Vaisman N, Katzman H, Carmiel-Haggai M, Lusthaus M, Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92(1):137-140.
doi pubmed - Kusnik A, Hunter N, Rasbach E, Miethke T, Reissfelder C, Ebert MP, Teufel A. Co-medication and nutrition in hepatocellular carcinoma: potentially preventative strategies in hepatocellular carcinoma. Dig Dis. 2021;39(5):526-533.
doi pubmed - Gluud LL, Dam G, Les I, Cordoba J, Marchesini G, Borre M, Aagaard NK, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;2:CD001939.
doi pubmed - Shirabe K, Yoshimatsu M, Motomura T, Takeishi K, Toshima T, Muto J, Matono R, et al. Beneficial effects of supplementation with branched-chain amino acids on postoperative bacteremia in living donor liver transplant recipients. Liver Transpl. 2011;17(9):1073-1080.
doi pubmed - Al-Obaid LN, Bazarbashi AN, Cohen ME, Kim J, Lei Y, Axelrad JE, Fox A, et al. Enteric tube placement in patients with esophageal varices: Risks and predictors of postinsertion gastrointestinal bleeding. JGH Open. 2020;4(2):256-259.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


