| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 17, Number 1, February 2024, pages 37-40
Primary Non-Function of Hepatic Allograft With Preexisting Microvesicular Steatosis/Foamy Degeneration and Mild Large-Droplet Macrovesicular Steatosis
Melissa E. Limiaa, Xiu Li Liua, Jennifer Yub, Kathleen Byrnesa, c
aDepartment of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, USA
bDepartment of Surgery, Washington University School of Medicine, St. Louis, MO, USA
cCorresponding Author: Kathleen Brynes, Department of Pathology and Immunology, Washington University School of Medicine, Campus PO Box 8118, St. Louis, MO 63110, USA
Manuscript submitted November 17, 2023, accepted December 30, 2023, published online February 28, 2024
Short title: Failure of Liver Graft With Microvesicular Fat
doi: https://doi.org/10.14740/gr1687
| Abstract | ▴Top |
It has been established that more than mild large-droplet macrovesicular steatosis (LD-MAS) is associated with increased risk of graft non-function. In contrast, even severe small-droplet macrovesicular steatosis (SD-MAS) has been found to be less prognostically significant. It remains unclear if a donor liver with diffuse microvesicular steatosis is associated with an increased risk of graft dysfunction. A 56-year-old male with alcoholic cirrhosis was transplanted with a liver from a 42-year-old overweight male donor after brain death. The frozen section of the donor liver biopsy taken at harvest showed diffusely enlarged clear/foamy hepatocytes and mild LD-MAS (about 5-10% of total tissue). The reperfusion liver biopsy taken at time 0 of transplantation showed hemorrhage, pale and enlarged hepatocytes, and mild LD-MAS (about 10% of total tissue) with lipopeliosis. The graft became non-functional, and the patient was re-transplanted 24 h after the initial transplantation. Histologic examination of the failed liver allograft showed extensive hemorrhagic necrosis, neutrophilic inflammation, diffuse microvesicular steatosis, and large extracellular fat droplets (about 20% of total tissue). This case demonstrates that precautions are needed to avoid using livers with diffuse and severe microvesicular steatosis.
Keywords: Donor liver; Foamy degeneration; Frozen section; Large-droplet macrovesicular steatosis; Liver transplant; Microvesicular steatosis; Primary nonfunction
| Introduction | ▴Top |
As the demand for liver donation increases, new strategies have been devised to expand the donor pool, including “extended criteria”. One such extended criterion is using fatty livers, with steatosis assessment typically made during intraoperative consultation. Recent Banff Consensus Recommendations for Steatosis Assessment in Donor Livers provides definitions for evaluating steatosis, including quantifying and describing steatosis [1]. Specifically, large-droplet macrovesicular steatosis (LD-MAS) describes a fat droplet that peripherally displaces the hepatocyte’s nucleus, and microvesicular steatosis describes tiny fat droplets imparting a “foamy” appearance (also known as foamy degeneration). Small-droplet macrovesicular steatosis (SD-MAS) describes steatosis that does not fit either criterion.
Current practice allows for transplantation of livers with moderate steatosis (30-60%), though this is center-dependent. Several studies note that more than mild LD-MAS (> 30%) is associated with increased risk of graft non-function. In contrast, even severe SD-MAS (> 60%) has been found to be less prognostically significant [2]. As such, pathologists agree that the degree of LD-MAS be assessed prior to transplantation to determine donor liver suitability [1]. The significance of microvesicular steatosis, however, is more ambiguous, potentially due to heterogeneous definitions and limited research. It remains unclear if a donor liver with diffuse microvesicular steatosis is associated with an increased risk of graft dysfunction.
Herein, we report a case of primary non-function of an allograft with severe microvesicular steatosis and mild LD-MAS.
| Case Report | ▴Top |
The donor was a 42-year-old overweight male who sustained severe head trauma after a fall while intoxicated, with deterioration to brain death. The donor’s clinical history was remarkable for alcohol use (30 beers/day for 17 years) and smoking (54 pack-years). Infectious serologies (hepatitis B and C, cytomegalovirus, and Epstein-Barr virus) were non-reactive. No evidence of acute liver failure was seen on liver function tests (Table 1).
