| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Short Communication
Volume 16, Number 6, December 2023, pages 334-341
Serum Activin A Is a Novel Biomarker of Endoscopic Activity in Ulcerative Colitis
Ryohei Ogiharaa, c, Hiroki Kurumia, Tsutomu Kandaa, Kazuo Yashimaa, Hajime Isomotoa, Naoyuki Yamaguchib
aDivision of Gastroenterology and Nephrology, Department of Multidisciplinary Internal Medicine, Tottori University Faculty of Medicine, Yonago, Japan
bDepartment of Endoscopy, Nagasaki University Hospital, Nagasaki, Japan
cCorresponding Author: Ryohei Ogihara, Division of Gastroenterology and Nephrology, Department of Multidisciplinary Internal Medicine, Tottori University Faculty of Medicine, Yonago, Japan
Manuscript submitted October 10, 2023, accepted November 16, 2023, published online December 9, 2023
Short title: Serum Activin A: Novel Biomarker in UC
doi: https://doi.org/10.14740/gr1677
| Abstract | ▴Top |
Background: Endoscopic healing (EH) is the long-term therapeutic goal for ulcerative colitis (UC). Since repeated colonoscopies are inconvenient and invasive, a surrogate biomarker for endoscopic activity is needed. Activin A is one of the transforming growth factor-β superfamily of proteins and has been shown to be associated with intestinal inflammation.
Methods: This single-center observational study included 27 Japanese patients with UC in clinical remission who underwent colonoscopy and blood sampling. We investigated the correlations between laboratory parameters, including serum activin A levels, and endoscopic activity, classified by the Mayo endoscopic subscore (MES) in these patients.
Results: This study included 15 males and 12 females. The median age was 44.0 years. In terms of endoscopic activity, five patients were diagnosed with MES 0, 14 patients with MES 1, seven patients with MES 2, and one patient with MES 3. The median serum activin level was 134.8 pg/mL (interquartile range (IQR), 105.3 - 188.1). Serum activin A levels were significantly correlated with the MES (Spearman’s rank correlation coefficient r = 0.591, P = 0.001), which was better than that of C-reactive protein (CRP) (r = 0.487, P = 0.010). In the comparison between the EH group (MES 0) and non-EH group (MES 1-3), patients without EH had significantly higher serum activin A levels (Mann-Whitney U test, P = 0.047). A cutoff value of 133.6 pg/mL indicated non-EH with a sensitivity and specificity of 0.682 and 1.000, respectively. The area under the curve (AUC) of serum activin A for detecting non-EH was 0.791 (95% confidence interval (CI), 0.618 - 0.964), while that of CRP was 0.723 (95% CI, 0.504 - 0.941).
Conclusions: The serum activin A level is a potential novel biomarker of endoscopic activity in UC.
Keywords: Ulcerative colitis; Endoscopic healing; Endoscopic activity; Biomarker; Serum activin A
| Introduction | ▴Top |
Ulcerative colitis (UC) is an idiopathic chronic inflammatory bowel disease characterized by a relapsing-remitting course. As there is no radical cure for UC, current treatments aim to achieve and maintain clinical remission. In recent years, endoscopic healing (EH) following clinical remission has become a long-term therapeutic goal for UC treatment [1]. Patients who achieve EH have lower relapse and surgery rates [2, 3]. Although some studies defined EH as a Mayo endoscopic subscore (MES) of 0 or 1, patients with MES 0 are reported to have a lower relapse rate than those with MES 1 [4, 5]. Endoscopic assessment is the gold standard for UC treatment; however, repeated colonoscopies are inconvenient and invasive.
Biomarkers for UC are often used instead of colonoscopy to predict endoscopic activity; however, they have problems in terms of accuracy and convenience. Common biomarkers include C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), leucine-rich alpha-2 glycoprotein (LRG), and fecal calprotectin (FC). CRP, ESR, and LRG are serological biomarkers, and CRP is often used clinically because it is easily accessible. CRP has been shown to correlate with endoscopic activity in many studies but is not sensitive enough to detect mild intestinal inflammation [6, 7]. The correlation between ESR and endoscopic activity has been reported to be lower than that between CRP levels and endoscopic activity [8]. Although some studies have shown that LRG is correlated with endoscopic activity, there is little evidence because it is a relatively new biomarker [9, 10]. FC is a fecal biomarker derived from inflammatory and epithelial cells in the intestine mucosa [11]. Since FC can selectively evaluate intestinal inflammation, it has been reported to correlate better with endoscopic activity than with serological markers [7, 12]. However, collecting stool samples is inconvenient and poorly accepted by patients [13]. Therefore, it is necessary to develop highly accurate and convenient surrogate biomarkers.
