| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 17, Number 1, February 2024, pages 41-51
Delays in Colorectal Cancer Screening for Latino Patients: The Role of Immigrant Healthcare in Stemming the Rising Global Incidence of Colorectal Cancer
Eleazar E. Montalvan-Sancheza, d , Renato Beasa
, Ahmad Karkasha, Ambar Godoya, Dalton Argean Norwoodb
, Michael Doughertyc
aDepartment of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, USA
bDivision of Preventive Medicine, University of Alabama at Birmingham, AL 35294, USA
cUNC Rex Digestive Healthcare, Raleigh, NC 27514, USA
dCorresponding Author: Eleazar E. Montalvan-Sanchez, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Manuscript submitted January 5, 2024, accepted February 1, 2024, published online February 28, 2024
Short title: Latino Health: Tackling CRC Screening Delays
doi: https://doi.org/10.14740/gr1697
| Abstract | ▴Top |
The significant global burden of colorectal cancer accentuates disparities in access to preventive healthcare in most low- and middle-income countries (LMICs) as well as large sections of underserved populations within high-income countries. The barriers to colorectal cancer screening in economically transitioning Latin America are multiple. At the same time, immigration from these countries to the USA continues to increase. This case highlights the delays in diagnosis experienced by a recent immigrant from a country with no established colorectal cancer screening program, to an immigrant population in the USA with similar poor screening coverage. We discuss common challenges faced by Latinos in their home countries and the USA, as well as strategies that could be implemented to improve screening coverage in US immigrant populations.
Keywords: Colorectal cancer; Screening delays; Latino patients; Immigrant healthcare; Rising global incidence; Colorectal health; Cancer prevention; Healthcare disparities
| Introduction | ▴Top |
Colorectal cancer (CRC) is one of the most common cancers worldwide and causes a high burden of disease in both high- and low-resource countries. Globally, incident CRC increased dramatically from 842,098 in 1990 to 2.17 million in 2010 [1]. CRC screening allows for early detection of early stage and precancerous lesions, allowing for curative treatment or even interrupting the adenoma-to-carcinoma sequence [2]. Screening has clearly contributed to reduced mortality from CRC globally [3, 4] and in the USA [5], and is recommended for average risk adults by most national and international guidelines starting around age 50 [6-8].
Despite recent declines in CRC incidence and mortality rates in high-income regions, these rates are increasing dramatically throughout the rest of the world [1]. Nowhere is this more pronounced than in Latin America, where Central and Andean Latin America has experienced a greater percentage increase in overall deaths and disability-adjusted life years (DALY) due to CRC than any other region [1]. Overall incidence rates are still lower in most of Latin America than in the USA and Europe, except in the southern cone of South America (Argentina and Uruguay), where rates are as high as any in the world [9].
Most screening programs exist in high-income countries with high CRC incidence rates and healthcare resources [10], while access to screening and surveillance remains limited in low-resource settings. At the intersection of these spheres, the large US immigrant population seeking to improve their situation by moving to a high-resource country often experience the same health inequity in the form of lack of access to screening even after arriving to the USA. The largest immigrant subpopulation in the USA, as well as the largest and fastest growing ethnic minority in the country, are those with Latin American ancestry and cultural heritage, also known as Latinos [11]. Historically, the term “Latino” has included individuals from Mexico, Central America, parts of the Caribbean, and most of South America, regardless of race [11, 12]. Despite many similarities, this group is quite heterogenous, with different subgroups and races including white, black, Native American, Mestizo (European-native American), Mulato (European-African ancestry), and Zambo (African-native American) [13]. There is a corresponding high level of genetic diversity, though self-identified Latino populations do share some unique variants and predisposition to certain diseases [14]. Accordingly, Latino immigrants are usually studied together, juxtaposed to immigrants from other continents.
Where studied in Latin America, CRC screening programs have shown benefit, but they have not been prioritized for implementation [15]. Most Latin American countries lack organized CRC screening programs altogether [16], leading to gaps in healthcare that have repercussions in the US health system among the immigrant population. We report a case of CRC in a Latino patient and highlight the barriers to care contributing to his late presentation, in the context of the current literature on CRC screening in Latin America and the USA.
| Case Report | ▴Top |
A 52-year-old man originally from South America, who immigrated to the USA 2 years before presentation, with a past medical history of diabetes mellitus type 2, hypertension and alcohol use disorder, presented to his first primary care clinic appointment in the USA with report of unintentional weight loss. The patient named lack of insurance or financial resources, fear and anxiety about a potentially complicated medical diagnosis, as well as a language barrier among the reasons for delay in the decision to seek health care. On this first visit, he reported having lost 18 pounds in 6 months, despite eating more greasy and processed foods. He had noticed his stools becoming thinner caliber, without any change in frequency. He also complained of abdominal discomfort that was relieved after defecation, and 2 years of intermittent, scant bright red blood per rectum (BRBPR). There was no rectal pain and no melena. A colonoscopy revealed an infiltrative, partially obstructing polypoid mass in the proximal rectum, about 5 cm in length (Fig. 1). Computed tomography (CT) of the abdomen and pelvis demonstrated thickening of the rectal wall and prominent adjacent adenopathy, but no distant metastases. A magnetic resonance imaging (MRI) demonstrated a middle to upper third rectal mass with involvement of the muscularis propria, with numerous perirectal nodular deposits/perirectal lymph nodes, compatible with stage T3b disease.
