| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 17, Number 1, February 2024, pages 32-36
Fulminant Emphysematous Pancreatitis: Diagnosis Time Counts
Basel Darawshaa, Subhi Mansoura, b, Tawfik Fahouma, Naseem Azzama, Yoram Klugera, b, Ahmad Assaliaa, c, Safi Khuria, b, d
aDepartment of General Surgery, Rambam Medical Center, Haifa, Israel
bBilio-Pancreatic Surgery Service, HPB and Surgical Oncology Unit, Rambam Medical Center, Haifa, Israel
cAdvanced Laparoscopic and Bariatric Surgery Unit, Rambam Medical Center, Haifa, Israel
dCorresponding Author: Safi Khuri, Department of General Surgery, Rambam Medical Center, Haifa, Israel
Manuscript submitted September 1, 2023, accepted October 2, 2023, published online February 28, 2024
Short title: Emphysematous Pancreatitis
doi: https://doi.org/10.14740/gr1671
| Abstract | ▴Top |
Emphysematous pancreatitis (EP), a severe form of necrotizing infection of the pancreas, is an extremely rare medical emergency with high rates of mortality. It is characterized by intraparenchymal pancreatic or peri-pancreatic air due to either monomicrobial or polymicrobial infection with gas-forming bacteria or due to entero-pancreatic fistula. EP is classified according to timing from disease onset when air bubble signs were detected on computed tomography (CT) scan, as early onset (within 2 weeks from disease onset) or late (more than 2 weeks from disease onset). While most cases of acute pancreatitis are resolved with supportive care alone, clinical outcomes of EP, especially the early onset subtype, are very poor with high rates of morbidity and mortality. These two case reports present the clinical features, diagnostic investigations, and management of two patients admitted to our hospital with early onset fulminant EP, each investigated and managed with different approaches. The first patient underwent a more conservative treatment, with diagnosis being made 52 h following admission, and thus, intensive care unit (ICU) admission and surgery were postponed, while the second patient was diagnosed a few hours following presentation with earlier ICU admission. In this article, we will present the critical importance of early diagnosis of the aforementioned rare entity of severe pancreatitis and will consider the consequences of rapid diagnosis on disease course, morbidity and mortality.
Keywords: Emphysematous pancreatitis; ICU; Drainage; Surgery; Mortality
| Introduction | ▴Top |
Acute pancreatitis, a common medical emergency caused mostly by alcohol or gallbladder stones, is usually defined as an inflammatory process affecting the pancreas gland with potential for local and systemic complications [1]. According to the revised Atlanta classification [2], acute pancreatitis could be classified either based on disease severity - into mild, moderate, or severe, or by pancreatitis form - into edematous versus necrotizing pancreatitis, with the former being more common at 80% of acute pancreatitis cases. Up to 20% of pancreatitis patients suffer necrosis of either pancreatic parenchyma, peri-pancreatic tissues or both. This entity is referred to as necrotizing pancreatitis. Necrotizing pancreatitis is usually sterile, yet infection, although rare, is known to occur. Infected necrotizing pancreatitis carries high rates of morbidity and mortality, due to sepsis and multi-organ failure [3]. Emphysematous pancreatitis (EP), a form of fatal acute necrotizing pancreatitis, is an extremely rare subtype characterized by the presence of gas within or around the necrotic pancreatic tissues. The source of the air bubbles is either monomicrobial or polymicrobial infection with gas-forming bacteria, or due to an entero-pancreatic fistula. EP is classified according to timing from disease onset when air bubble signs were detected on computed tomography (CT) scan as early onset (within 2 weeks from disease onset) or late (more than 2 weeks from disease onset) [4]. Moreover, EP could be categorized based on intra-pancreatic or peri-pancreatic air bubble distribution into extensive EP (when more than 50% of the pancreatic/peri-pancreatic necrosis is involved and connected into sheets) or common EP (when less than 50% is involved). While most cases of acute pancreatitis are resolved with supportive care alone, clinical outcomes of EP, especially the early onset subtype, are very poor with high rates of morbidity and mortality. Early onset EP may present as a fulminant disease, causing mortality within a few hours/days following admission and diagnosis [5]. Due to the previously mentioned apprehensions, timely diagnosis and initiation of aggressive management, especially for the fulminant type, is crucial for survival. Treatment usually includes intensive care unit (ICU) admission, aggressive fluid therapy, broad-spectrum antibiotics, and drainage, either percutaneous or surgical [5]. Herein, we present the clinical features, diagnostic investigations, and management of two patients admitted to our hospital with early onset fulminant type EP, each investigated and managed with different approaches.