 Click to view | Table 1. Liver Function Tests |
Procurement was uneventful. The donor liver weighed 2,400 g and had standard arterial anatomy. This was transplanted into a 56-year-old male recipient with alcoholic cirrhosis complicated by non-occlusive portal vein and superior mesenteric vein thrombosis, but with ultimately normal cardiac function (ejection fraction (EF) 70%). Cold and warm ischemic times were 272 and 26 min, respectively. Thrombectomy of the portal and superior mesenteric veins were performed, and adequate portal vein (2.1 L/min) and hepatic artery (550 mL/min) blood flows were noted post-transplantation. During the hepatectomy, the patient was on minimal pressor support, and he received one unit of packed red blood cells (PRBCs). Unfortunately, the patient became severely hemodynamically unstable upon reperfusion and coagulopathic, requiring massive transfusions and significant pressor support. The graft became non-functional, with marked transaminitis and acidosis (Table 1).
Ultimately, the failed allograft was explanted 24 h after transplantation, and the patient relisted urgently as status 1A for primary non-function. The patient received a second liver allograft 20 h after explantation, with subsequent liver function normalization by time of discharge (post-operative day 20). As of his 1-year follow-up, the patient’s liver function remains normal.
Four specimens were available for histologic evaluation, including: a donor core liver biopsy at harvest (frozen and permanent sections), a donor liver biopsy after transplantation and reperfusion (time 0 biopsy), a biopsy of the failing allograft (post-transplant day 1, frozen and permanent sections), and the explanted failed allograft. Histologically, hepatocellular fat droplets that were large enough to displace the nucleus were classified as LD-MAS, while more minute fat droplets that were smaller than the nucleus and imparted a foamy appearance to the cytoplasm were classified as microvesicular steatosis. Pathologic findings in these specimens are summarized in Table 2, and photos of pertinent features are collected in Figure 1.
 Click to view | Table 2. Pathologic Findings in Liver Specimens |
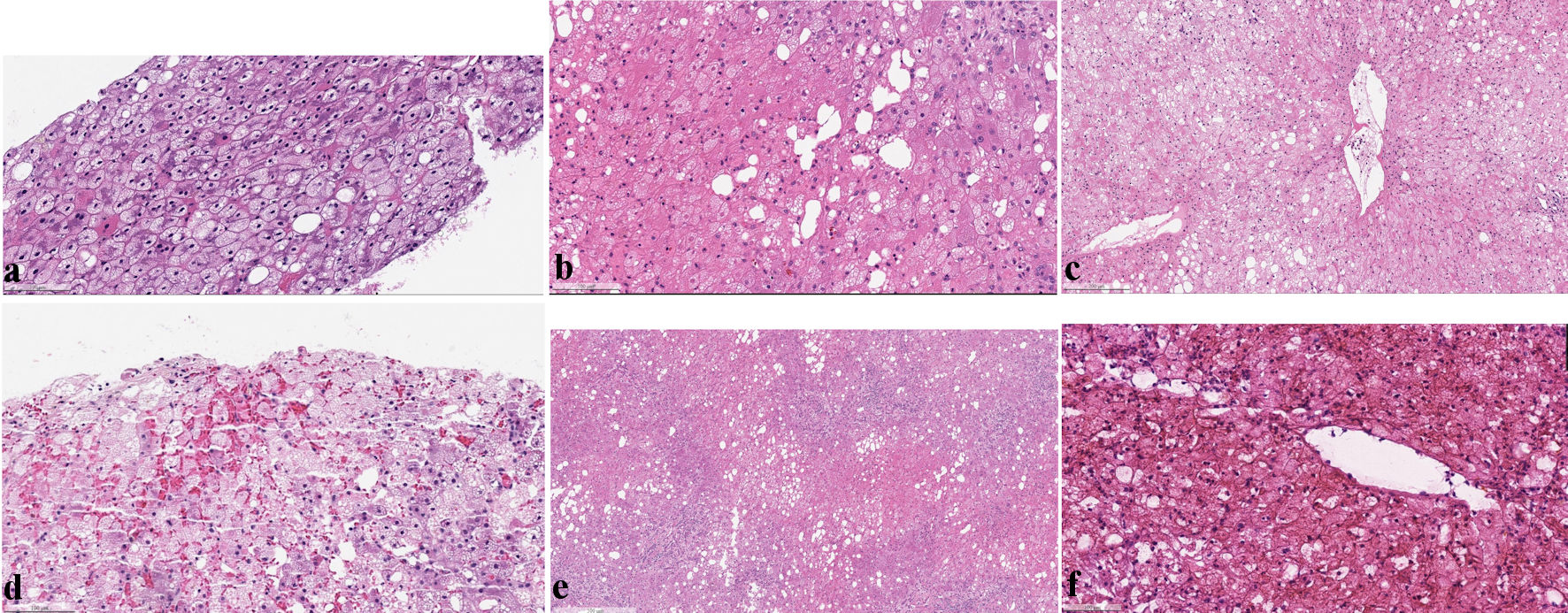 Click for large image | Figure 1. (a) Permanent sections of liver core biopsy taken at time of harvest showed diffuse microvesicular steatosis, mild LD-MAS (5% total), and no evidence of hepatocyte necrosis or inflammation (H&E stain). (b) Core needle biopsy taken at reperfusion (time 0 biopsy) showed diffuse microvesicular steatosis, hemorrhage, hepatic ischemic injury (loss of nuclear staining) and fat droplets in sinusoids (lipopeliosis) (H&E stain). (c, d) Permanent sections of the failing allograft at 24 h post-transplant showed diffuse microvesicular steatosis, hemorrhagic necrosis, mild LD-MAS, and no evidence of acute cellular or antibody-mediated rejection (H&E stain). (e) Sections of the failed allograft after explantation showed extensive hepatocyte necrosis, inflammation, and ductular reaction without evidence of cellular rejection (H&E stain). (f) High power view showed diffuse microvesicular steatosis, ischemic necrosis, mild LD-MAS in hepatocytes (20%) and large, irregular vacuoles in the sinusoids (10% total) (H&E stain). H&E: hematoxylin and eosin; LD-MAS: large-droplet macrovesicular steatosis. |
