| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 1, February 2024, pages 15-22
A Real-World Experience on a Chinese Population of Patients With Unresectable Hepatocellular Carcinoma Treated With Nivolumab
Shou-Wu Leea, b, c, g, Sheng-Shun Yanga, b, d, e, f, Teng-Yu Leea, b
aDivision of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung 40705, Taiwan, Republic of China
bSchool of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan, Republic of China
cDepartment of Post-Baccalaureate Medicine, College of Medicine, Chung Hsing University, Taichung 40227, Taiwan, Republic of China
dSchool of Medicine, National Yang Ming Chiao Tung University, Taipei 11221, Taiwan, Republic of China
ePh.D. Program in Translational Medicine, Chung Hsing University, Taichung 40227, Taiwan, Republic of China
fInstitute of Biomedical Sciences, Chung Hsing University, Taichung 40227, Taiwan, Republic of China
gCorresponding Author: Shou Wu Lee, Division of Gastroenterology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taichung 40705, Taiwan, Republic of China
Manuscript submitted November 5, 2023, accepted January 3, 2024, published online February 28, 2024
Short title: HCC Treated With Nivolumab
doi: https://doi.org/10.14740/gr1684
| Abstract | ▴Top |
Background: For unresectable hepatocellular carcinoma (HCC), nivolumab (anti-programmed death receptor-1 (PD-1)) is used as non-curative interventions. The aim of the study was to focus on the real-world experience of nivolumab applied to patients with HCC.
Methods: Unresectable HCC patients receiving nivolumab treatments at Taichung Veterans General Hospital, from June 2018 to May 2020, were recruited. Exclusion criteria were Child-Pugh stage C, poor performance status, a lack of compliance or intolerable to drug treatments. The tumor radiological responses and survival outcomes of enrolled patients were collected and analyzed.
Results: Among a total of 57 patients, most of them were classified as Child-Pugh stage A (70.2%) and Barcelona Clinic Liver Cancer (BCLC) stage C (66.7%). Nivolumab was given to 14 (24.6%) as the primary-line, and 43 patients (75.4%) as the secondary-line systemic treatments. The mean therapeutic duration was 6.5 months. Objective response rate (ORR) was 24.6%, and disease control rate (DCR) was 42.1%. The overall median progression-free survival (PFS) was 5.8 months (95% confidence interval (CI): 1.1 - 10.6), and overall survival (OS) was 11.5 months (95% CI: 4.3 - 17.8). Immune-related adverse event (IRAE) was 8.8%. Presence of alpha-fetoprotein (AFP) response (a decline in AFP ≥ 10% from baseline) during therapy predicted the tumor radiological response (to objective response: hazard ratio (HR): 4.89, 95% CI: 1.14 - 21.00; to disease control: HR: 4.71, 95% CI: 1.32 - 16.81). Those with tumor radiological responses showed longer PFS and OS.
Conclusions: Decline in AFP during therapy has a predicting role on HCC radiological responses to nivolumab. Achieving radiological responses had better survival outcomes.
Keywords: Alpha-fetoprotein; Hepatocellular carcinoma; Nivolumab
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related morbidity worldwide, and most HCC cases occur with chronic liver inflammation [1]. Treatments of HCC depend on disease stages, typically according to the Barcelona Clinic Liver Cancer (BCLC) staging, which considers prognosis-related factors, such as tumor burden, liver function and performance status [2, 3]. In patients with intermediate (BCLC stage B) or advanced (BCLC stage C) HCC, non-curative interventions are used, with the aim to prolong survival by slowing tumor progression. These interventions are as follows: for patients with intermediate HCC, transarterial chemoembolization (TACE) [4], and for patients with advanced HCC or intermediate HCC with unsuitable for locoregional therapy, systemic therapy with tyrosine kinase inhibitor (TKI) or immunotherapeutic agents [5, 6].
Currently, four first-line treatments options are available, which include sorafenib (SOR), lenvatinib (LEN), the combination of atezolizumab with bevacizumab (Beva), and the combination of tremelimumab with durvalumab, based on the successful phase 3 studies [7].