| Case Reports | ▴Top |
Case 1
Investigations
A 78-year-old female patient, with a past medical history of hypertension and hyperlipidemia, presented to our emergency department with complaints of severe epigastric pain of few hours’ duration. The pain was sharp and radiating to the back, with a characteristic belt pattern. The patient also complained of nausea and vomiting. She denied having fever, chills, or rigors. She denied alcohol consumption and cigarette use as well.
Diagnosis
On physical examination upon her admission, her vital signs were within normal limits. An abdominal examination revealed bilateral upper abdominal tenderness with guarding. No abdominal or inguinal masses were palpated. Digital rectal exam was normal. Complete blood count (CBC) showed increased white blood cells of 19,000 cells/µL, with 11% bands. Kidney and liver function tests, as well as serum electrolytes were within normal limits. blood amylase level was significantly elevated at 3,400 IU/L, the urine amylase level was likewise elevated at 4,981 IU/L.
An upright chest and abdominal X-ray showed no evidence of free air in the peritoneum. Abdominal ultrasonography (US) indicated a normal gallbladder without any stones but an enlarged edematous pancreas with fluid accumulation and fat stranding, indicative for acute pancreatitis. She was admitted to the general surgery ward with clinical, laboratory and sonographic diagnosis of acute pancreatitis.
Treatment
The patient was admitted to the general surgery ward for further evaluation and supportive management with nil per os (NPO), intravenous (IV) fluids and painkillers. She received 3 L of IV 1/2 standard fluids during the first 24 h following admission. Further investigations to detect the etiology of acute pancreatitis such as triglyceride levels, calcium levels and immunoglobulin (Ig) G4 antibodies were undertaken, with the results within normal limits.
Thirty-six hours following admission, the patient complained of severe pain. Although she was hemodynamically stable, she suffered from anuria, thus; a Foley catheter was inserted to monitor hourly urine output along with intensive fluid resuscitation.
Repeat blood tests revealed worsening blood amylase level at 3,700 IU/L and a considerable increase in C-reactive protein (CRP) levels (from 0.23 at admission to 26.7 mg/dL). Arterial blood gas analysis revealed metabolic acidosis with pH levels of 7.18 and lactate levels of 8 mmol/L. Several hours later, she developed signs of acute respiratory distress syndrome (ARDS) with severe hypoxia and bilateral wheezing. Chest X-ray was indicative for ARDS. Repeated blood tests showed severe lactic acidosis and marked leukocytosis. Despite supportive treatment with IV hydrocortisone and IV diuretics, the patient eventually required intubation and mechanical ventilation due to respiratory failure. Consequently, she was transferred (46 h following admission) to the ICU for continuous monitoring and advanced management. Blood cultures taken on the day of admission revealed the presence of gram-negative bacteria, indicating infection; in consequence, an antibiotic treatment of piperacillin/tazobactam was initiated following the results. The patient’s condition continued to deteriorate with hemodynamic signs of deep septic shock and multiorgan failure, prompting the initiation of IV noradrenaline and vasopressin. However, there was no significant improvement, and the antibiotic treatment was changed to meropenem and amikacin pending full blood culture results. An abdomino-pelvic CT scan (52 h following admission) showed an enlarged and edematous pancreas with hypodense regions and free air throughout its length (Fig. 1). A multilocular pancreatic collection involving the lesser sac was observed, with no discriminating plane from the stomach containing air, suggesting a possible direct involvement of the duodenum and stomach. Severe peripancreatic fat stranding was also observed, which was consistent with acute EP.