Activins are members of the transforming growth factor-β superfamily of proteins and involved in a wide range of biological processes including development, differentiation, tissue repair, inflammation, fibrosis and carcinogenesis [14, 15]. Several types of activins have been identified, with activin A being a homodimer of two βA subunits. Activin A and activin receptors have been reported to be expressed in epithelial cells of the rat small intestine and involved in the regulation of such cellular functions as proliferation and differentiation [16]. In dextran sodium sulfate (DSS)-induced colitis mice, the expression of activin βA subunit and activin receptors was found increased in intestinal macrophages and epithelial cells respectively [17]. In addition, plasma activin A levels were elevated in these mice. In a study using human intestinal tissue, activin receptors were expressed in both patients with inflammatory bowel disease (IBD) and controls, whereas activin A was expressed only in patients with IBD [18]. Another study showed that the expression of activin βA subunits increased markedly in surgical specimens collected from the intestines of patients with UC, and the levels of the expression correlated with the severity of intestinal inflammation [19]. Thus, serum activin A levels may reflect endoscopic activity in patients with UC. In the present study, we investigated the correlation between serum activin A levels and endoscopic activity in patients with UC.
| Materials and Methods | ▴Top |
Patients and procedure
This single-center observational study included 27 Japanese patients with UC in clinical remission. All patients who underwent colonoscopy and blood sampling at the Tottori University Hospital, Tottori, Japan, between January 2022 and March 2023 were enrolled. After endoscopic assessment and measurement of laboratory parameters, including serum activin A levels, the correlations were analyzed. Furthermore, patients were divided into EH and non-EH groups, and laboratory parameters were compared between the two groups. Diagnosis of UC was based on standard Japanese diagnostic criteria [20]. Clinical remission was defined as a partial Mayo score < 3 and no subscore > 1 [1, 4]. Laboratory parameters were also analyzed for correlations with patient characteristics and with clinical activity classified by the partial Mayo score. This study was approved by the Tottori University Institutional Review Board and was conducted in compliance with the ethical standards of the responsible institution for human subjects as well as with the Helsinki Declaration.
Endoscopic assessment
All patients underwent a total colonoscopy to evaluate endoscopic activity. Endoscopic activity in each patient was assessed at the most severely inflamed site and classified using the MES. All endoscopic examinations were performed by experienced endoscopists who assessed endoscopic activity. EH was defined as an MES of 0 [1, 4], while non-EH was defined as an MES of 1-3.
Measurement of serum activin A levels
Patient blood samples were centrifuged to obtain serums, divided into aliquots, and stored frozen at -30 °C until use. Serum activin A levels were measured using an ELISA kit (Human/Mouse/Rat Activin A Quantikine ELISA Kit, Lot No. P318823, R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s protocol. First, anti-activin A biotinylated antibody was conjugated to streptavidin-coated microplates. Next, activin A standards and serum samples were added to the wells of the microplate and incubated for 3 h at room temperature on a horizontal orbital microplate shaker set at approximately 500 rpm. After washing each well with wash buffer for six times, horseradish peroxidase-conjugate antibody specific for the βA subunit was added to into each well and incubated for 1 h at room temperature on the shaker. After washing each well with wash buffer six times, the substrate solution was added to each well and incubated for 30 min at room temperature on a benchtop. When the stop solution was added to each well, the color developed in proportion to the amount of bound activin A. Finally, the absorbance at 450 and 540 nm was measured using a microplate spectrophotometer (Vientonano, DS Pharma Biochemical Co., Ltd, Osaka, Japan), and the absorbance at 450 nm was corrected by subtracting the absorbance at 540 nm. The activin A concentration in each sample was calculated based on the absorbance of the activin A standard. The following laboratory parameters were also measured in blood samples: CRP, white blood cell (WBC) count, ESR, and LRG.