Two common immunotherapies clinically used for second-line therapies on patients with HCC are nivolumab and pembrolizumab, which both target programmed death receptor-1 (PD-1) [6]. According to the phase 1/2 CheckMate 040 trial, nivolumab is effective as the second-line therapies for patients with HCC after failed responses to SOR [8].
The aim of our study was to focus on the real-world experience of nivolumab in treating patients with unresectable HCC.
| Materials and Methods | ▴Top |
HCC patients, diagnosed at Taichung Veterans General Hospital in accordance with American Association for the Study of Liver Disease (AASLD) guideline [9], were recruited for study during the period from June 2018 to May 2020. The inclusion criterion was unresectable HCC receiving nivolumab treatments. Exclusion criteria were those with cirrhotic Child-Pugh stage C, poor performance status, a lack of compliance or intolerability to drugs within the following day or missing any follow-ups. Clinical parameters were collected for all enrolled patients, including age, gender, liver function such as total bilirubin, albumin, alpha-fetoprotein (AFP), presence of chronic hepatitis B virus (HBV), hepatitis C virus (HCV), macroscopic vascular invasion (MVI), extrahepatic spread (EHS), cirrhotic Child-Pugh stage, albumin-bilirubin (ALBI) grade and BCLC stage. During the study period, the uses of combined TKI, such as SOR or LEN, or local-regional therapy (LRT), such as TACE or radiofrequency ablation (RFA), were also recorded.
After initial administration of nivolumab, patients returned for follow-up appointments at the outpatient clinic every 2 weeks and received continuous nivolumab treatments. The dosage of nivolumab each patient received was determined by the hepatologist. Nivolumab usage was discontinued if tumor progression had been found from follow-up imaging studies. The therapeutic duration, as well as dosage of nivolumab for each of the enrolled patients were recorded.
Throughout the study period, patients were assessed every 4 to 8 weeks by dynamic imaging. Any death, disease progression or treatment failure with nivolumab were recorded. Immune-related adverse events (IRAEs) were also recorded. AFP response was defined as a decline in AFP level ≥ 10% from baseline during therapy. To assess tumor responses, mRECIST criteria [10] was adapted and classified in four response categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients with CR or PR were considered having objective response, and patients with CR, PR or SD were considered having disease control. Objective response rate (ORR) and disease control rate (DCR) were calculated. Progression-free survival (PFS) was defined as the time from the start of study until disease progression or death. Overall survival (OS) was defined as the time from the start of study until death within the study period.
Data were expressed as mean and standard deviation of each measured parameter. The positive rates of individual stratified groups were expressed as percentages of the individual groups. Statistical comparisons were made using univariate or multivariate logistic regression to determine strengths of association between clinical parameters and tumor responses following nivolumab. Hazard ratio (HR) and 95% confidence interval (CI) were calculated, and statistical significance was set at P ≤ 0.05. Survival analyses were carried out using the Kaplan-Meier method.
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Taichung Veterans General Hospital approved this study (CF21236B).
| Results | ▴Top |
A total of 57 patients were enrolled for study. Their characteristics are shown in Table 1. Their median age was 66.7 years, with male predominance (84.2%). Chronic HBV was found in 28 patients (49.1%) and HCV infection in 14 patients (24.6%). The majority of them were classified as cirrhotic Child-Pugh stage A (70.2%), and BCLC stage C (66.7%) at the time of enrollment. ALBI grade 1 was found in 25 cases (43.9%), grade 2 in 30 cases (52.6%) and grade 3 in two cases (3.5%).
 Click to view | Table 1. The General Data of Patients |
There were 21 patients (36.8%) with MVI, and 25 patients (43.9%) with EHS. Among all patients, 14 received nivolumab as the primary systemic therapy (24.6%), and 43 as the secondary-line treatment (75.4%). During the nivolumab therapeutic course, nine patients (15.8%) had combined TKI, and eight patients (14.0%) had LRT. The average nivolumab dosage was 2.5 mg/kg, and the mean duration of medication was 6.5 months.