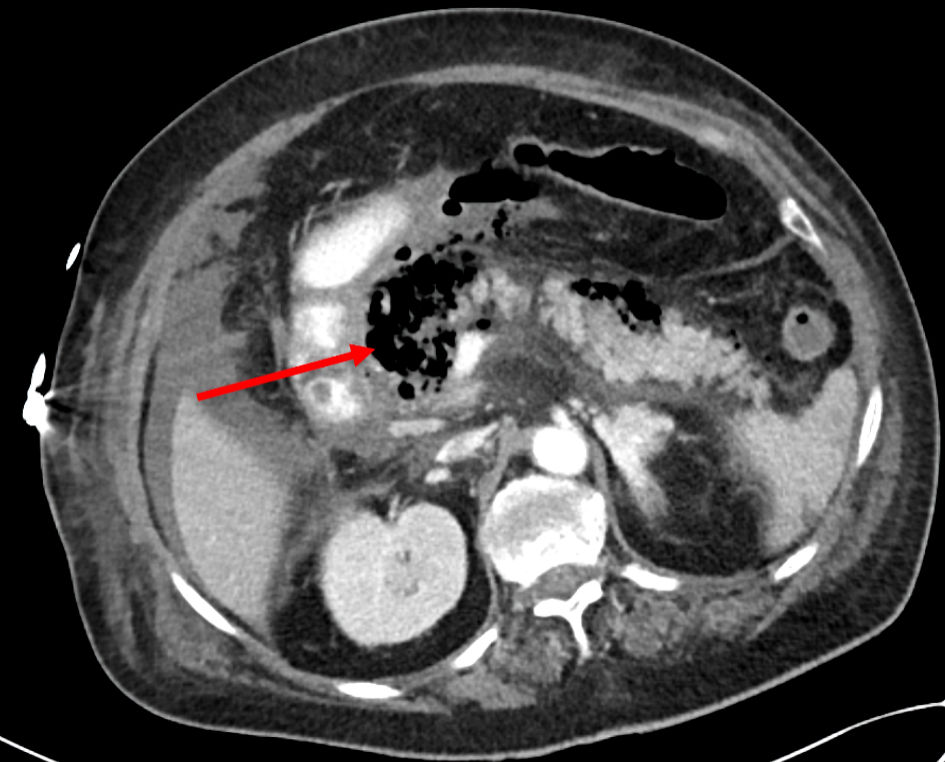 Click for large image | Figure 1. An axial abdomino-pelvic CT scan showed multilocular pancreatic collection involving the lesser sac containing air (arrow), consistent with acute emphysematous pancreatitis. CT: computed tomography. |
The CT findings were discussed, and several treatment options were considered, including radiologically guided percutaneous drainage of the pancreatic collection, Endoscopic ultrasound (EUS)-guided drainage, or surgical drainage. Initially, a maximal supportive non-surgical treatment was adopted. Several hours later, the patient’s condition continued to deteriorate, necessitating an additional vasopressor medication. Considering the severity of the patient’s condition and the lack of improvement with conservative non-operative measures, it was decided to proceed with surgical intervention for optimal drainage and debridement.
The patient underwent an exploratory laparotomy (72 h following admission), during which necrotic and hemorrhagic content was found in the lesser sac with a large amount of turbid fluid in the abdominal cavity. The necrotic fluid was meticulously suctioned, and intensive irrigation was performed in the lesser sac and the abdominal cavity. The first and second duodenal parts were intact without perforation. Methylene blue test by nasogastric (NG) tube was negative for duodenal leak. To facilitate drainage and manage the pancreatic collection, two Jackson-Pratt drains were placed (one in the lesser sac and the other in the Morrison pouch).
Follow-up and outcomes
Unfortunately, despite aggressive supportive medical management and surgical intervention, the patient’s condition did not improve. Eighty-two hours following admission, the patient had a loss of vital signs, and her death was announced.
Case 2
Investigations
A 72-year-old female with a medical history of chronic obstructive pulmonary disease (COPD) and aortic stenosis presented to the emergency department with complaints of epigastric pain, nausea and vomiting of 3 h duration. Five years earlier, she had been admitted to another hospital due to severe pancreatitis, which necessitated ICU admission for a period of 2 weeks. Despite a thorough investigative process involving blood tests for triglycerides, calcium and IgG4 levels, EUS, abdominal magnetic resonance imaging (MRI), CT scan, and US, the etiology of pancreatitis remained unidentified. She was assigned the title of idiopathic pancreatitis.