Statistical analysis
Continuous variables were expressed as medians with interquartile ranges (IQRs). Categorical variables are expressed as percentages. Correlation analysis between each laboratory parameter and the MES was performed using Spearman’s rank correlation coefficient. Differences between the two groups were analyzed using the Mann-Whitney U test. A receiver-operating characteristic (ROC) curve was generated to estimate the area under the curve (AUC) and the cutoff for detecting non-EH. The optimal cutoff values were determined by minimizing the square of the distance between a point (sensitivity of 1, 1-specificity of 0) and any point on the ROC curve. Statistical significance was set at P < 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [21].
| Results | ▴Top |
Patient characteristics
There were 27 patients in total, 15 male patients and 12 female patients (Table 1). The median age was 44.0 years. The disease type was pancolitis in 17 patients, left-sided colitis in six patients, and proctitis in four patients. The treatment was 5-aminosalicylic acid in 23 patients, and corticosteroids, immunomodulators, and biologics in five patients each. The median serum activin level was 134.8 pg/mL (IQR, 105.3 - 188.1). Median CRP level, WBC count, ESR, and LRG level were 0.03 mg/dL (IQR, 0.02 - 0.09), 4,700/µL (IQR, 3,700 - 5,500), 3.0 mm/h (IQR, 2.0 - 9.5), and 10.8 µg/mL (IQR, 9.4 - 13.1), respectively. The MES details were as follows: MES 0, five patients; MES 1, 14 patients; MES 2, seven patients; and MES 3, one patient. In other words, five patients achieved EH and 22 patients did not. Median serum activin levels in the EH and non-EH group were 103.2 (IQR, 99.8 - 126.8) and 148.7 (IQR, 111.0 – 190.4), respectively.
 Click to view | Table 1. Patient Characteristics |
Serum activin A levels, CRP levels, ESR, and LRG levels were significantly correlated with patients’ ages (Spearman’s rank correlation coefficient r = 0.770, P < 0.001; r = 0.432, P = 0.025; r = 0.646, P < 0.001; r = 0.608, P < 0.001, respectively). Patients’ ages were also significantly correlated with endoscopic activity classified by the MES (r = 0.556, P = 0.003), with older patients tending to have higher endoscopic activity. No laboratory parameter had significant correlations with patients’ sex or disease types. In terms of the associations between treatment agents and laboratory parameters, WBC count was significantly higher in steroid users (Mann-Whitney U test, P = 0.042), and ESR was significantly higher in immunomodulator users (Mann-Whitney U test, P = 0.018). No laboratory parameter showed significant correlations with the other treatment agents or with clinical activity classified by the partial Mayo score.
Correlation between serum activin A levels and endoscopic activity
Serum activin A levels exhibited a significant correlation with endoscopic activity classified by the MES and increased with higher endoscopic disease activity. (Spearman’s rank correlation coefficient r = 0.591, P = 0.001) (Fig. 1). Among the other laboratory parameters, CRP levels were significantly correlated (r = 0.487, P = 0.010), whereas WBC count, ESR, and LRG levels were not significantly correlated (r = 0.236, P = 0.237; r = 0.281, P = 0.155; r = 0.173, P = 0.387, respectively). In the comparison between the EH and non-EH groups, serum activin A levels showed a significant difference (Mann-Whitney U test, P = 0.047), with the non-EH group having higher serum activin A levels (Fig. 2). However, CRP levels were not significantly different between the two groups (Mann-Whitney U test, P = 0.130). A serum activin A cutoff value of 133.6 pg/mL indicated non-EH, with a sensitivity and specificity of 0.682 and 1.000, respectively (Fig. 3). The AUC of serum activin A for detecting non-EH was 0.791 (95% confidence interval (CI), 0.618 - 0.964), while that of CRP was 0.723 (95% CI, 0.504 - 0.941). There was no statistically significant difference between the AUCs (P = 0.429).
