| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 17, Number 1, February 2024, pages 10-14
Alcohol Relapse After Early Liver Transplantation in Patients With Alcoholic Liver Disease: A Meta-Analysis
Yousaf Zafara, g, Ahmed Kamal Siddiqib, Nafhat Shaikhc, Maria Imrand, Syed Sarmad Javaidd, Laila Manzoora, Arsalan Zafar Iqbale, Jan Petrasekf
aDepartment of Medicine, University of Mississippi Medical Center, Jackson, MS, USA
bDepartment of Medicine, Ziauddin Medical University, Karachi, Pakistan
cDepartment of Medicine, Liaquat University of Medical & Health Sciences, Jamshoro, Pakistan
dDepartment of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan
eFMH Lahore Medical College, Lahore, Pakistan
fDepartment of Digestive Diseases, University of Mississippi Medical Center, Jackson, MS, USA
gCorresponding Author: Yousaf Zafar, Department of Medicine, University of Mississippi Medical Center, Jackson, MS, USA
Manuscript submitted September 12, 2023, accepted January 19, 2024, published online February 28, 2024
Short title: Alcohol Relapse After LT
doi: https://doi.org/10.14740/gr1674
| Abstract | ▴Top |
Background: Alcohol use disorder (AUD) is a significant source of end-stage liver disease and liver failure and an indication for liver transplant (LT). Historically, LT for alcoholic liver disease (ALD) required 6 months of alcohol abstinence. Recently, it has been demonstrated that early LT (< 6 months of abstinence) in strictly selected group of patients provides survival benefit while keeping the relapse to harmful drinking at acceptable levels. This practice has been reflected in the Dallas consensus, but more data are needed to appropriately risk stratify the patient from the perspective of return to harmful alcohol drinking post-transplant. This “6-month rule” has been highly debated and recent data demonstrated that the duration of pre-transplant sobriety is not related with an increased risk of relapse to alcohol post-transplant. We performed a meta-analysis to compare the rate of alcohol relapse in individuals having standard vs. early LT.
Methods: MEDLINE and SCOPUS were searched for randomized controlled trials (RCTs), observational studies, and case-control studies from their inception through June 2022. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMSA) 2009 checklist guidelines were followed for this meta-analysis. Studies comparing post-transplant outcomes, such as alcohol relapse, in individuals following standard vs. early LT, were included. Reviews, case studies, conference abstracts, clinical trials with only an abstract, and studies with inadequate data for extraction were all disqualified. The data were retrieved, gathered, and examined. The random effects model was used to generate forest plots. For the analysis, a P-value of 0.05 was considered significant.
Results: Thirty-four studies were discovered in the initial search. Three studies were included in this systematic review and meta-analysis incorporating 367 patients. Mean age was 51.7 years. Out of 367 patients, 173 (47%) underwent early LT. Out of three studies included, one study demonstrated decreased probability of alcohol relapse in patients undergoing early LT, whereas the other two showed the opposite result. All of the included studies were analyzed and had minimal risk of bias. Pooled analysis demonstrates that the difference in alcohol relapse between early vs. standard LT was insignificant (odds ratio: 1.24, 95% confidence interval: 0.75 - 2.06, P = 0.40).
Conclusion: Our results show that early LT is not associated with increased risk of alcohol relapse post-transplant when compared with a mandatory 6-month abstinence period. Hence, individuals with ALD should not be categorically rejected from LT merely on the criteria of 6 months of abstinence. Other selection criteria based on the need and post-transplant outcomes should be utilized.
Keywords: Alcohol use disorder; Post-transplant survival rates; 6-month abstinence rule; Selection criterion; Post-transplant outcomes
| Introduction | ▴Top |
The most prevalent chronic liver disease worldwide is alcoholic liver disease (ALD) [1]. About half of cirrhosis deaths worldwide are ascribed to alcohol consumption [2]. The United States have the second largest yearly per capita alcohol consumption after Europe [3].
ALD is one of the most widespread reasons for a liver transplant (LT) [4]. Studies suggest that ALD patients undergoing LT have survival rates similar to those receiving transplants for other indications [5]. However, alcohol relapse negatively impacts post-transplant survival rates due alcohol-induced damage in the allograft, exacerbation of pre-existing extrahepatic manifestations of ALD and, in some transplant recipients, lack of post-operative medical compliance [4, 5].