Diagnosis
Upon her admission, the patient’s vital signs were within normal limits. Physical examination revealed epigastric tenderness with no signs indicative of peritonitis. No abdominal or inguinal masses were palpated. Digital rectal exam was normal. Laboratory results showed no leukocytosis, blood amylase was elevated at 1,873 IU/L, aspartate aminotransferase (AST) level was measured at 76 IU/L, CRP at 0.42 mg/dL, and lactate at 2.2 mmol/L. Kidney function tests and serum electrolytes were within normal limits.
An abdomino-pelvic CT (Fig. 2) scan revealed an enlarged pancreas with hypodense areas, indicative of potential necrosis, along with fat stranding and retroperitoneal fluid accumulation. Heterogeneous enhancement of the pancreas and irregular contours were noted, accompanied by substantial gas within and around the necrotic pancreatic tissues. The CT-severity index score was 9, indicative of severe acute pancreatitis. The previous findings were supportive of the diagnosis of EP.
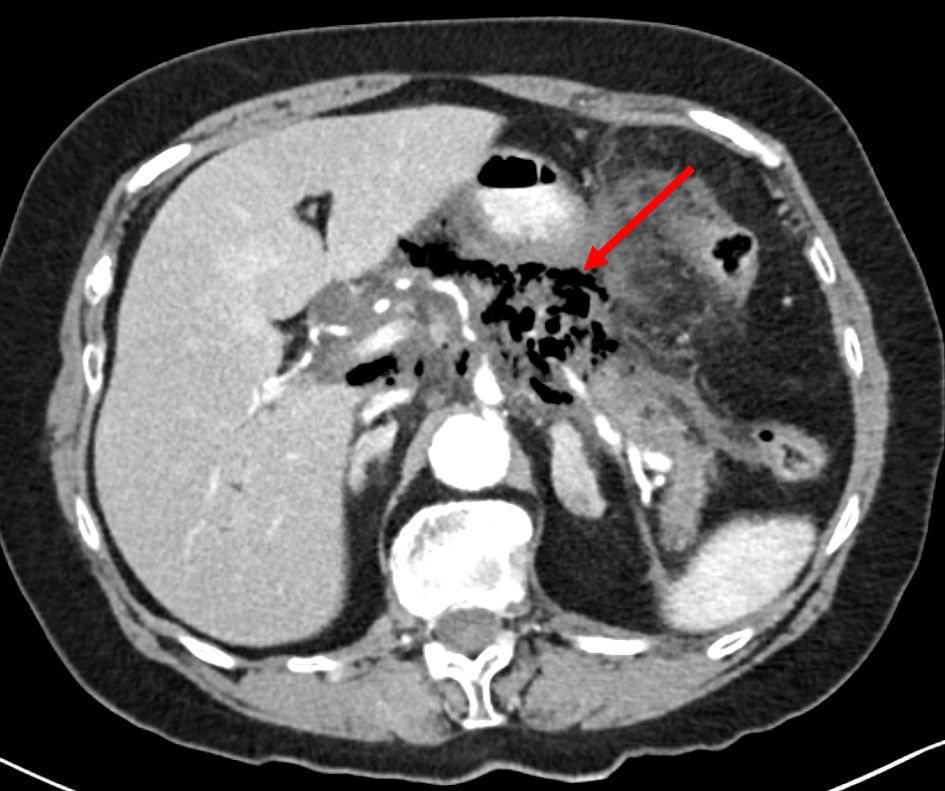 Click for large image | Figure 2. An axial abdominal CT scan demonstrates heterogeneous enhancement of the pancreas and irregular contours, accompanied by substantial gas within and around the necrotic pancreatic tissues (arrow). These findings were supportive of the diagnosis of emphysematous pancreatitis. CT: computed tomography. |
Due to the rapidity and severity of her presentation, the patient was promptly admitted to the ICU.
Further workup in the ICU
Upon transfer to the ICU, the patient’s condition was closely monitored. She remained alert, maintaining blood oxygen saturation of 93% with the assistance of an oxygen mask. Arterial blood gas analysis revealed metabolic acidosis with PCO2 level of 33 mm Hg. Lactate levels surged, peaking at 6 mmol/L in recurrent arterial blood gas tests. Despite these challenges, urine output remained satisfactory. She received 3 L of IV 1/2 standard fluids during the first 24 h following admission. Abdominal examination revealed severe abdominal tenderness, while bilirubin levels were measured at 1.2 mg/dL (direct) and 0.6 mg/dL (indirect).