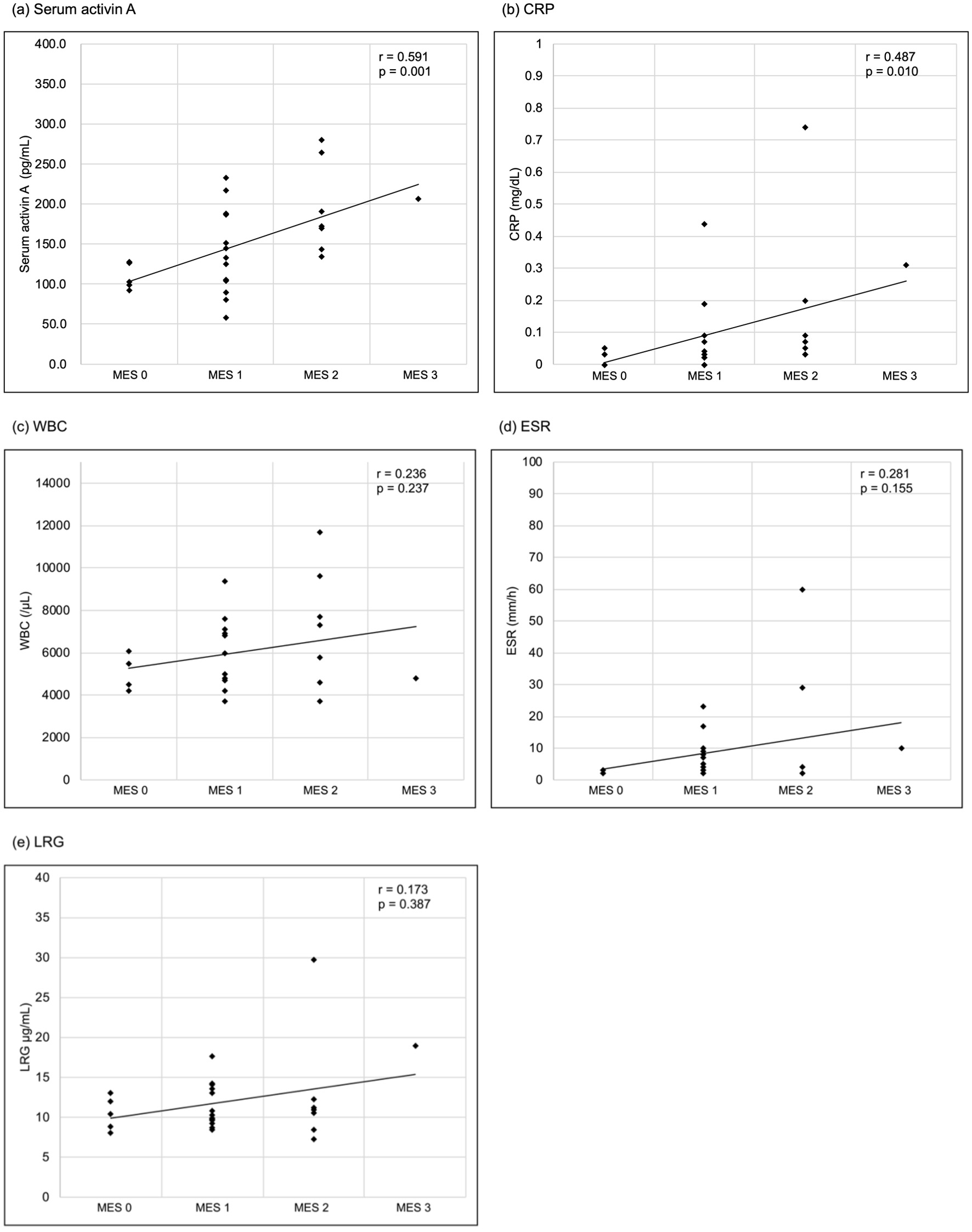 Click for large image | Figure 1. Correlations between laboratory parameters and the MES. (a) Serum activin A, (b) CRP, (c) WBC, (d) ESR, (e) LRG. Serum activin A and CRP significantly correlated with the MES (Spearman’s rank correlation coefficient r = 0.591, P = 0.001; r = 0.487, P = 0.010, respectively), while WBC, ESR and LRG had no significant correlation (r = 0.236, P = 0.237; r = 0.281, P = 0.155; r = 0.173, P = 0.387, respectively). CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; LRG: leucine-rich alpha-2 glycoprotein; MES: Mayo endoscopic score; WBC: white blood cell. |