Therapeutic responses of these patients with nivolumab are listed in Table 2. The radiological responses are as follows: two (3.5%) with CR, 12 (21.1%) with PR, 10 (17.5%) with SD, and 33 cases (57.9%) with PD. Overall, ORR was 24.6%, and DCR was 42.1%. For all patients, their median PFS was 5.8 months (95% CI: 1.1 - 10.6), and OS was 11.5 months (95% CI: 4.3 - 17.8). Only five patients (8.8%) reported IRAE to nivolumab treatments, which included three cases of skin-related adverse effects and two with hepatic adverse effects. Positive AFP responses were noted in 17 patients (30.4%).
 Click to view | Table 2. The Therapeutic Responses of Patients Treated With Nivolumab |
Logistic analyses of the patients with objective responses to nivolumab are listed in Table 3. According to the univariable analysis, presence of HCV infection (HR: 5.14, 95% CI: 1.36 - 19.33, P = 0.015) and AFP response (HR: 7.65, 95% CI: 2.01 - 29.14, P = 0.003) had significant positive impacts to achieve the tumor objective response. Patients with age ≤ 65 years old (HR: 0.17, 95% CI: 0.03 - 0.88, P = 0.034) had a negative impact. After adjustments by the multivariable analysis, AFP response (HR: 4.89, 95% CI: 1.14 - 21.00, P = 0.033) still had the statistical strength in predicting the tumor objective response.
 Click to view | Table 3. The Strength of Association Between Clinical Parameters and Tumor Objective Response Following Nivolumab Usage |
Logistic analyses of patients with disease control to nivolumab are listed in Table 4. According to the multivariable analysis, AFP response (HR: 4.71, 95% CI: 1.32 - 16.81, P = 0.017) also had the statistical strength in predicting tumor disease control.
 Click to view | Table 4. The Strength of Association Between Clinical Parameters and Tumor Disease Control Following Nivolumab Usage |
Logistic analyses of OS of patients are listed in Table 5. According to the logistic analysis, none of the clinical parameters, including age, gender, Child-Pugh stage, ALBI grade, BCLC stage, presence of MVI and EHS, and baseline or during therapy AFP levels, was associated with the length of OS. Similarly, as primary or secondary-line systemic therapy, dosage of nivolumab, presence of IRAE, and combination of TKI or LRT had no impacts to the OS of our patients.
 Click to view | Table 5. The Strength of Association Between Clinical Parameters and Overall Survival Following Nivolumab Usage |
As shown in Figure 1 and Table 6, the median PFS for patients with tumor objective response to nivolumab was 8.4 months (95% CI: 2.5 - 14.3), and for those without such response, it was 4.9 months (95% CI: 0.8 - 9.0). Their difference was statistically significant (HR: 2.63, 95% CI: 1.23 - 5.66, P = 0.013). For those with tumor disease control to nivolumab, their mean PFS was 8.3 months (95% CI: 2.7 - 13.9), and for those without such control, it was 3.9 months (95% CI: 0.8 - 7.0). Again, their difference was statistically significant (HR: 2.89, 95% CI: 1.56 - 5.37, P = 0.008).