During her hospitalization, the patient exhibited a fever of 38 °C, leukocytosis (12,660 cells/µL), and a considerable increase in CRP levels (from 0.74 at admission to 25 mg/dL). The presence of gram-negative bacteria in the blood warranted immediate intervention. Consequently, treatment with meropenem, a broad-spectrum antibiotic, was initiated to address the infectious component.
On the second day at the ICU, a new onset atrial fibrillation (AF) was diagnosed, characterized by a heart rate ranging from 130 to 160 beats per minute (bpm), while maintaining normal blood pressure. The patient was treated with IV amiodarone for rhythm control and IV esmolol for rate control. Subcutaneous (SC) heparin was also administered due to high CHADS-VASC score. However, a decrease in blood pressure to 90/60 mm Hg during fibrillation was documented with a heart rate of 160 bpm. It was suspected that abdominal pathology is the trigger for the new onset of atrial fibrillation. A multi-disciplinary meeting including ICU physicians, acute care surgery specialists and pancreas surgeons was held bedside, in which it was decided to proceed with electrical cardioversion initially, in case the patient continues to be hemodynamically unstable following cardioversion, surgical debridement will follow, especially as the patient exhibited a bloated abdomen and rigidity upon abdominal examination with decreased tolerance for parental feeding. To address this, interventions included withholding oral intake (NPO), insertion of a NG tube for decompression, administration of IV fluids, total parenteral nutrition (TPN) feeding, and supplementation with thiamine (B1) were undertaken. Fortunately, following cardioversion, the patient regained hemodynamic stability.
Follow-up and outcomes
Significant clinical improvement was observed over the course of 8 days in the ICU. A repeated abdomino-pelvic CT scan showed lower amount of intra-parenchymal and peripancreatic free air compared to the previous CT scan.
The patient’s sustained progress and successful enteral feeding tolerance, coupled with normal blood values, culminated in her discharge on day 19 following admission.
| Discussion | ▴Top |
EP, known also as gangrenous pancreatitis, is an uncommon fatal complication of necrotizing pancreatitis [6]. According to the classification system based on timing by Li et al [4], early onset EP is defined as EP diagnosed within 2 weeks of disease onset. The time period for this classification is highly challenging, as some patients, including ours, have intra/peri-pancreatic air bubbles as a sign of EP on the initial presentation. Most of these patients suffer from a fulminant course of the disease with very high mortality rates [5, 7, 8]. Thus, due to the above, fulminant EP is a more appropriate title for this subtype of early onset EP and should be regarded as a stand-alone disease and not a complication of acute necrotizing pancreatitis. Reviewing the current English literature revealed a scarce amount of case reports of fulminant EP.
Both case reports emphasize the challenges in managing EP. Early recognition, accurate diagnosis and a tailored approach to management are crucial in addressing this complex and lethal condition. These cases underscore the importance of a multidisciplinary approach. Furthermore, they emphasize the need for a prompt response and intensive monitoring for patients with fulminant EP.
Most patients suffering from fulminant type EP present with severe upper abdominal pain accompanied by nausea and vomiting [5, 8]. In our cases, both patients presented with severe upper abdominal pain as the main complaint.
Abdomino-pelvic CT scan is the radiological modality of choice for the diagnosis of EP, with high specificity and sensitivity for detecting typical findings, mainly intra-parenchymal and pancreatic or peri-pancreatic free gas [9]. Both patients in our case reports were diagnosed with a CT scan demonstrating typical signs of severe pancreatitis along with free gas.
The most common pathogens causing EP are gram-negative bacteria, with Escherichia coli being the most frequently isolated [6]. Occasionally, other pathogens, such as Klebsiella, Enterobacter, Pseudomonas or polymicrobial infection are encountered. Blood cultures were positive for Escherichia coli in both our cases. The exact pathogenesis for EP and bacterial translocation into the pancreatic tissues is unknown. Several hypotheses have been mentioned including either lymphatic or hematogenous dissemination, biliopancreatic reflux, or direct translocation from an entero-pancreatic fistula [6, 10].