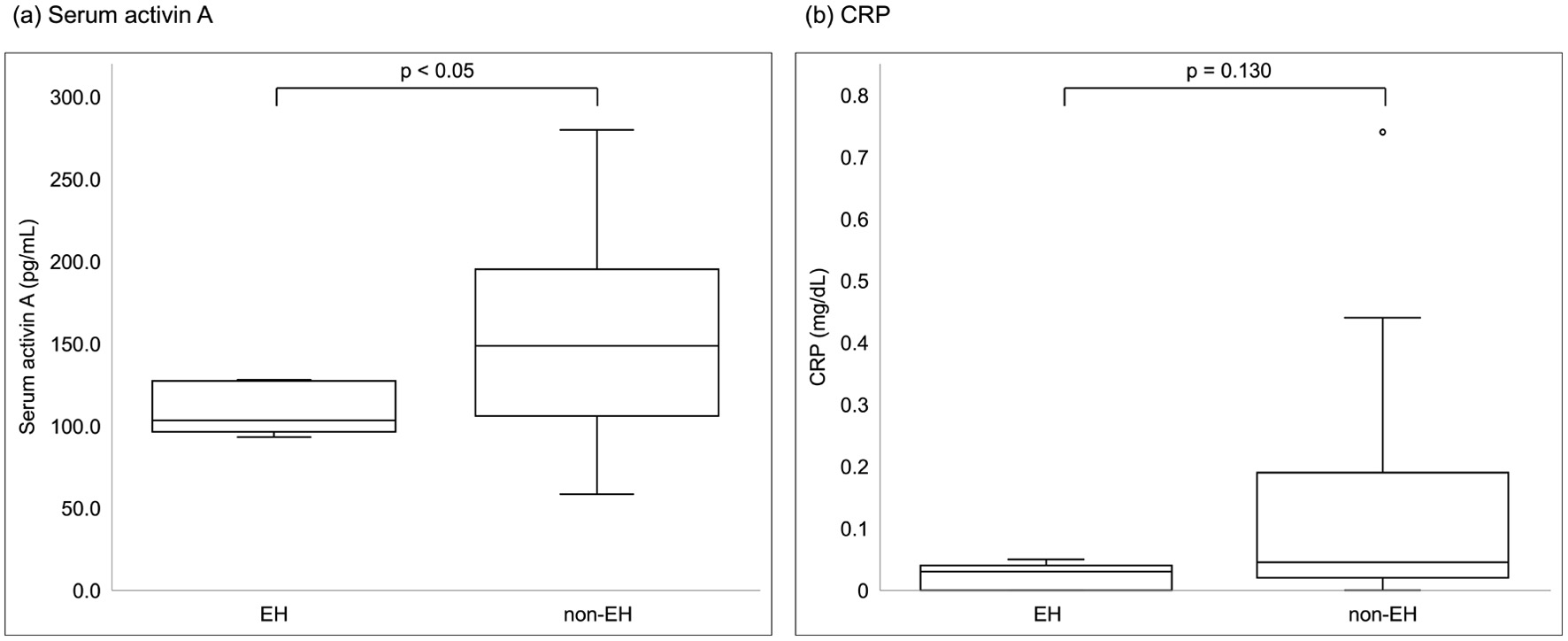 Click for large image | Figure 2. Comparisons of laboratory parameters between patients with EH and non-EH. (a) Serum activin A, (b) CRP. Among laboratory parameters, only serum activin A was significantly different between the groups (Mann-Whitney U test, P = 0.047). CRP: C-reactive protein; EH: endoscopic healing. |
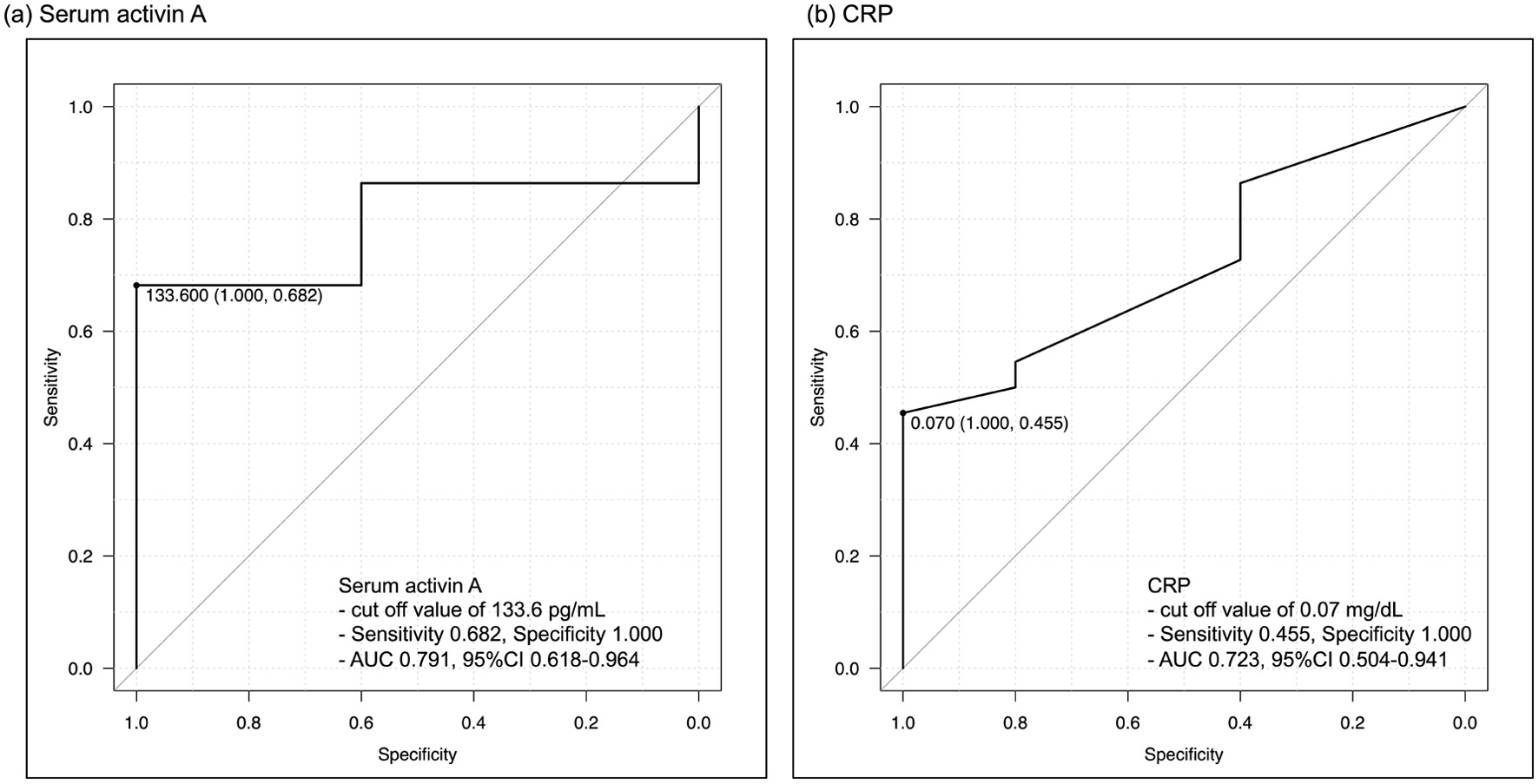 Click for large image | Figure 3. Receiver-operating characteristic curves for detecting non-EH. (a) Serum activin A, (b) CRP. The best cut-off value of serum activin A is 133.6 pg/mL, with a sensitivity of 0.682 and specificity of 1.000. The AUC of serum activin A is 0.791, while that of CRP is 0.723. AUC: area under the curve; CRP: C-reactive protein; EH: endoscopic healing. |
| Discussion | ▴Top |
This study demonstrated a correlation between serum activin A levels and endoscopic activity, as classified by the MES, in patients with UC. We also found that serum activin A levels correlated better with endoscopic activity than with other clinical serological biomarkers. Patients without EH have significantly higher serum activin A levels than those with EH. Thus, patients with high serum activin A levels may have endoscopic inflammation, and may be considered for optimization of therapy.