The frozen section of the liver biopsy taken at harvest showed diffusely enlarged clear/foamy hepatocytes and mild LD-MAS (about 5-10% of total tissue). At high power, the enlarged hepatocytes contained numerous tiny fat droplets, consistent with severe microvesicular steatosis (involving > 60% of total tissue). Permanent section confirmed these findings and demonstrated periportal and focal centrilobular fibrosis (stage 2 of 4) using the Kleiner et al (2005) staging system for non-alcoholic fatty liver disease [3]. No necrosis or inflammation was seen.
The reperfusion liver biopsy taken at time 0 of transplantation showed hemorrhage, pale and enlarged hepatocytes, and mild LD-MAS (about 10% of total tissue). High power examination showed diffuse, severe microvesicular steatosis (> 60% of total tissue) and focal inflammation. Ischemic injury was also seen (loss of hepatocyte nuclear staining) and fat droplets within the sinusoids (a phenomenon known as lipopeliosis).
Frozen section of the failing allograft on post-transplant day 1 showed extensive hemorrhagic necrosis, neutrophilic inflammation, diffuse microvesicular steatosis (> 60% of total tissue), and large extracellular fat droplets (about 20% of total tissue). The explanted allograft showed similar features without evidence of T cell-mediated/acute cellular or antibody-mediated rejection.
| Discussion | ▴Top |
LD-MAS has an established association with non-functional allografts, as described by Todo et al (1989), where they reported two cases of graft failure in donor livers with severe LD-MAS [4]. In their report, they noted large extrahepatocellular fat droplets within the sinusoids of the failed grafts and postulated that the fat was released by random hepatocyte rupture during organ preservation. They posit the fat droplets may have gone on to compromise sinusoidal microvasculature, ultimately causing acute allograft failure. Reticulin architectural disruption, hemorrhage, vascular congestion, and focal hepatocellular necrosis were also seen in the failed allografts.
In our case, while there was an increase in LD-MAS (5% at harvest vs. 20% at reperfusion), the degree of steatosis remained within the acceptable range for transplantation based on current guidelines. However, the pattern of steatosis in the donor liver was predominantly microvesicular, and no specific transplantation guidelines are available at this time due to a lack of consensus regarding the safety of transplanting livers with this pattern of steatosis [5]. Yoong et al (1999) reviewed 116 retransplanted livers and observed that those with severe microvesicular steatosis (> 66% of hepatocytes) was associated with significantly reduced 1-year graft survival, with a median survival of 1.5 months [6]. A potential mechanism for this poor post-transplantation graft function may be related to the mitochondrial dysfunction associated with microvesicular steatosis [6]. Meanwhile, more recent studies claim that there is no significant risk of graft dysfunction in donor livers with microvesicular steatosis [7]. However, others suggest that errors in classification may be confounding the issue, making donor livers with microvesicular steatosis appear safer than they are. Sharkey et al (2011) [5] reviewed 161 post-reperfusion donor liver biopsies to assess post-operative outcomes, and as part of their assessment proposed subcategorizing microvesicular steatosis into low- and high-grades. They defined low-grade microvesicular steatosis as having only a few fatty vesicles without hepatocyte enlargement, while hepatocytes with high-grade microvesicular steatosis are enlarged and have multiple small, fatty vesicles that fill the cytoplasm [5]. In their study, they reported that the presence of high-grade microvesicular steatosis was significantly associated with delayed hepatic function [5].