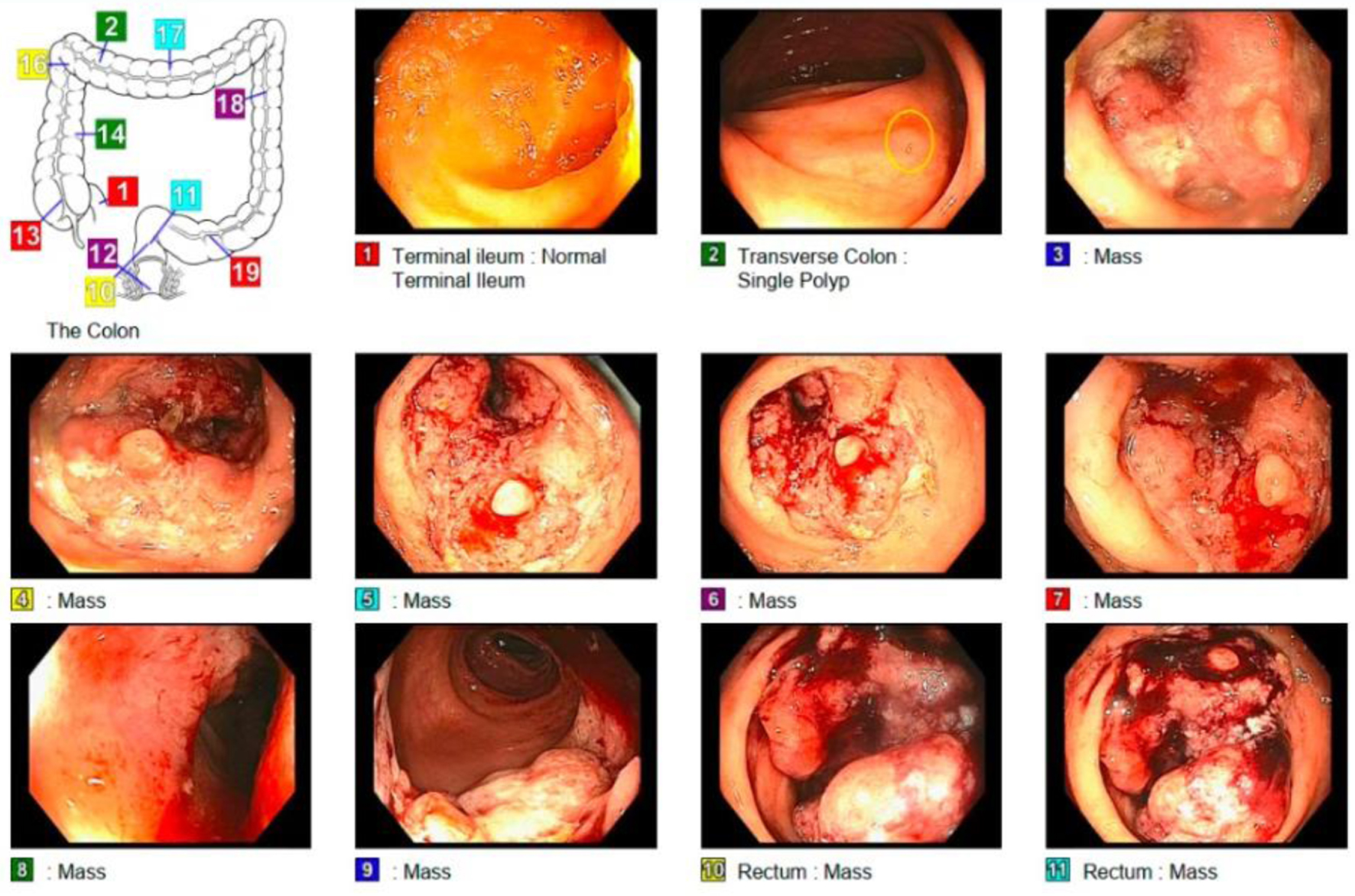 Click for large image | Figure 1. The examined portion of the ileum looks normal. One 2 mm polyp in the transverse colon. Obstructing tumor in the rectum malignant-appearing. A biopsy from a colorectal lesion was taken confirming adenocarcinoma. Figure created by the first author Eleazar Montalvan-Sanchez. |
The patient underwent total mesorectal excision via robotic low anterior resection, with neoadjuvant chemoradiation with capecitabine and oxaliplatin, according to the RAPIDO trial regimen [9]. The surgery was performed 8 months after his initial encounter with the primary care physician (PCP). One year after that clinic visit, he had a successful takedown of diverting ileostomy and no symptoms were reported on follow-up.
| Discussion | ▴Top |
Our patient presented with an advanced stage of CRC due to lack of access to CRC screening, as well as timely diagnostic colonoscopy. While patient characteristics and tumor molecular pathology lead to a wide variation in rates, some data suggest tumors double in size in a median of 211 days, with the majority increasing their T stage at 150 days [17]. This suggests our patient may not have required as aggressive therapy with both chemotherapy and radiation if he had presented even 6 - 12 months earlier, much less the 2 years earlier when he was first eligible for CRC screening in the USA, and less symptomatic. We present this case as an example of the difference that could be made by improving access to recommended cancer screening to the US immigrant population, which is increasing in size as incidence of CRC is rising in their countries of origin.
Risk factors for CRC in Latin America
The reasons behind the rising incidence of CRC in Latin America are not completely understood, though are largely a function of a country’s stage of development. As development index increases in middle- and upper-middle-income countries, there is greater adoption of Western lifestyle patterns (processed food, red meat, and resultant obesity, accompanied by changes in the gut microbiota) [9, 18-21], though still without adequate coverage by organized CRC screening programs. Red meat consumption in Argentina and Uruguay are thought to drive the very high incidence of CRC in those countries [22, 23], but CRC is also more frequent in urban areas of Mexico than rural [24], presumably due to the same lifestyle factors. Our patient was from an urban center of South America, possibly reflecting lifestyle risk factors elevating his risk closer to that of individuals in developed countries. He also endorsed significant lifetime alcohol consumption, which has been associated with an up to 60% increase in the likelihood of developing CRC [25, 26]. While US Latinos are more likely to abstain completely from alcohol than non-Latino Whites, Latino drinkers on average consume more than non-Hispanic White drinkers, perhaps contributing to a unique risk factor for this subpopulation [27].
Barriers to CRC screening in Latin America: economic and access to care
Even in countries with newly established screening programs, as in Brazil, the coverage of screened patients remains suboptimal owing mainly to limited resources and low population awareness, among other factors [28, 29]. With financial barriers being the most important obstacle to a standardized screening program, cost-effectiveness analyses take into account the type of screening test and/or cascade [30, 31] counterposed to the high costs (medical and non-medical) of cancer treatments that can be avoided by early detection, to assess the cost per DALY or death averted. Research suggests that CRC screening is still cost-effective in some low- and middle-income countries (LMICs), at least via stool-based screening, especially as age-standardized rate (ASR) rises above 14 [28, 32, 33]. However, prevention programs are an investment for the future health of a population, and still a hard sell to struggling economies and health systems with more immediate concerns before them. As a result, very few LMICs, including our patient’s country of origin, have established screening programs [28, 32].
Barriers to CRC screening in Latin America: knowledge and beliefs
There are other barriers to screening uptake besides healthcare resources however. While there is not much direct evidence regarding CRC screening programs in Latin America, experience with other screen-detectable cancers can shed light on some of the specific cultural barriers to and motivations for cancer screening in immigrants [34]. Lack of knowledge about cancer and screening is an important driver of lower screening uptake in Latin America. Fear of finding cancer is another important factor that discouraged cancer screening in Brazil and Chile [35, 36]. Fatalism, or the belief that cancer is due to bad luck or God’s will, and there is nothing a person can do about it, was noted as a barrier for cervical cancer screening [37]. In some settings, gender roles have also prevented screening, as men have not allowed wives to undergo pelvic examination for cervical cancer screening [38, 39]. Another study showed that women place greater priority on taking care of their children, family, and work, leading them to neglect their own health [40, 41]. In addition to providing screening programs, addressing such social factors with education aimed at changing attitudes toward cancer is necessary to improve screening rates.