One of the purposes of colonoscopy in the treatment of UC is to determine whether patients in clinical remission have achieved EH. It is difficult to estimate endoscopic activity from clinical symptoms alone, as one study reported that approximately half of patients with UC in clinical remission had endoscopic inflammation (MES > 0) [22]. Therefore, only patients in clinical remission were included in this study and most had no or mild intestinal inflammation. Nevertheless, serum activin A levels are correlated with endoscopic activity, indicating that serum activin A may be useful in real-world clinical practice.
In addition to serum activin A, this study examined the correlations between CRP levels, ESR, LRG, and endoscopic activity. CRP is synthesized by hepatocytes and rapidly produced following the release of proinflammatory cytokines such as interleukin (IL)-6 [23]. ESR indicates the amount of supernatant formed by the sinking of blood cell components. In acute inflammation, ESR is increased owing to high concentrations of fibrinogen, globulin, and complement. Approximately 60-70% of the ESR depends on fibrinogen, which is produced by the liver in response to IL-6, IL-1, and tumor necrosis factor (TNF) [24]. LRG is produced in inflamed organs as well as in the liver and is upregulated by not only IL-6 but also IL-1β, TNF-α, IL-22 [25]. In this study, among the three biomarkers, only the CRP level correlated significantly with endoscopic activity; however, the serum activin A level correlated better than the CRP level. Some laboratory parameters, including serum activin A levels, showed significant correlations with patients’ ages. Patients’ ages were also significantly correlated with endoscopic activity. This may explain why some laboratory parameters were significantly correlated with patients’ ages.
In mice, serum activin A increased after lipopolysaccharide (LPS) stimulation and regulated cytokines, such as IL-6 and TNF [26]. Furthermore, activin A was released early in the cascade of circulatory cytokines during inflammation roughly coincident with TNF-α and before IL-6. This suggests that activin A may be able to detect mild intestinal inflammation that is undetectable by biomarkers that depend on cytokines released later in the cascade, such as IL-6.
Although it is not clear how activin A is involved in the pathogenesis of UC, a study on mice with DSS-induced colitis showed that activin A produced by intestinal macrophages promotes intestinal inflammation and inhibits the proliferation of intestinal epithelial cells [17]. Serum activin A has also been reported to be elevated in chronic inflammatory diseases, such as rheumatoid arthritis, osteoarthritis, and bronchial asthma [27, 28]. In these diseases, activin A is elevated in the joint fluid, bronchial lavage fluid, and serum. Therefore, serum activin A may directly reflect local inflammation, unlike CRP, which is produced in the liver. In UC, it is possible that increased activin A from the inflamed intestine may be transferred into the bloodstream, resulting in the elevation of serum activin A. To clarify this hypothesis, it is necessary to investigate the association between serum activin A levels, the severity of histological inflammation, and the level of activin A expression in intestinal tissues.
This study had several limitations. First, this was a single-center study with a relatively small sample size. In this study, the accuracy of serum activin A in detecting EH was not significantly different from that of CRP, possibly due to the small sample size. Second, healthy subjects and patients with UC were not compared. Third, the correlations between fecal biomarkers such as FC and endoscopic activity were not investigated. FC measurements were also conducted in this study; however, some individuals did not submit their stool samples to our hospital, likely due to the home collection process. Consequently, we were unable to gather enough data for FC, and it was excluded from the analysis. Finally, serial measurements should be performed to investigate the changes in serum activin A levels before and after treatment.
Conclusion
Serum activin A is a potential novel biomarker of endoscopic activity in patients with UC. Further research is required to evaluate the usefulness of serum activin A as a novel biomarker.
Acknowledgments
We thank the staff of the Division of Gastroenterology and Nephrology, Department of Multidisciplinary Internal Medicine, Tottori University.
Financial Disclosure
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors. No external funding was received for this study.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Informed consent was obtained from all patients involved in the study.