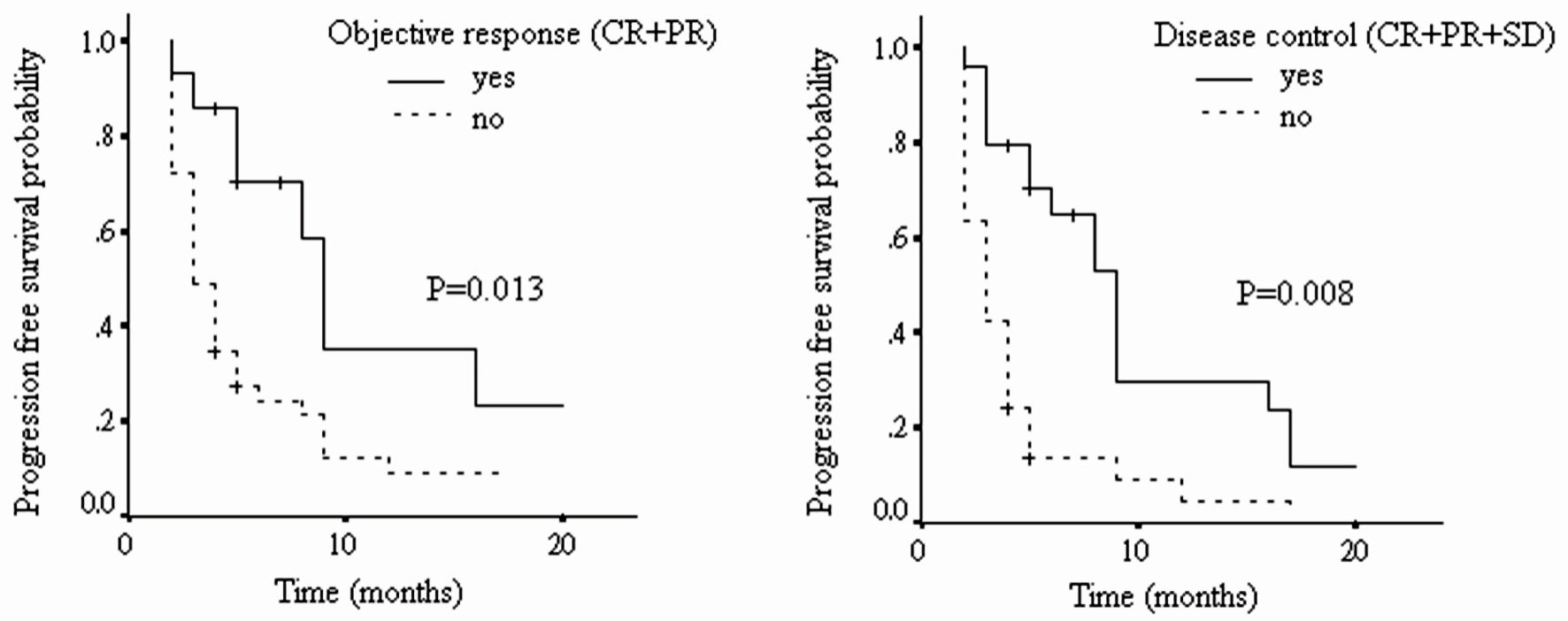 Click for large image | Figure 1. The progression-free survival of patients with different tumor radiological responses (CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease). |
 Click to view | Table 6. The Strength of Association Between Survival Outcomes and Best Tumor Radiological Responses Following Nivolumab Usage |
As shown in Figure 2 and Table 6, patients with tumor disease control to nivolumab had significantly longer OS than those without (median OS: 13.8 vs. 9.8 months, HR: 2.09, 95% CI: 1.07 - 4.08, P = 0.032). However, no significant difference was found between those with tumor objective response to nivolumab and those without (median OS: 13.5 vs. 10.8 months, HR: 1.53, 95% CI: 0.71 - 3.34, P = 0.284).