Immigrant healthcare is another means to address the problem of screening in lower resource countries, albeit only partially. Immigrants from lower-incidence regions typically retain a lower incidence of CRC than destination countries, but this increases with time even within the same generation, and certainly in subsequent ones [9, 42, 43]. While immigrants’ primary motivation is usually economic opportunity, the health equity opportunity should not be lost on destination countries. If high-income countries hold global health equity as a goal, the immigrant to a high-income country has not only become a much easier client to serve, by dropping right into an established health system, but the immigrant is now also a potentially productive member of the economy, whom it befits the host country to keep as healthy as possible. Maintaining immigrant health should include age-appropriate screening. However, immigrants (and particularly “language-discordant” minorities such as our patient, whose preferred language differs from the predominant language of the healthcare system and their own provider’s) are consistently the least up-to-date on health maintenance items in the United States [44-46].
Resource-related barriers to CRC screening among US immigrants
Factors influencing US immigrants’ CRC screening uptake can be categorized as resource-related or culture-related. Health insurance coverage and screening costs are significant resource-related factors. Chinese and Korean American immigrants were less likely to undergo screening colonoscopy if they lacked health insurance [47]. In addition, many insured immigrants are still unaware that colonoscopy is covered [47]. Other resource-related barriers mentioned previously in the literature include lack of time from work or childcare and difficulty in navigation or accessing complex healthcare systems [48].
Specifically among Latinos, data from the National Health Interview Survey from 2010 and 2015 showed a low prevalence of being up-to-date with CRC screening for all Latinos subgroups (between 23% and 29% up-to-date) [49], though worst for Mexicans and possibly Central/South Americans [50-52], relative to Latinos of Caribbean heritage (Cubans, Dominicans, or Puerto Ricans, depending on study). These differences are likely related to social determinants of health among the different subgroups of Latinos [51, 49, 53]. Similar to other immigrant and disadvantaged non-immigrant communities in the USA, the most significant determinant of screening has been health insurance [49, 51]. Accordingly, large differences in Latino screening uptake between states are likely driven by different healthcare-related policies, though differing makeups of Latino subpopulations could contribute as well [54]. Low income, no recent physician visits, or no high school education were associated with decreased CRC screening in US Latinos [51, 49, 53, 55], as well as living in the USA for less than 15 years [49] and having less “acculturation” [56]. Compared to second- and third-generation Latinos (those who were born in the United States or who have spent a significant amount of their lives in the United States), first-generation Latinos (those who were born outside the United States) generally have lower rates of CRC screening uptake [49, 51, 57], though the effect of US nativity may vary by Latino subgroup. Being born in the USA was associated with increased screening rates in Mexican-Americans, no change in rate for individuals of Puerto Rican descent, and decreased screening rates among Cuban-Americans [58]. Our patient was a first-generation immigrant with low socioeconomic status and healthcare access, contributing to his delayed presentation.
Culture-related barriers among US immigrants
A major culture-related factor impeding screening uptake among immigrants is the language barrier. A study of Chinese and Korean American immigrants showed that patients prefer language-concordant physicians to whom they can “express their concerns” without the need of a translator, and more easily understand medical terminologies [48]. Beyond language however, another culture-related factor is disease misconception. For example, some US Asian immigrants consider cancer a “Western disease”, assuming their risk is low due to their different lifestyle and healthier eating habits [47]. However, a study showed that most epidemiologic patterns of GI cancer including CRC in Chinese American immigrants were closer to those of the people of their new country of residence compared to those of their original country, indicating a shift in environmental exposures which may change cancer risk [59]. Fatalistic beliefs present in cultures of origin persist and affect adherence to screening [47, 60-63]. In addition, many cultures have a bias towards first seeking complementary and alternative medicine, where screening does not feature as prominently [47, 64]. One culture-related barrier that has been studied specifically in Latinos is the concept of machismo in Latino men [65, 66]. This cultural construct, a conglomerate of attitudes and behaviors associated with masculinity widespread throughout Latin America and US Hispanic populations, contributes to the reluctance of some male Latinos to get a screening colonoscopy, in the sense that rectal instrumentation is associated with fear, embarrassment and a “transformative” threat to their masculinity [64, 65, 67].
Many of these beliefs and practices regarding cancer screening may not be deeply held, however, and modifiable through education. As changing beliefs can take time, early initiation of cancer screening discussions through culturally tailored public health messaging and contact with primary care are important. It is notable that studies on Latino immigrants’ perspectives on CRC screening are few. Machismo should be considered in approaches to CRC screening of Latino populations in order to adequately support screen-eligible Latino men. More research is needed to further elucidate the specific cultural beliefs and norms relevant to screening the largest subgroup of US immigrants (Fig. 2).