Author Contributions
Conceptualization: RO and HI; methodology: RO, HI, and NY; validation: RO, HI, and NY; formal analysis: RO; investigation: RO, HK, and TK; resources: RO, HK, TK, KY, and HI; data curation: RO; original draft preparation: RO; review and editing: HK, TK, KY, HI, and NY; and supervision: HI. All authors have read and agreed to the published version of the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
AUC: area under the curve; CI: confidence interval; CRP: C-reactive protein; EH: endoscopic healing; ESR: erythrocyte sedimentation rate; FC: fecal calprotectin; IBD: inflammatory bowel disease; IL: interleukin; LRG: leucine-rich alpha-2 glycoprotein; MES: Mayo endoscopic subscore; ROC: receiver-operating characteristic; TNF: tumor necrosis factor; UC: ulcerative colitis; WBC: white blood cell
| References | ▴Top |
- Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, et al. STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583.
doi pubmed - Froslie KF, Jahnsen J, Moum BA, Vatn MH, IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133(2):412-422.
doi pubmed - Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194-1201.
doi pubmed - Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625-1629.
doi pubmed - Barreiro-de Acosta M, Vallejo N, de la Iglesia D, Uribarri L, Baston I, Ferreiro-Iglesias R, Lorenzo A, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J Crohns Colitis. 2016;10(1):13-19.
doi pubmed - Krzystek-Korpacka M, Kempinski R, Bromke M, Neubauer K. Biochemical biomarkers of mucosal healing for inflammatory bowel disease in adults. Diagnostics (Basel). 2020;10(6):367.
doi pubmed pmc - Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, Sandborn WJ, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802-819; quiz 820.
doi pubmed - Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014;59(4):829-837.
doi pubmed - Shinzaki S, Matsuoka K, Iijima H, Mizuno S, Serada S, Fujimoto M, Arai N, et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis. 2017;11(1):84-91.
doi pubmed pmc - Yasutomi E, Inokuchi T, Hiraoka S, Takei K, Igawa S, Yamamoto S, Ohmori M, et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci Rep. 2021;11(1):11086.
doi pubmed pmc - Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34(1):50-54.
doi pubmed - Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, Schmidt C, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19(2):332-341.
doi pubmed - Osborne JM, Flight I, Wilson CJ, Chen G, Ratcliffe J, Young GP. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient Prefer Adherence. 2018;12:1825-1836.
doi pubmed pmc - Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986;321(6072):779-782.
doi pubmed - Namwanje M, Brown CW. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harb Perspect Biol. 2016;8(7):a021881.
doi pubmed pmc - Sonoyama K, Rutatip S, Kasai T. Gene expression of activin, activin receptors, and follistatin in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;278(1):G89-97.
doi pubmed - Zhang YQ, Resta S, Jung B, Barrett KE, Sarvetnick N. Upregulation of activin signaling in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2009;297(4):G768-780.
doi pubmed pmc - Dignass AU, Jung S, Harder-d'Heureuse J, Wiedenmann B. Functional relevance of activin A in the intestinal epithelium. Scand J Gastroenterol. 2002;37(8):936-943.
doi pubmed - Hubner G, Brauchle M, Gregor M, Werner S. Activin A: a novel player and inflammatory marker in inflammatory bowel disease? Lab Invest. 1997;77(4):311-318.
pubmed - Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, Saruta M, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489-526.
doi pubmed pmc - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed pmc - Rosenberg L, Lawlor GO, Zenlea T, Goldsmith JD, Gifford A, Falchuk KR, Wolf JL, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19(4):779-784.
doi pubmed pmc - Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313-336.
pubmed - Breda L, Nozzi M, De Sanctis S, Chiarelli F. Laboratory tests in the diagnosis and follow-up of pediatric rheumatic diseases: an update. Semin Arthritis Rheum. 2010;40(1):53-72.
doi pubmed - Serada S, Fujimoto M, Terabe F, Iijima H, Shinzaki S, Matsuzaki S, Ohkawara T, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012;18(11):2169-2179.
doi pubmed - Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, Phillips DJ. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A. 2007;104(41):16239-16244.
doi pubmed pmc - El-Gendi SS, Moniem AE, Tawfik NM, Ashmawy MM, Mohammed OA, Mostafa AK, Zakhari MM, et al. Value of serum and synovial fluid activin A and inhibin A in some rheumatic diseases. Int J Rheum Dis. 2010;13(3):273-279.
doi pubmed - Samitas K, Poulos N, Semitekolou M, Morianos I, Tousa S, Economidou E, Robinson DS, et al. Activin-A is overexpressed in severe asthma and is implicated in angiogenic processes. Eur Respir J. 2016;47(3):769-782.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