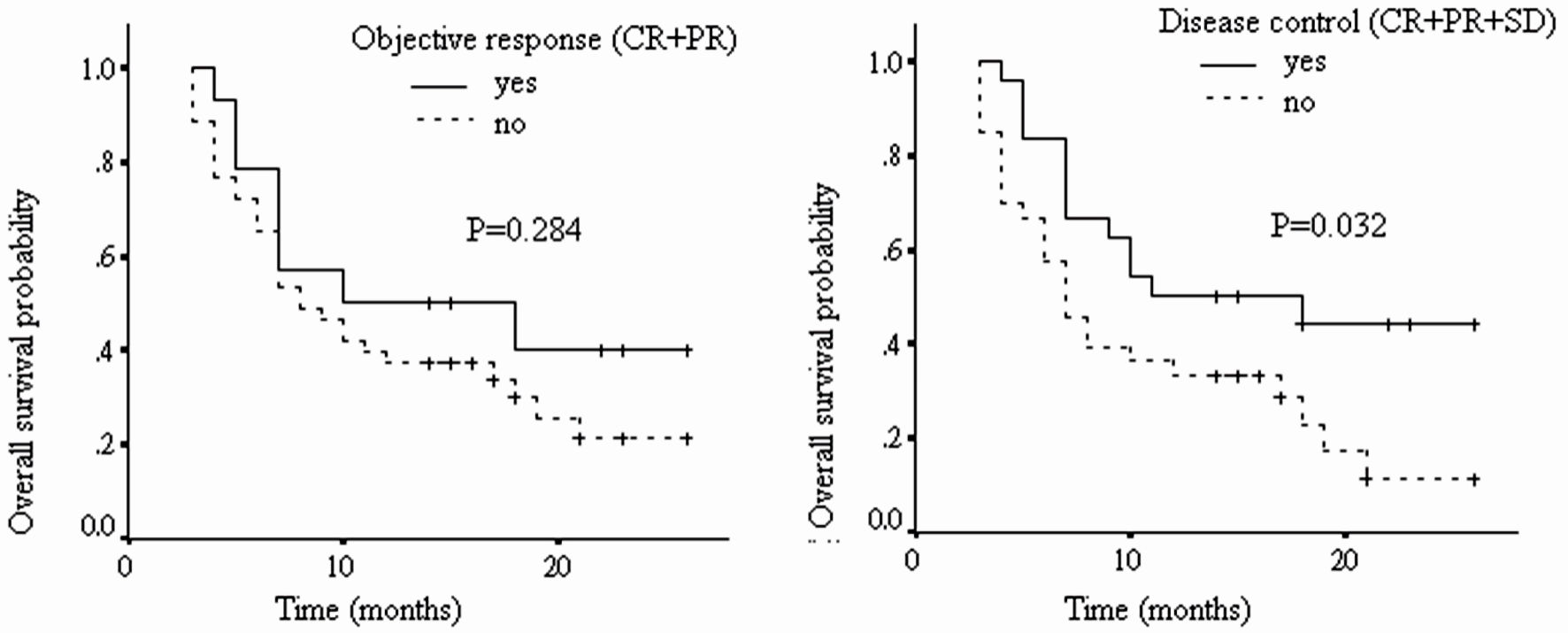 Click for large image | Figure 2. The overall survival of patients with different tumor radiological responses (CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease). |
| Discussion | ▴Top |
Liver cancer is a common cancer worldwide, and HCC accounts for 90% of primary liver cancer cases. It occurs predominantly in patients with underlying chronic liver disease and cirrhosis [11]. For the treatment of unresectable HCC, LRT with TACE and systemic therapy with targeted or immunotherapeutic agents are recommended [4-6].
SOR was the first TKI approved for clinical use as the first-line treatment for unresectable HCC. Its application started in 2007 according to two phase 3 studies [5, 12]. Another TIK, LEN, was also approved for first-line treatment of advanced or unresectable HCC based on a phase 3 non-inferiority report [13]. More recently, atezolizumab in combination with bevacizumab was also reported effective in the first-line treatment of unresectable HCC according to a phase 3 trial evaluation [14]. Since 2017, other multi-kinase inhibitors, like regorafenib, cabozantinib and ramucirumab (AFP ≥ 400 ng/mL), have been approved for treating advanced HCC after prior SOR treatment [15-17].
Nivolumab is a recombinant anti-PD-1 monoclonal antibody. It is administered through intravenous infusion. In a single-arm phase 1/2 CheckMate 040 trial, it was designed as a second-line therapy for HCC who did not respond to SOR. The ORR with nivolumab was 20%, and DCR was 64% [7, 18, 19]. Thus, the drug received accelerated approval as a second-line therapy for unresectable HCC. However, in the phase 3 CheckMate 459 trial, aimed as a potential first-line systemic treatment for HCC, the OS of patients with nivolumab treatments was not statistically better than those with SOR treatments (HR: 0.85, 95% CI: 0.72 - 1.02; P = 0.0752) [20].