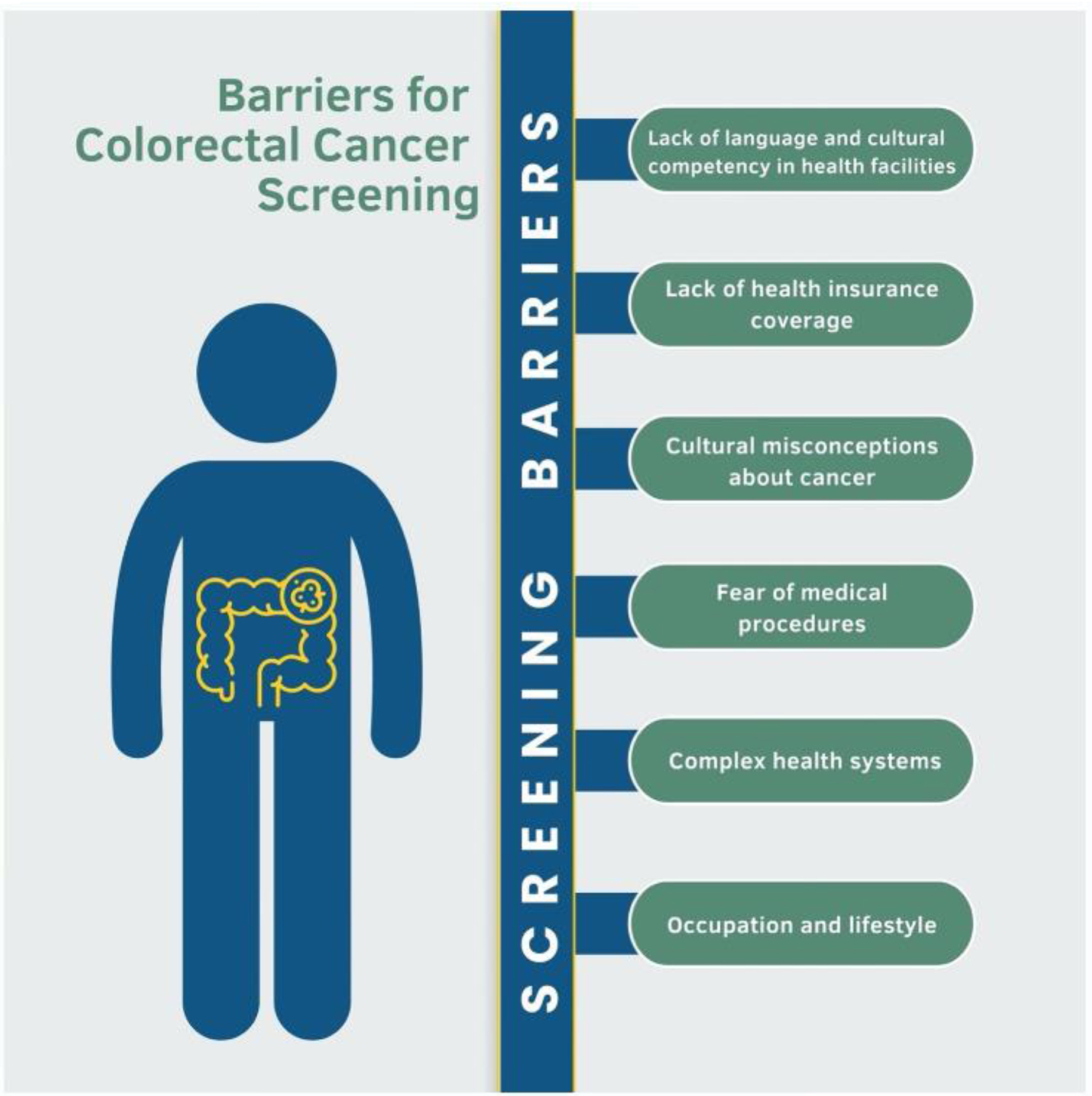 Click for large image | Figure 2. Barriers to screening for colorectal cancer encountered by the immigrant population when moving to the USA. Figure created by the first author Eleazar Montalvan-Sanchez. |
Health systems barriers for CRC screening
The process for CRC screening differs from country to country, and even between regions of the USA. There are organized screening programs within the USA, though the majority of the population is approached for screening through opportunistic means (via a provider visit). Provider-related factors certainly affect immigrant adherence to screening guidelines, especially in opportunistic settings. Studies of the Chinese American community have found limited provider time to contribute to inadequate cancer screening [68, 69]. Improving clinician-patient communication skills in screening discussions is ideal [70], but making the recommendation to get screened at all has been shown to be important at least in Chinese and Latino immigrants [56, 68, 70-74]. Technological advancements through machine learning and deep learning (including the Internet of Things concept) may be able to increase trainee and global health practitioner expertise in CRC diagnosis, such as via enhanced classification of histopathologic images used in diagnosis as well as enhanced effectiveness and safety of surgery [75-77]. If CRC screening and diagnosis is easier and more efficient through technology, it may be more available to more individuals at lower cost.
As for systems-level interventions to improve screening uptake, the first step is addressing the non-cultural barriers of access to care, increasing both supply and demand for healthcare among immigrants. Targeted messaging to immigrant populations could highlight the importance of accessing insurance coverage and medical care, as well as the importance of CRC screening and resources for covering the associated costs. Once having accessed primary care, providers can discuss cultural beliefs about cancer and screening as well as specific screening tests available, and introduce immigrants to the new country’s health care system [78]. Ideally, patient education and counselling should take place early, to avoid situations such as our case where an immigrant does not seek medical care until they are symptomatic. Effective patient-level measures include patient reminders, financial incentives, and reducing barriers such as transportation and language issues [5, 79, 80]. Language barriers can be overcome not only by offering translators but also by providing multi-language advertising media. Further, multi-level and/or multi-component interventions have been shown to be the most effective for increasing screening uptake [5, 81, 82]. This has held true in Latino and Asian immigrant populations when tested, for instance with patient navigation [83, 84]. Multicomponent interventions include patient navigation, interventions that combine patient with provider-level interventions, and those that address multiple structural barriers to screening [85, 86].
Many such structural barriers involve financial and insurance constraints as discussed above, and can also be addressed by patient navigators, or else via policy-level changes to make health coverage more accessible. However, many US immigrants still remain uninsured, due to requirements for > 5 years of residency, undocumented status, or just persistent unmitigated socioeconomic stresses overrepresented in this population. Foreign-born individuals (especially undocumented and language-discordant populations) face greater resource-related barriers to healthcare access and access less preventive care than US-born individuals of the same socioeconomic status [87]. A more aggressive policy change to address immigrant health disparities might consist of a basic public health insurance coverage (e.g. Medicaid) for a temporary period (6 - 12 months) to enhance the chance of the immigrant getting introduced to the healthcare system as they gain their financial footing, and are able to transition into an employer-based or health insurance marketplace plan.
While health insurance coverage may not be as crucial of an issue to legal immigrants to developed countries with universal healthcare, there are still disparities in access at least for recent immigrants [88-91]. In all settings, highly effective screening measures such as FIT kit distribution (or self-administered cervical HPV DNA testing) could also likely be implemented with a good value on a programmatic basis for new immigrants within eligible age ranges [92]. Patient navigation can improve access to the available system resources, and in a study of New York Latinos reversed the association of acculturation and time in the USA with lower screening rates, at least for Dominicans and Central Americans [93]. Community-based coalitions can advise and coordinate the planning, development and implementation of such cancer prevention programs to include education and other measures culturally relevant to their own community. Leveraging digital platforms that are specifically relevant to immigrant populations (such as WhatsApp or WeChat) could enhance test completion and navigation to follow-up [92]. Audits of such programs with registries of clinical outcomes and cost-effectiveness analyses will be crucial for sustainability and even expansion of funding. Access for the undocumented will remain a large problem, but counterposed to the legal concerns regarding undocumented immigration, there are practical reasons to consider supporting high-value preventive healthcare for all. If we are not prepared to deny any human life-saving care (which would be morally reprehensible), it may be more cost-effective to prevent common life-threatening conditions, such as the case of emergency vs. regular dialysis [94].
Finally, even non-health-related policy changes affect immigrant healthcare utilization, through fostering generally more or less restrictive immigrant policy “climates” [95]. “English-only” laws, “Public Charge” rules, and other policies that promote fear of deportation and overall limit integration into the larger community of the destination country thereby reduce participation in preventive care (which immigrants often perceive as less essential). Such policies are not aligned with the “Health in All Policies” approach espoused by the WHO and CDC.