Similar to the treatment of severe pancreatitis, treatment of EP usually necessitates a multidisciplinary team approach, including ICU physicians, gastroenterologists, acute care surgeons, invasive radiologists and pancreas surgeons. Treatment of EP defers from patient to patient and depends mainly on hemodynamic status. Treatment options include either conservative non-operative management or surgical therapy. Non-operative management includes fluid resuscitation, analgesics, broad-spectrum antibiotics with or without endoscopic or percutaneous debridement [11]. As fulminant type EP carries high rates of mortality, early diagnosis is crucial for early aggressive conservative therapy and antibiotic treatment initiation. Patients who deteriorate in spite of aggressive non-surgical therapy should be considered for early surgical intervention in the form of necrosectomy/debridement. As have been mentioned before, diagnosis of EP in our first case was delayed, and thus, ICU admission, aggressive treatment as well as surgical intervention were all delayed and the patient passed away 82 h following admission. In contrast, the second patient was diagnosed early, with favorable consequences due to early ICU admission and broad-spectrum antibiotics therapy.
Although mortality rates of 10-36% for EP have been mentioned in the literature, based on single case reports and case series, the exact mortality rate is yet to be known as no systematic meta-analysis has been performed and the exact definition of such a rare entity is yet to be known as well [6, 12].
Table 1 depicts the differences in the workup timeline for the two patients. This table emphasized how critical the time factor for EP diagnosis is: early diagnosis may save lives.
 Click to view | Table 1. The Differences in the Workup Timeline for the Two Patients |
In conclusion, these case reports highlight the divergent outcomes that can arise in cases of EP. The lessons drawn from these cases contribute to a better understanding of the disease and reinforce the importance of early, comprehensive, and coordinated management strategies for improved patient outcomes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
BD, TF and SK drafted the manuscript. SM, YK, AA and SK reviewed the manuscript and consistently improved its content. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
EP: emphysematous pancreatitis; ICU: intensive care unit; CT: computed tomography
| References | ▴Top |
- Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75(12):1073-1079.
doi pubmed - Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-111.
doi pubmed - Ashley SW, Perez A, Pierce EA, Brooks DC, Moore FD, Jr., Whang EE, Banks PA, et al. Necrotizing pancreatitis: contemporary analysis of 99 consecutive cases. Ann Surg. 2001;234(4):572-579; discussion 579-580.
doi pubmed pmc - Li J, Zhu S, Cao X, Lin C, Ning C, Huang G. Classification of emphysematous pancreatitis and its relation to prognosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(11):1348-1354.
doi pubmed - Xi Terence LY, Jia GY, Madhavan K. Fulminant emphysematous pancreatitis. Clin Gastroenterol Hepatol. 2019;17(3):A32.
doi pubmed - Wig JD, Kochhar R, Bharathy KG, Kudari AK, Doley RP, Yadav TD, Kalra N. Emphysematous pancreatitis. Radiological curiosity or a cause for concern? JOP. 2008;9(2):160-166.
pubmed - Porter NA, Lapsia SK. Emphysematous pancreatitis: a severe complication of acute pancreatitis. QJM. 2011;104(10):897.
doi pubmed - Komatsu H, Yoshida H, Hayashi H, Sakata N, Morikawa T, Onogawa T, Motoi F, et al. Fulminant type of emphysematous pancreatitis has risk of massive hemorrhage. Clin J Gastroenterol. 2011;4(4):249-254.
doi pubmed - Bul V, Yazici C, Staudacher JJ, Jung B, Boulay BR. Multiorgan failure predicts mortality in emphysematous pancreatitis: a case report and systematic analysis of the literature. Pancreas. 2017;46(6):825-830.
doi pubmed pmc - Itai Y, Ohtomo K, Kokubo T, Nagai H, Atomi Y, Kuroda A. CT demonstration of gas in dilated pancreatic duct. J Comput Assist Tomogr. 1986;10(6):1052-1053.
doi pubmed - Martinez D, Belmonte MT, Kosny P, Ghitulescu MA, Florencio I, Aparicio J. Emphysematous pancreatitis: a rare complication. Eur J Case Rep Intern Med. 2018;5(11):000955.
doi pubmed pmc - Nadkarni N, D'Cruz S, Kaur R, Sachdev A. Successful outcome with conservative management of emphysematous pancreatitis. Indian J Gastroenterol. 2013;32(4):242-245.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