Until recently a 6-month period of abstinence was required by many transplant programs. This mandatory period of sobriety was thought to allow time for the recovery of liver function and to preclude the need for unnecessary LT. In addition, it was thought to reduce the probability of post-operative alcohol relapse [6]. However, this so-called “6-month rule” has been highly debated and recent data demonstrated that the duration of pre-transplant abstinence is unrelated to the likelihood of post-transplant alcohol relapse. Consequently, early LT for alcohol-associated hepatitis is now increasingly being accepted by many liver centers. A seminal Franco-Belgian study demonstrated dramatically improved survival rates by early LT in patients with alcoholic hepatitis (AH) who did not respond to treatment with steroids [7]. The results of this study were reproduced by the US-based ACCELERATE-AH consortium [8].
Previous studies have shown inconsistent results regarding alcohol relapse in standard LT as compared to early LT. A clinical trial, which assessed the effects of early LT in patients with ALD, demonstrated that only three out of 26 patients who underwent early LT had alcohol relapse, with no relapse during first 6 months after the transplants [7]. Due to this ambiguity, we aimed to pool data from all the relevant studies and conduct a meta-analysis to assess the alcohol relapse rate in patients undergoing standard LT versus early LT.
| Materials and Methods | ▴Top |
Literature search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMSA) 2009 checklist guidelines were used for this meta-analysis [9]. Because the data were freely available, no ethical review board approval was necessary for this research. From inception through September 2022, a complete search was conducted utilizing electronic databases such as MEDLINE and SCOPUS, with no time or language limitations. A thorough search technique is shown in Supplementary Table 1 (www.gastrores.org). The bibliographies of recognized papers, gray/unpublished literature, clinical trial registries, and reviews on the issue were also included in the literature search.
Study selection
Included studies satisfied the following criteria for eligibility: 1) randomized controlled trials (RCTs) or any observational study including cohort, cross-sectional or case-control; 2) adult patients aged 18 years or above; 3) patients having any ALD who underwent LT; 4) reporting alcohol relapse or alcohol recurrence as primary outcome; and 5) studies assessing alcohol relapse in early versus standard LT. The criteria for exclusion included the following: 1) reviews, case reports, conference abstracts and clinical trials with only abstract; and 2) studies with insufficient data for extraction.
Data extraction and quality assessment
EndNote X9 (Thomson Reuters, Toronto, Ontario, Canada) was used to import all of the selected studies, and duplicates were detected and eliminated. The remaining studies were reviewed by AKS and NS based on their title and abstract. The complete text was thoroughly evaluated against the inclusion and exclusion criteria for the final study selection. AMR was engaged as a third reviewer to evaluate and resolve any differences. The first investigator, AKS, extracted the data, which were then double-checked for correctness by the second investigator, NS. The data gathered comprised baseline characteristics, with alcohol relapse serving as the primary outcome.
Risk of bias
The Cochrane risk of bias method for RCTs was used to assess the study’s quality [10]. We used the Newcastle-Ottawa quality assessment to assess the risk of bias in two retrospective cohort studies. The risk of bias in the clinical trial included was assessed with the Jadad scale, sometimes referred to as the Oxford quality scoring system.
Statistical analysis
All statistical analysis was performed using RevMan software (Review Manager Version 5.3.5, The Nordic Cochrane Centre, Copenhagen). For each trial, the rate of alcohol relapse following early and standard LT was assessed. The results were collected and presented as odd ratios (ORs) with 95% confidence intervals (CIs), and the random effects model was used to combine them. A forest plot was created to visually analyze the outcomes (Fig. 1). Table 1 illustrates all of the data utilized in the meta-analysis. The Cochrane Q statistic was used to quantify heterogeneity; P of 0.1 indicates considerable heterogeneity. The Higgins I2 test was used to measure the heterogeneity among the studies, with a score of 25% indicating low risk, 25-75% indicating moderate risk, and > 75% indicating high risk. P-values were all two-sided, and a P-value of 0.05 or lower was always regarded as significant.