Conclusion
Our patient lacked health insurance, an established healthcare provider, English language proficiency, and was a recently immigrated, foreign-born individual of South American ethnicity, all factors associated with worse uptake of CRC screening in the USA. His educational level or prior knowledge of CRC screening are not known, nor if he was affected by certain cultural beliefs such as fatalism or machismo, but these could have played a role as well. It is likely that an earlier contact with culturally sensitive, language concordant support services to facilitate access to healthcare could have led to earlier detection of his CRC, potentially with a less morbid outcome. Fortunately his long-term prognosis is optimistic, though this is still not the case for a disproportionate number of US immigrants.
CRC is a global challenge of increasing importance, with disease burden rising across the globe in places where proven screening strategies to reduce morbidity and mortality are not available. The impact of this disparity in screening access could be partially alleviated by screening immigrants from LMICs to high-income countries. To-date such screening has been inadequate, though there are evidence-based strategies to address this, on both patient and policy levels (Fig. 3). These strategies depend on a commitment from high-income countries to advance global health equity within their own borders.
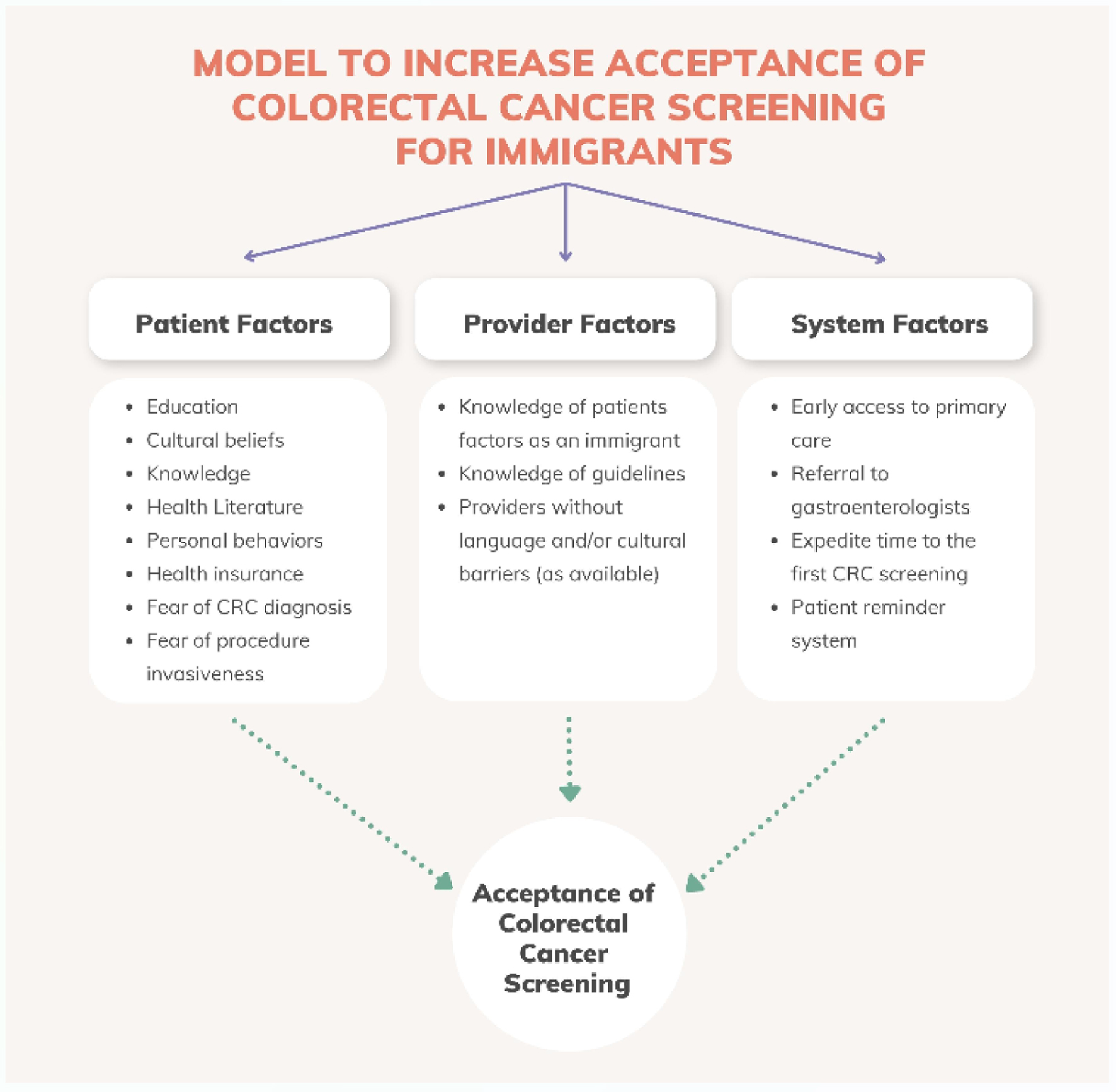 Click for large image | Figure 3. Model to increase uptake of colorectal cancer screening in immigrants. Figure created by the first author Eleazar Montalvan-Sanchez. |
Learning points
1) CRC screening programs should be encouraged in all countries with significant burdens of disease, while taking into account competing financial burdens of the health system.
2) Lack of access, lack of awareness, and language discordance are the main barriers that the healthcare system must address to increase CRC screening uptake among US immigrants.
3) Outreach and bilingual patient navigation are some of the many methods that may decrease barriers and increase screening adherence among immigrants. Funding for such programs, and generally more support for immigrant health coverage, should be part of equity-informed health systems.
4) More research is needed on how to best increase preventive health uptake for the breadth and diversity of ethnic groups and subgroups that make up immigrant populations.
Acknowledgments
We acknowledge the following individual, Mirian Ramirez-Rojas from Ruth Lily Medical Library, for the support and literature review provided to the Latino Gastroenterology Research Group in Indiana University School of Medicine.
Financial Disclosure
The authors have no financial conflict of interest disclosed.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was provided by the patient.
Author Contributions
EEMS, AG, AK and RB: the leading role in patient care and diagnosis. EEMS, RB, AG, DN and MD executed the study literature review. EEMS designed the figures and table. All authors provided the overall data interpretation and oversight. All authors provided a critical review of the manuscript and approved the final manuscript.