Some real-world studies were reported, focusing on the effectiveness of nivolumab in patients with unresectable HCC. One international, multicenter, real-world cohort, enrolled 64 patients with advanced HCC, who were given PD-1 immunotherapy, including nivolumab (n = 34) or pembrolizumab (n = 31). They found ORR of 12%, and DCR of 49%. Their median PFS was 4.6 months (95% CI: 3.0 - 6.2), and median OS was 11.0 months (95% CI: 8.2 - 13.8). Further investigations found significantly shorter OS in cases with Child-Pugh stage B compared with cases with stage A (8.6 vs. 16.7 months, P = 0.065) [21]. Another retrospective single-center study was conducted in Taiwan, on 95 patients with unresectable HCC receiving PD-1 immunotherapy, including nivolumab (n = 92) and pembrolizumab (n = 3). They found ORR of 24.4%, and DRR of 36.7%. Declines in AFP > 10% within 4 weeks was the independent predictor of achieving tumor objective response (HR: 7.259, P = 0.001). Their median OS was 11.9 months (95% CI: 5.6 - 18.2), and early decline AFP, ALBI grade and Child-Pugh stage were also independent factors associated with OS [22].
One recent international multicenter observational study, on 233 patients receiving nivolumab as HCC treatment, reported ORR of 22.4%, and DCR of 52.1%. Their median PFS was 10.1 months (95% CI: 6.1 - 14.2), and OS was 12.2 months (95% CI: 8.4 - 16.0). The OS was shorter for those in Child-Pugh stage B than in stage A (7.3 vs. 16.3 months, P < 0.001), and also in post-first line use (10.4 vs. 16.3 months, P = 0.05). Achievements of tumor objective response were predictive of improved OS (25.4 vs. 13.2 months, P < 0.001) [23]. One retrospective single-center study in Korea evaluated 203 patients with advanced HCC under nivolumab treatment. They found a shorter ORR for patients in Child-Pugh stage B than in stage A (2.8% vs. 15.9%, P = 0.010). Their median OS was also shorter in Child-Pugh stage B patients (11.3 vs. 42.9 weeks, adjusted hazard ratio (aHR) 2.10, P < 0.001) [24]. Another retrospective study was conducted in Taiwan on 87 patients with unresectable HCC over a median nivolumab treatment period of 2.53 months. Their final outcomes were ORR of 19.5%, and DCR of 39.1%, respectively. Declined AFP levels of ≥ 20% within the first 3 months of treatment was a predictor of achieving OR (OR: 5.997, P = 0.042). Their median PFS was 2.67 months, and OS was 5.87 months. The lack of MVI, combination therapy, and AFP response were predictors of PFS. Cancer of the Liver Italian Program (CLIP) scores of 0 to 2 (HR: 3.717, P = 0.004) and grade 1 to 2 IRAE (HR: 2.217, P = 0.049) were also predictors of OS [25].
Our present findings in our patients show an ORR of 24.6% and DCR of 42.1%, which are comparable to previous studies. The only significant factor of radiological responses to the HCC patients receiving nivolumab treatments was the AFP response. That was defined as a decline AFP levels ≥ 10% with reference to baseline during nivolumab therapy. Other factors, including age, gender, Child-Pugh stage, ALBI grade, BCLC stage, presence of MVI and EHS, baseline AFP, whether nivolumab was used as primary or secondary-line systemic therapy, dosage of nivolumab, presence of IRAE, combination of TKI or LRT, were not at all found to be associated with the best tumor radiological responses.
The median PFS and OS of all our enrolled cases were 5.8 and 11.5 months, respectively. Achievements of tumor objective response or disease control were associated with longer PFS, and achievements of tumor disease control predicted better OS. Therefore, in absence of molecular predictors, the achievement of radiological response following nivolumab can help clinicians identify patients who are likely to derive long-term benefit from nivolumab therapy.