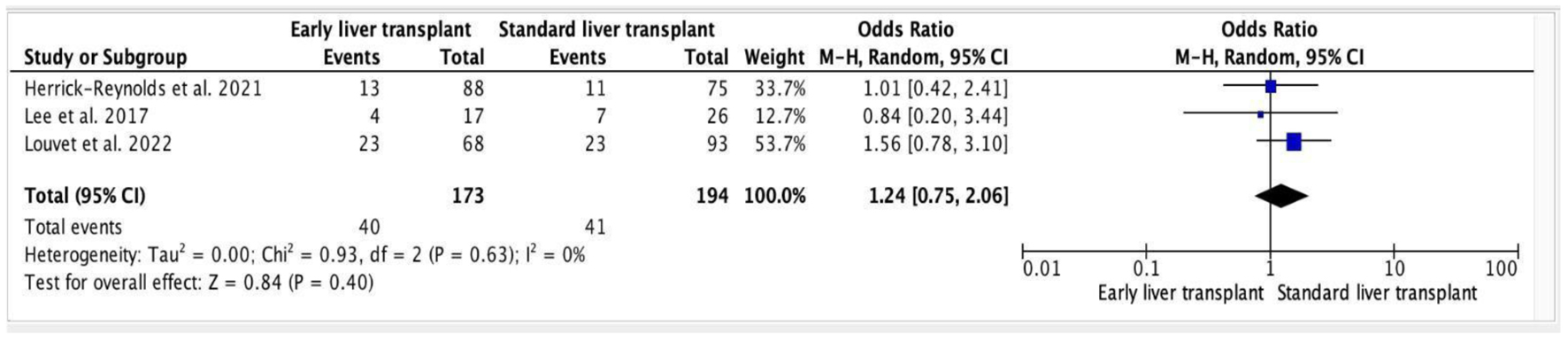 Click for large image | Figure 1. Forest plot of alcohol relapse post-transplant (early vs. standard). |
 Click to view | Table 1. Baseline Characteristics of the Included Studies |
| Results | ▴Top |
Study selection
An extensive literature search of articles was conducted by two independent reviewers. Thirty-four studies were discovered in the initial search. Twenty-nine articles were excluded after the screening based on abstract and title. Out of the five remaining articles, two did not fit the criteria for inclusion. As a result, the remaining three studies were included in this meta-analysis. The findings of our literature search are described in the PRISMA flow chart (Fig. 2).
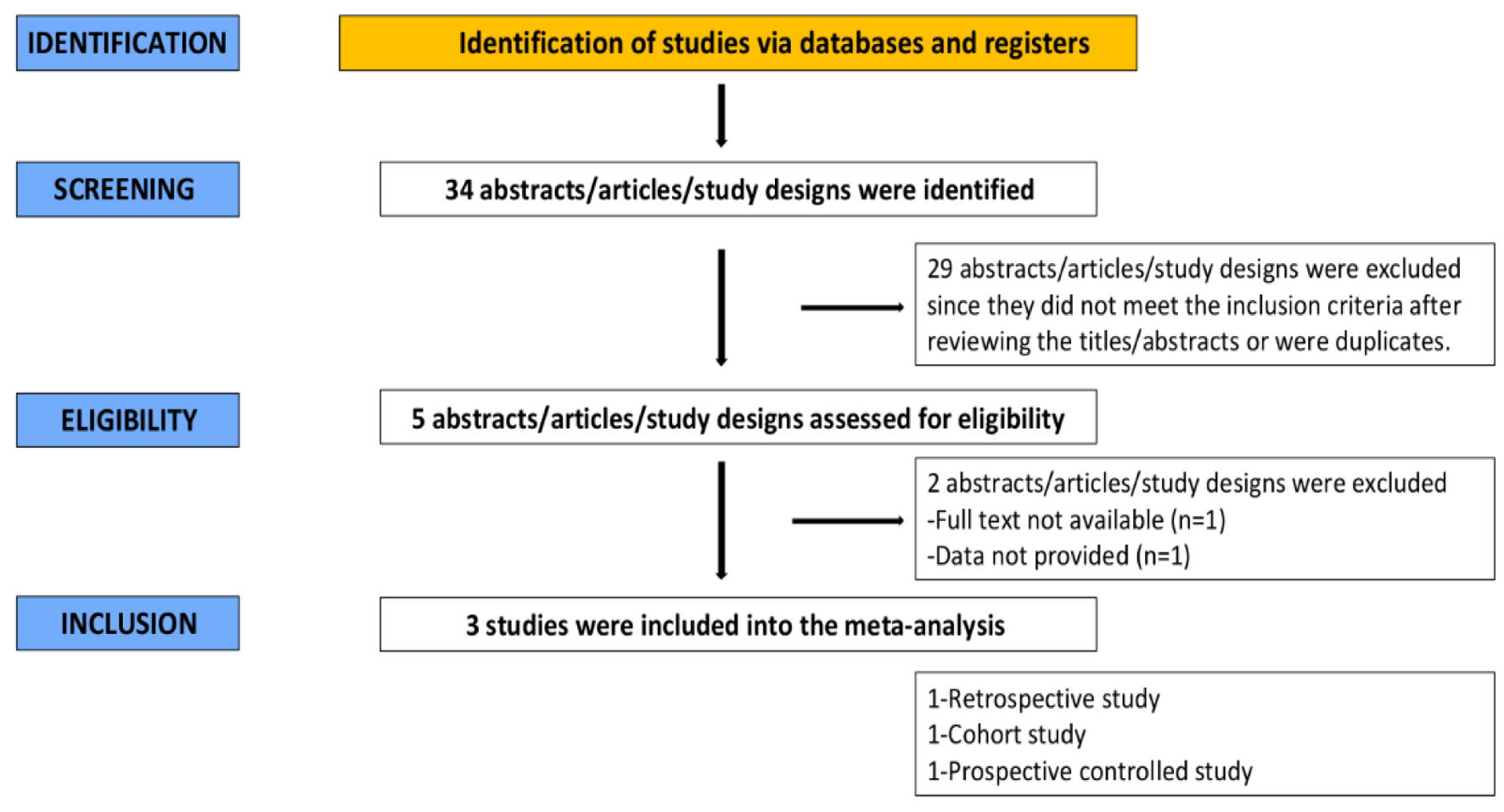 Click for large image | Figure 2. PRISMA flow chart. |
Table 1 shows details of the included trials. The total number of participants was 367 with a mean age of 51.7 years. Out of 367 patients, 173 (47%) underwent early LT. Among the included trials, two trials included participants with severe alcoholic hepatitis (SAH) [11, 12]. One trial included participants with any ALD [13].
Alcohol relapse in early versus standard LT
Out of three studies included, one study demonstrated decreased probability of alcohol relapse in patients going through an early LT [12], whereas the other two showed the opposite result [11, 13]. However, when the results of all three studies were taken together, they were statistically non-significant. Meta-analysis of the studies depicted that early LT was statistically insignificant when associated with increased alcohol relapse rates (OR: 1.24, 95% CI: 0.75 - 2.06, P = 0.40). In addition, the heterogeneity among the trials was low (heterogeneity: tau2 = 0.00; chi2 = 0.93, df = 2 (P = 0.63); I2 = 0%).
Publication bias
All of the included studies had a low risk of bias. Using the Newcastle-Ottawa quality assessment, the cohort conducted by Herrick-Reynolds et al [13] scored eight stars in total: four, one, and three in the selection, comparability, and outcome domains, respectively with a good quality score. The retrospective study by Lee et al [12] was also classified as good quality using the same assessment. Accordingly, in compliance with the Jadad scale, sometimes referred to as the Oxford quality scoring system, the clinical trial carried out by Louvet et al [11] was awarded 4 points. A thorough assessment of each study is given in Supplementary Tables 2 and 3 (www.gastrores.org).
| Discussion | ▴Top |