After allograft failure, histologic examination redemonstrated diffuse microvesicular steatosis, and new large extracellular fat droplets were observed as well. As hepatocytes ruptured during organ procurement, these small fat droplets may have coalesced and compromised the hepatic vasculature, like the mechanism described by Todo (1989), despite the donor liver’s steatosis pattern being primarily microvesicular [4]. The mitochondrial dysfunction associated with microvesicular steatosis and long-term alcohol use (given the donor’s substance use history) may have also contributed to the allograft’s failure. Additionally, the patient’s postoperative hemodynamic instability may have also contributed to his primary non-function. Hemodynamic instability immediately following reperfusion is not unusual, and depends on an array of factors, such as difficulty of the native hepatectomy and associated blood loss, underlying comorbidities including renal disease or diminished cardiac function, and oxygen free radicals and inflammatory cytokines released due to cellular disturbances at the time of reperfusion. Steatotic grafts in general are much less tolerant of this instability given that ischemia-reperfusion injury is exacerbated in these more marginal donor livers and thus are more prone to primary non-function.
To our knowledge, this is the first described case of primary non-function of a hepatic allograft with pre-existing severe microvesicular steatosis and mild LD-MAS in the absence of overt liver failure in the donor, with well-documented histopathology. This case demonstrates that precautions are needed to avoid using livers with diffuse and severe microvesicular steatosis. Additionally, donor liver frozen section reporting should note the presence and degree of true microvesicular steatosis and take care not to confuse it with the separate entity of SD-MAS. Transplant surgeons should also be aware of the risk that diffuse and severe microvesicular steatosis poses to allograft functioning.
We think this case highlights the potential impact of microvesicular steatosis on graft function, and that liver function in this setting may be compromised even without overt liver failure, with ischemia/reperfusion during transplantation potentially exacerbating occult liver dysfunction, leading to graft failure.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
All authors contributed to the study conception and design. LME, LX, YJ, and BK were responsible for collection of the case history and follow-up. LME wrote the manuscript, and LX was responsible for the submission. All authors reviewed the manuscript and agreed to the final version of the case report to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The authors declares that data supporting the findings of this study are available within the article.
Abbreviations
LD-MAS: large-droplet macrovesicular steatosis; SD-MAS: small-droplet macrovesicular steatosis; H&E: hematoxylin and eosin; EF: ejection fraction; PRBCs: packed red blood cells; FFP: fresh frozen plasma
| References | ▴Top |
- Neil DAH, Minervini M, Smith ML, Hubscher SG, Brunt EM, Demetris AJ. Banff consensus recommendations for steatosis assessment in donor livers. Hepatology. 2022;75(4):1014-1025.
doi pubmed pmc - Fishbein TM, Fiel MI, Emre S, Cubukcu O, Guy SR, Schwartz ME, Miller CM, et al. Use of livers with microvesicular fat safely expands the donor pool. Transplantation. 1997;64(2):248-251.
doi pubmed - Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321.
doi pubmed - Todo S, Demetris AJ, Makowka L, Teperman L, Podesta L, Shaver T, Tzakis A, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47(5):903-905.
doi pubmed pmc - Sharkey FE, Lytvak I, Prihoda TJ, Speeg KV, Washburn WK, Halff GA. High-grade microsteatosis and delay in hepatic function after orthotopic liver transplantation. Hum Pathol. 2011;42(9):1337-1342.
doi pubmed - Yoong KF, Gunson BK, Neil DA, Mirza DF, Mayer AD, Buckels JA, McMaster P. Impact of donor liver microvesicular steatosis on the outcome of liver retransplantation. Transplant Proc. 1999;31(1-2):550-551.
doi pubmed - Andert A, Ulmer TF, Schoning W, Kroy D, Hein M, Alizai PH, Heidenhain C, et al. Grade of donor liver microvesicular steatosis does not affect the postoperative outcome after liver transplantation. Hepatobiliary Pancreat Dis Int. 2017;16(6):617-623.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