Data Availability
All data underlying the results are available as part of the article, and no additional source data are required.
| References | ▴Top |
- Collaborators GBDCC. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7(7):627-647.
doi pubmed pmc - Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med. 2019;170(5):319-329.
doi pubmed - Morgan DR, Malik PR, Romeo DP, Rex DK. Initial US evaluation of second-generation capsule colonoscopy for detecting colon polyps. BMJ Open Gastroenterol. 2016;3(1):e000089.
doi pubmed pmc - Garcia-Dominic O, Lengerich EJ, Wray LA, Parrott R, Aumiller B, Kluhsman B, Renderos C, et al. Barriers to CRC screening among Latino adults in Pennsylvania: ACCN results. Am J Health Behav. 2012;36(2):153-167.
doi pubmed - Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, Cubillos L, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(12):1645-1658.
doi pubmed pmc - Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39:7.
doi pubmed pmc - Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458-479.
doi pubmed - Patel SG, May FP, Anderson JC, Burke CA, Dominitz JA, Gross SA, Jacobson BC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. multi-society task force on colorectal cancer. Gastroenterology. 2022;162(1):285-299.
doi pubmed - Pineros M, Laversanne M, Barrios E, Cancela MC, de Vries E, Pardo C, Bray F. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg Health Am. 2022;13:100294.
doi pubmed pmc - Ding H, Lin J, Xu Z, Chen X, Wang HHX, Huang L, Huang J, et al. A global evaluation of the performance indicators of colorectal cancer screening with fecal immunochemical tests and colonoscopy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(4):1073.
doi pubmed pmc - Siatkowski AA. Hispanic acculturation: a concept analysis. J Transcult Nurs. 2007;18(4):316-323.
doi pubmed - Humes KJ, Ramirez RR. Overview of race and Hispanic origin: 2010. 2011 [cited Feb 20, 2023]; Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf.
- Jaimes N, Londono V, Halpern AC. The term Hispanic/Latino: a note of caution. JAMA Dermatol. 2013;149(3):274-275.
doi pubmed - Anwar MY, Baldassari AR, Polikowsky HG, Sitlani CM, Highland HM, Chami N, Chen HH, et al. Genetic pleiotropy underpinning adiposity and inflammation in self-identified Hispanic/Latino populations. BMC Med Genomics. 2022;15(1):192.
doi pubmed pmc - Montalvan-Sanchez EE, Norwood DA, Dougherty M, Beas R, Guranizo-Ortiz M, Ramirez-Rojas M, Morgan DR, et al. Colorectal cancer screening programs in Latin America: a systematic review and meta-analysis. JAMA Netw Open. 2024;7(2):e2354256.
doi pubmed pmc - Schliemann D, Ramanathan K, Matovu N, O'Neill C, Kee F, Su TT, Donnelly M. The implementation of colorectal cancer screening interventions in low-and middle-income countries: a scoping review. BMC Cancer. 2021;21(1):1125.
doi pubmed pmc - Burke JR, Brown P, Quyn A, Lambie H, Tolan D, Sagar P. Tumour growth rate of carcinoma of the colon and rectum: retrospective cohort study. BJS Open. 2020;4(6):1200-1207.
doi pubmed pmc - Sierra MS, Forman D. Burden of colorectal cancer in Central and South America. Cancer Epidemiol. 2016;44(Suppl 1):S74-S81.
doi pubmed - Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691.
doi pubmed - Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27.
doi pubmed - Gonzalez-Pons M, Cruz-Correa M. Colorectal cancer disparities in Latinos: genes vs. environment. In: Ramirez AG, Trapido EJ, eds. Advancing the science of cancer in Latinos. Cham (CH). 2020; p. 35-41.
doi pubmed pmc - Reich M, Buki LP. Colorectal cancer screening in Uruguay: current assessment and roadmap for the future. Psicol Reflex Crit. 2021;34(1):20.
doi pubmed pmc - Sanguinetti JM, Lotero Polesel JC, Piscoya A, Saenz Fuenzalida R. Colorectal cancer screening: a South American perspective. Rev Gastroenterol Peru. 2020;40(3):238-245.
pubmed - Espinosa-Tamez P, Suazo-Zepeda E, Sanchez-Blas H, Meneses-Medina M, Huitzil-Melendez FD, Van Loon K, Potter M, et al. National and state-level colorectal cancer mortality trends in Mexico, 1998-2018. Salud Publica Mex. 2021;64(1):5-13.
doi pubmed - Slattery ML, Potter J, Caan B, Edwards S, Coates A, Ma KN, Berry TD. Energy balance and colon cancer—beyond physical activity. Cancer Res. 1997;57(1):75-80.
pubmed - Jayasekara H, MacInnis RJ, Room R, English DR. Long-term alcohol consumption and breast, upper aero-digestive tract and colorectal cancer risk: a systematic review and meta-analysis. Alcohol Alcohol. 2016;51(3):315-330.
doi pubmed - Alcoholism N.I.o.A.A.a. Alcohol and the hispanic community. 2023. [cited July 30, 2023] Available from: https://www.niaaa.nih.gov/sites/default/files/publications/Alcohol_and_the_Hispanic_Community_0.pdf.
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637-1649.
doi pubmed - Organization PAH. Colorectal cancer screening in the Americas situation and challenges. Website of the Pan American Health Organization. 2016. [cited April 2022] Available from: https://www.paho.org/hq/dmdocuments/2016/Colorectal-Cancer-Screening-Landscape-English.pdf.
- Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33(1):88-100.
doi pubmed pmc - Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):96-104.
doi pubmed - Espinola N, Palacios A. Evaluando la costo-efectividad de pruebas de tamizaje en cancer colorrectal. Un caso de estudio para Argentina. Acta gastroenterol Latinoam. 2016;46(1):8-17.
- Dan YY, Chuah BY, Koh DC, Yeoh KG. Screening based on risk for colorectal cancer is the most cost-effective approach. Clin Gastroenterol Hepatol. 2012;10(3):266-271.e261-266.
doi pubmed - Lee DC, Liang H, Chen N, Shi L, Liu Y. Cancer screening among racial/ethnic groups in health centers. Int J Equity Health. 2020;19(1):43.
doi pubmed pmc - dos Santos GD, Chubaci RY. [Awareness about breast cancer and mammography in elderly women who frequent Daycare Centers in Sao Paulo (SP, Brazil)]. Cien Saude Colet. 2011;16(5):2533-2540.
doi pubmed - Wood MF, Vial MC, Martinez-Gutierrez J, Mason MJ, Puschel K. Examining barriers for mammography screening compliance within a private hospital and an underserved primary care clinic in Santiago, Chile. J Am Coll Radiol. 2013;10(12):966-971.
doi pubmed - Abdullahi A, Copping J, Kessel A, Luck M, Bonell C. Cervical screening: Perceptions and barriers to uptake among Somali women in Camden. Public Health. 2009;123(10):680-685.
doi pubmed - Garrett JJ, Barrington C. 'We do the impossible': women overcoming barriers to cervical cancer screening in rural Honduras—a positive deviance analysis. Cult Health Sex. 2013;15(6):637-651.