This meta-analysis, comprised of three studies with a total of 367 participants, does not demonstrate any association between early LT and increased post-transplant alcohol relapse rates, and was consistent with previously published studies reporting that the period of alcohol abstinence prior to transplant is not predictive of the probability of post-transplant relapse.
Prior studies on this subject have demonstrated inconsistent results. A meta-analysis by Chuncharunee et al highlighted alcohol abstinence of less than 6 months to be one of the strong predictors of post-transplant alcohol relapse. However, this study was limited by publication bias due to absence of negative studies on abstinence less than 6 months [4]. Other studies represented no significant association between alcohol relapse and early LT [11-13]. To the best of our knowing, this meta-analysis is the first to exclusively compare alcohol relapse incidence in early LT with standard LT and has no heterogeneity.
All three observational studies included in our analysis strictly included participants with no prior transplant history to generate a homogenous comparison sample between early LT and standard LT. Every study had alcohol relapse as one of their primary outcomes; however, two studies assessed relapse-free survival rates while the third took 2-year survival rates as the outcome. Two of our studies included patients with ALD whereas study by Louvet et al studied patients having SAH who were unresponsive to medical treatment [11-13]. The pooled analysis of the aforementioned studies deduced that there was no significant relationship between incidence of alcohol relapse and early LT and therefore suggests that the 6-month abstinence rule is not beneficial in reducing post-transplant relapse. This may be due to the fact that studies have also shown evidence of alcohol relapse being strongly linked to psychiatric co-morbidities and psychosocial variables such as poor family support and family history of alcohol abuse [4, 14]. In addition, a study has also shown significant association of alcohol relapse with younger age [13].
Certain limitations should be considered while analyzing the findings of our meta-analysis. Firstly, we have only included studies published in peer-reviewed journals and we did not include conference abstracts. Second, there is no general definition of alcohol relapse, therefore it differed among the studies. Third, post-transplant alcohol relapse assessment was neither reliable nor uniform across the studies. This was more often done by interviewing patients on follow-up visits than laboratory screening. Lastly, the studies pooled in our analysis vary in design, cohorts and follow-up duration.