Different from previous studies, our results found no association between OS and Child-Pugh stage (B vs. A: 9.9 vs. 12.1 months, HR: 0.75, 95% CI: 0.38 - 1.49, P = 0.418). The reason could be related to the limited ample size and short follow-up periods. We also found a lower ratio of IRAE (8.8%) with nivolumab compared with previous studies. For example, in the phase 3 CheckMate 459 trial, they found a ratio of 22% grade 3/4 IRAE, which led to a 4% discontinuation [20]. The explanation of the discrepancy in results could be due to the exclusion of patients intolerant to nivolumab in our study, resulting in an underestimated incidence of IRAE.
Our study had several limitations. First, this is a retrospective study, and selection or reporting bias may have existed. Second, our sample size was relatively small, and the follow-up period was relatively short. Third, the PD-L1 expression in the tumor or immune cells was not assessed. Further prospective studies are needed on more patients and with more variables.
In conclusion, we found that the efficacy of nivolumab for unresectable HCC is acceptable. The decline in AFP during therapy is a predictor of tumor radiological responses, and achieving radiological responses predicted better survival outcomes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
This study was designed and conducted in accordance with the IRB and consisted of retrospective chart review. The information in the manuscript is fully confidential and does not contain any individual patient identifiers. All the informed consents from the patients for publication of the manuscript were obtained.
Author Contributions
SW Lee and TY Lee designed and coordinated this study. SW Lee, SS Yang and TY Lee were responsible for chart review and data collection. SW Lee and TY Lee wrote the manuscript and all subsequent revisions. SW Lee performed the statistical analysis and created all tables and figures for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Furthermore, the raw data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462.
doi pubmed - Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329-338.
doi pubmed - Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301-1314.
doi pubmed - Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429-442.
doi pubmed - Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
doi pubmed - Rimassa L, Worns MA. Navigating the new landscape of second-line treatment in advanced hepatocellular carcinoma. Liver Int. 2020;40(8):1800-1811.
doi pubmed pmc - Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, Gores GJ, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. 2022;3(4):386-401.
doi pubmed pmc - El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.
doi pubmed pmc - Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358-380.
doi pubmed - Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60.
doi pubmed - European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236.
doi pubmed - Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
doi pubmed - Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173.
doi pubmed - Cheng AL, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(Suppl 9):LBA3.
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
doi pubmed - Merle P, Rimassa L, Ryoo B, et al. Assessment of tumor response, AFP response, and time to progression in the phase 3 CELESTIAL trial of cabozantinib versus placebo in advanced hepatocellular carcinoma (HCC). Ann Oncol. 2018;29(Suppl 5):v104.
- Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282-296.
doi pubmed - Crocenzi TS, El-Khoueiry AB, Yau TC, et al. Nivolumab (nivo) in sorafenib (sor)-naive and-experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol. 2017;35(15 Suppl):4013.
- Melero I, Sangro B, Yau TC, et al. Nivolumab dose escalation and expansion in patients with advanced hepatocellular carcinoma (HCC): The CheckMate 040 study. J Clin Oncol. 2017;35(4 Suppl):226. trial. Lancet. 2017;389:2492-2502.
- Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30(Suppl 5):v874-v875.
- Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, Koch S, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333.
doi pubmed pmc - Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel). 2020;12(1):182.
doi pubmed pmc - Fessas P, Kaseb A, Wang Y, Saeed A, Szafron D, Jun T, Dharmapuri S, et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer. 2020;8(2):e001033.
doi pubmed pmc - Choi WM, Lee D, Shim JH, Kim KM, Lim YS, Lee HC, Yoo C, et al. Effectiveness and safety of nivolumab in child-pugh B patients with hepatocellular carcinoma: a real-world cohort study. Cancers (Basel). 2020;12(7):1968.
doi pubmed pmc - Hsu WF, Chuang PH, Chen CK, Wang HW, Tsai MH, Su WP, Chen HY, et al. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience. Am J Cancer Res. 2020;10(12):4547-4560.
pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