doi pubmed - Marvan ML, Ehrenzweig Y, Catillo-Lopez RL. Knowledge about cervical cancer prevention and psychosocial barriers to screening among Mexican women. J Psychosom Obstet Gynaecol. 2013;34(4):163-169.
doi pubmed - Conde-Ferraez L, Suarez Allen RE, Carrillo Martinez JR, Ayora-Talavera G, Gonzalez-Losa Mdel R. Factors associated with cervical cancer screening amongst women of reproductive age from Yucatan, Mexico. Asian Pac J Cancer Prev. 2012;13(9):4719-4724.
doi pubmed - Paz-Soldan VA, Bayer AM, Nussbaum L, Cabrera L. Structural barriers to screening for and treatment of cervical cancer in Peru. Reprod Health Matters. 2012;20(40):49-58.
doi pubmed pmc - Paszat L, Sutradhar R, Liu Y, Baxter NN, Tinmouth J, Rabeneck L. Risk of colorectal cancer among immigrants to Ontario, Canada. BMC Gastroenterol. 2017;17(1):85.
doi pubmed pmc - Stern MC, Fejerman L, Das R, Setiawan VW, Cruz-Correa MR, Perez-Stable EJ, Figueiredo JC. Variability in cancer risk and outcomes within US Latinos by National Origin and Genetic Ancestry. Curr Epidemiol Rep. 2016;3:181-190.
doi pubmed pmc - Shahidi NC, Homayoon B, Cheung WY. Factors associated with suboptimal colorectal cancer screening in US immigrants. Am J Clin Oncol. 2013;36(4):381-387.
doi pubmed - Santiago-Rodriguez EJ, Shariff-Marco S, Gomez SL, Hiatt RA. Disparities in colorectal cancer screening by time in the U.S. and Race/Ethnicity, 2010-2018. Am J Prev Med. 2023;65(1):74-82.
doi pubmed - Cofie LE, Hirth JM, Cuevas AG, Farr D. A national study of gender and racial differences in colorectal cancer screening among foreign-born older adults living in the US. J Behav Med. 2020;43(3):460-467.
doi pubmed - Jung MY, Holt CL, Ng D, Sim HJ, Lu X, Le D, Juon HS, et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2018;23(8):847-866.
doi pubmed pmc - U. S. Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965-1977.
doi pubmed - Calderon-Mora J, Alvarado L, Dwivedi A, Shokar N. Prevalence of colorectal cancer screening among Hispanic subgroups in the United States: NHIS 2010 and 2015 Data. Hisp Health Care Int. 2022;20(3):202-211.
doi pubmed - Miranda PY, Yao N, Snipes SA, BeLue R, Lengerich E, Hillemeier MM. Citizenship, length of stay, and screening for breast, cervical, and colorectal cancer in women, 2000-2010. Cancer Causes Control. 2017;28(6):589-598.
doi pubmed - Castaneda SF, Gallo LC, Nodora J, Talavera GA, Penedo FJ, Evenson KR, Lopez-Gurrola M, et al. Colorectal cancer screening among Hispanics/Latinos in the HCHS/SOL sociocultural ancillary study. Prev Med Rep. 2019;15:100947.
doi pubmed pmc - Vargas Bustamante A, Chen J, Rodriguez HP, Rizzo JA, Ortega AN. Use of preventive care services among Latino subgroups. Am J Prev Med. 2010;38(6):610-619.
doi pubmed - White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, Geiger AM, et al. Cancer screening test use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201-206.
doi pubmed pmc - Viramontes O, Bastani R, Yang L, Glenn BA, Herrmann AK, May FP. Colorectal cancer screening among Hispanics in the United States: Disparities, modalities, predictors, and regional variation. Prev Med. 2020;138:106146.
doi pubmed - Castaneda-Avila MA, Oyinbo AG, Epstein MM, Ortiz-Ortiz KJ, Tortolero-Luna G, Lapane KL. Trends and factors associated with fecal occult blood test utilization among Hispanic adults in Puerto Rico and the United States: BRFSS 2012-2020. Cancer Prev Res (Phila). 2023;16(4):229-237.
doi pubmed pmc - Johnson-Kozlow M. Colorectal cancer screening of Californian adults of Mexican origin as a function of acculturation. J Immigr Minor Health. 2010;12(4):454-461.
doi pubmed - Yao JS, Paguio JA, Dee EC, Amen TB, Escota GV. Disparities in Access to Colorectal Cancer Screening Among US Immigrants. J Gen Intern Med. 2022;37(8):2126-2129.
doi pubmed pmc - Afable-Munsuz A, Liang SY, Ponce NA, Walsh JM. Acculturation and colorectal cancer screening among older Latino adults: differential associations by national origin. J Gen Intern Med. 2009;24(8):963-970.
doi pubmed pmc - Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8):djw322.
doi pubmed pmc - Tang TS, Solomon LJ, McCracken LM. Barriers to fecal occult blood testing and sigmoidoscopy among older Chinese-American women. Cancer Pract. 2001;9(6):277-282.
doi pubmed - Tang TS, Solomon LJ, McCracken LM. Cultural barriers to mammography, clinical breast exam, and breast self-exam among Chinese-American women 60 and older. Prev Med. 2000;31(5):575-583.
doi pubmed - Shokar NK, Nguyen-Oghalai T, Wu H. Factors associated with a physician's recommendation for colorectal cancer screening in a diverse population. Fam Med. 2009;41(6):427-433.
pubmed pmc - Shokar NK, Salinas J, Dwivedi A. Mediators of screening uptake in a colorectal cancer screening intervention among Hispanics. BMC Cancer. 2022;22(1):37.
doi pubmed pmc - Moreno PI, Yanez B, Schuetz SJ, Wortman K, Gallo LC, Benedict C, Brintz CE, et al. Cancer fatalism and adherence to national cancer screening guidelines: Results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Cancer Epidemiol. 2019;60:39-45.
doi pubmed pmc - Martens CE, Crutchfield TM, Laping JL, Perreras L, Reuland DS, Cubillos L, Pignone MP, et al. Why wait until our community gets cancer? Exploring CRC screening barriers and facilitators in the Spanish-Speaking Community in North Carolina. J Cancer Educ. 2016;31(4):652-659.
doi pubmed pmc - Getrich CM, Sussman AL, Helitzer DL, Hoffman RM, Warner TD, Sanchez V, Solares A, et al. Expressions of machismo in colorectal cancer screening among New Mexico Hispanic subpopulations. Qual Health Res. 2012;22(4):546-559.
doi pubmed pmc - Jo AM, Maxwell AE, Rick AJ, Cha J, Bastani R. Why are Korean American physicians reluctant to recommend colorectal cancer screening to Korean American patients? Exploratory interview findings. J Immigr Minor Health. 2009;11(4):302-309.