Our meta-analysis does not support the validity of 6-month abstinence rule as the primary selection criteria for LT. This is especially important for patients with SAH, a condition in which 75-90% deaths occur with 2 months [8]. SAH, if refractory to corticosteroids, has 6-month mortality rate as high as 70% [15]. Therefore, our study highlights the need to identify all the factors that may affect post-transplant outcomes and use them to carefully devise a thorough selection process for LT. These factors may include age, family history of alcoholism, quantity of pre-transplant alcohol use, social support, psychiatric co-morbidities, and history of smoking. This suggests that proper screening, psychiatric profile, and social variables should be included in pre-transplant and management intervals. Moreover, our analysis also draws attention to devise more reliable tools for alcohol use assessment and their widespread usage for efficient documentation.
Conclusion
Our meta-analysis demonstrated that the period of abstinence from alcohol before transplant does not indicate the likelihood of post-transplant relapse. As a result, our findings do not support the 6-month sobriety rule’s efficacy in choosing individuals with AH for LT. More research is needed to establish relapse patterns and the individual effects of social variables on alcohol relapse and post-transplant outcomes.
Learning points
A primary source of end-stage liver disease, liver failure, and a reason for LT is AUD. To compare the rate of alcohol relapse in patients receiving regular LT vs. early LT, we performed a meta-analysis. This systematic review and meta-analysis, which covered 367 patients, was comprised of three studies. Comparing early LT to a required 6-month abstinence interval, our findings show that there is no increased risk of alcohol relapse after transplantation. Consequently, those with alcoholic liver illness should not be absolutely denied from LT just because they do not meet the requirement of 6 months sobriety.
| Supplementary Material | ▴Top |
Suppl 1. Search Strategy.
Suppl 2. Quality Assessment Criteria Through Newcastle-Ottawa Scale (NOS).
Suppl 3. Quality Assessment Criteria Through Jaded Scale.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Yousaf Zafar and Laila Manzoor: acquisition of data, data analysis and interpretation, drafting the article, final approval; Ahmed Kamal Siddiqi and Nafhat Shaikh: data collection, analysis, and interpretation, drafting the article, final approval; Maria Imran and Syed Sarmad Javaid: data interpretation, manuscript revision, and final approval; Arsalan Zafar Iqbal: data interpretation, paper revision, final approval; Jan Petrasek: study idea and design, critical review, and final approval.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16.
doi pubmed - Stein E, Cruz-Lemini M, Altamirano J, Ndugga N, Couper D, Abraldes JG, Bataller R. Heavy daily alcohol intake at the population level predicts the weight of alcohol in cirrhosis burden worldwide. J Hepatol. 2016;65(5):998-1005.
doi pubmed - Higuera-de-la-Tijera F, Lira-Vera JE, Morales-Gutierrez O, Martinez-Castillo M, Medina-Avila Z, Servin-Caamano A, Perez-Hernandez JL, et al. Alcoholic liver disease. Clin Liver Dis (Hoboken). 2022;19(2):63-67.
doi pubmed pmc - Chuncharunee L, Yamashiki N, Thakkinstian A, Sobhonslidsuk A. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):150.
doi pubmed pmc - Neuberger J, Tang H. Relapse after transplantation: European studies. Liver Transpl Surg. 1997;3(3):275-279.
doi pubmed - Stickel F, Datz C, Hampe J, Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11(2):173-188.
doi pubmed pmc - Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365(19):1790-1800.
doi pubmed - Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, Im GY, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology. 2018;155(2):422-430.e421.
doi pubmed pmc - Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784.
doi pubmed - Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
doi pubmed pmc - Louvet A, Labreuche J, Moreno C, Vanlemmens C, Moirand R, Feray C, Dumortier J, et al. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol Hepatol. 2022;7(5):416-425.
doi pubmed - Lee BP, Chen PH, Haugen C, Hernaez R, Gurakar A, Philosophe B, Dagher N, et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg. 2017;265(1):20-29.
doi pubmed - Herrick-Reynolds KM, Punchhi G, Greenberg RS, Strauss AT, Boyarsky BJ, Weeks-Groh SR, Krach MR, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034.
doi pubmed pmc - Dew MA, DiMartini AF, Steel J, De Vito Dabbs A, Myaskovsky L, Unruh M, Greenhouse J. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl. 2008;14(2):159-172.
doi pubmed pmc - Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348-1354.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