doi pubmed pmc - Heyman JM, Nunez GG, Talavera V. Healthcare access and barriers for unauthorized immigrants in El Paso County, Texas. Fam Community Health. 2009;32(1):4-21.
doi pubmed - Jih J, Nguyen MP, Ly I, Tsoh JY, Le GM, Woo K, Chan E, et al. The role of physician recommendation in colorectal cancer screening receipt among immigrant Chinese Americans. J Immigr Minor Health. 2018;20(6):1483-1489.
doi pubmed pmc - Huei-Yu Wang J, Ma GX, Liang W, Tan Y, Makambi KH, Dong R, Vernon SW, et al. Physician intervention and Chinese Americans' colorectal cancer screening. Am J Health Behav. 2018;42(1):13-26.
doi pubmed pmc - Wolf MS, Baker DW, Makoul G. Physician-patient communication about colorectal cancer screening. J Gen Intern Med. 2007;22(11):1493-1499.
doi pubmed pmc - Jandorf L, Ellison J, Villagra C, Winkel G, Varela A, Quintero-Canetti Z, Castillo A, et al. Understanding the barriers and facilitators of colorectal cancer screening among low income immigrant hispanics. J Immigr Minor Health. 2010;12(4):462-469.
doi pubmed pmc - Fernandez ME, Wippold R, Torres-Vigil I, Byrd T, Freeberg D, Bains Y, Guajardo J, et al. Colorectal cancer screening among Latinos from U.S. cities along the Texas-Mexico border. Cancer Causes Control. 2008;19(2):195-206.
doi pubmed - Miranda-Diaz C, Betancourt E, Ruiz-Candelaria Y, Hunter-Mellado RF. Barriers for compliance to breast, colorectal, and cervical screening cancer tests among Hispanic patients. Int J Environ Res Public Health. 2015;13(1):ijerph13010021.
doi pubmed pmc - Otiniano ME, Wood RC, Poursani RS, Katerndahl DA, Siddiqui S, Nadeau MT. Association of knowledge, attitudes, and behaviors for colon cancer screening in Hispanic patients. Ethn Dis. 2013;23(3):343-348.
pubmed - Mulita F, Verras GI, Anagnostopoulos CN, Kotis K. A Smarter Health through the Internet of Surgical Things. Sensors (Basel). 2022;22(12):4577.
doi pubmed pmc - Bousis D, Verras GI, Bouchagier K, Antzoulas A, Panagiotopoulos I, Katinioti A, Kehagias D, et al. The role of deep learning in diagnosing colorectal cancer. Prz Gastroenterol. 2023;18(3):266-273.
doi pubmed pmc - Molokwu JC, Shokar N, Dwivedi A. Impact of targeted education on colorectal cancer screening knowledge and psychosocial attitudes in a predominantly Hispanic population. Fam Community Health. 2017;40(4):298-305.
doi pubmed - Butterly LF. Proven strategies for increasing adherence to colorectal cancer screening. Gastrointest Endosc Clin N Am. 2020;30(3):377-392.
doi pubmed - Doria-Rose VP, Lansdorp-Vogelaar I, McCarthy S, Puricelli-Perin DM, Butera V, Segnan N, Taplin SH, et al. Measures of longitudinal adherence to fecal-based colorectal cancer screening: Literature review and recommended approaches. Int J Cancer. 2021;149(2):316-326.
doi pubmed - Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, Melillo S, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97-118.
doi pubmed - Hendren S, Winters P, Humiston S, Idris A, Li SX, Ford P, Specht R, et al. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J Gen Intern Med. 2014;29(1):41-49.
doi pubmed pmc - Aragones A, Schwartz MD, Shah NR, Gany FM. A randomized controlled trial of a multilevel intervention to increase colorectal cancer screening among Latino immigrants in a primary care facility. J Gen Intern Med. 2010;25(6):564-567.
doi pubmed pmc - Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, Li L, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial. Cancer. 2006;107(5):959-966.
doi pubmed - U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr., Garcia FAR, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315(23):2564-2575.
doi pubmed - Montalvan-Sanchez EE, Beas R, Norwood DA, Alkashash AM, Rodriguez Murillo AA, Calderon G. Upper gastrointestinal cancer: delays in diagnosis and treatment caused by barriers to healthcare in the latino community. Gastroenterology Res. 2022;15(3):142-147.
doi pubmed pmc - Adigun S, Barroso C, Mixer S, Myers C, Anderson J. Minding the gaps: health care access for foreign-born people in the U.S.: an integrative review. J Health Care Poor Underserved. 2021;32(4):1653-1674.
doi pubmed - Higginbottom GMA, Evans C, Morgan M, Bharj KK, Eldridge J, Hussain B. Experience of and access to maternity care in the UK by immigrant women: a narrative synthesis systematic review. BMJ Open. 2019;9(12):e029478.
doi pubmed pmc - Mudyarabikwa O, Regmi K, Ouillon S, Simmonds R. Refugee and immigrant community health champions: a qualitative study of perceived barriers to service access and utilisation of the National Health Service (NHS) in the West Midlands, UK. J Immigr Minor Health. 2022;24(1):199-206.
doi pubmed pmc - Woodgate RL, Busolo DS, Crockett M, Dean RA, Amaladas MR, Plourde PJ. A qualitative study on African immigrant and refugee families' experiences of accessing primary health care services in Manitoba, Canada: it's not easy! Int J Equity Health. 2017;16(1):5.
doi pubmed pmc - Perez-Urdiales I, Goicolea I, Sebastian MS, Irazusta A, Linander I. Sub-Saharan African immigrant women's experiences of (lack of) access to appropriate healthcare in the public health system in the Basque Country, Spain. Int J Equity Health. 2019;18(1):59.
doi pubmed pmc - Fang CY, Ragin CC. Addressing disparities in cancer screening among U.S. immigrants: progress and opportunities. Cancer Prev Res (Phila). 2020;13(3):253-260.
doi pubmed pmc - Costas-Muniz R, Jandorf L, Philip E, Cohen N, Villagra C, Sriphanlop P, Schofield E, et al. Examining the impact of Latino nativity, migration, and acculturation factors on colonoscopy screening. J Community Health. 2016;41(5):903-909.
doi pubmed pmc - Nguyen OK, Vazquez MA, Charles L, Berger JR, Quinones H, Fuquay R, Sanders JM, et al. Association of scheduled vs emergency-only dialysis with health outcomes and costs in undocumented immigrants with end-stage renal disease. JAMA Intern Med. 2019;179(2):175-183.
doi pubmed pmc - Dondero M, Altman CE. Immigrant policies as health policies: State immigrant policy climates and health provider visits among U.S. immigrants. SSM Popul Health. 2020;10:100559.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


