| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Short Communication
Volume 16, Number 4, August 2023, pages 240-243
A Significant Effect of Pemafibrate on Hepatic Steatosis and Fibrosis Indexes in Patients With Hypertriglyceridemia
Hisayuki Katsuyamaa, Hidekatsu Yanaia, b, Hiroki Adachia, Mariko Hakoshimaa
aDepartment of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, Chiba, Japan
bCorresponding Author: Hidekatsu Yanai, Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted July 6, 2023, accepted August 16, 2023, published online August 26, 2023
Short title: Pemafibrate and HSI and Fibrosis
doi: https://doi.org/10.14740/gr1656
| Abstract | ▴Top |
Background: We previously reported that the selective peroxisome proliferator-activated receptor alpha modulator, pemafibrate, significantly reduced serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) and significantly increased serum albumin levels at 3, 6 and 12 months after the start of pemafibrate, with an improvement of atherogenic dyslipidemia, in patients with hypertriglyceridemia.
Methods: We performed a post hoc analysis of our previous data obtained from patients with hypertriglyceridemia who had been prescribed pemafibrate continuously for 1 year or longer. We compared the indexes for hepatic steatosis (hepatic steatosis index (HSI)) and fibrosis (nonalcoholic fatty liver disease (NAFLD) fibrosis score (NFS), AST to platelet ratio index (APRI) and FIB-4 index) at baseline with the data at 1 year after the start of pemafibrate.
Results: Pemafibrate significantly reduced HSI at 1 year after the start of pemafibrate. NFS did not show a significant change after 1 year. However, APRI was significantly reduced by pemafibrate after 1 year. FIB-4 index significantly decreased in patients with baseline FIB-4 index ≥ 1.45 at 1 year after the start of pemafibrate. HSI at baseline tended to be negatively correlated with change in HSI after 1 year. There was no significant correlation between NFS at baseline and change in this score after 1 year. APRI and FIB-4 index at baseline were significantly and negatively correlated with changes in APRI and FIB-4 index at 1 year after the start of pemafibrate.
Conclusions: The 1-year pemafibrate treatment improved hepatic steatosis and fibrosis indexes in patients with hypertriglyceridemia.
Keywords: Hepatic steatosis; Hepatic fibrosis; Metabolic dysfunction-associated steatotic liver disease; Pemafibrate; Triglyceride
| Introduction | ▴Top |
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are characterized by pathological accumulation of triglyceride (TG) and other lipids in hepatocytes [1]. Since the principal limitations of the terms NAFLD and NASH are the reliance on exclusionary confounder terms and the use of potentially stigmatizing language, the names chosen to replace NAFLD and NASH were metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH), respectively [2]. MASLD can progress from hepatic steatosis to steatohepatitis, and eventually end-stage liver diseases. MASLD is closely associated with systemic metabolic disorders such as insulin resistance and atherogenic dyslipidemia. When lesions consist of fatty and hydropic degeneration, inflammation, and eventually fibrosis, the condition is designated MASH [3]. Increased abdominal adipose tissue lipolysis, excessive fatty acid (FA) uptake by the liver, and a disturbed clearance of TG-rich lipoproteins, which are induced by insulin resistance, contribute to the development of MASH [3]. Both the inflammatory and hepatocellular degenerative components of MASH are attributed to oxidative stress, which induces lipid peroxidation in cell membranes, liver fibrosis, chronic inflammation, and apoptosis. Peroxisome proliferator-activated receptor alpha (PPARα) agonists reduce atherogenic lipoproteins such as TG-rich lipoproteins and have an anti-inflammatory effect and the property to reduce oxidative stress [4].
We previously reported that the selective PPARα modulator, pemafibrate, significantly reduced serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) and significantly increased serum albumin levels at 3, 6 and 12 months after the start of pemafibrate, with an improvement of atherogenic dyslipidemia [5]. Unexpectedly, we discovered such beneficial effects of pemafibrate on liver function. Therefore, to elucidate the effects of pemafibrate on the indexes for hepatic steatosis and fibrosis, we performed a post hoc analysis of the data at baseline and 1 year after the start of pemafibrate obtained from the previous study [5].
| Materials and Methods | ▴Top |
We performed a post hoc analysis of our previous data obtained from patients with hypertriglyceridemia who had been prescribed pemafibrate continuously for 1 year or longer [5]. We compared the indexes for hepatic steatosis and fibrosis at baseline with the data at 1 year after the start of pemafibrate. Hepatic steatosis was assessed by hepatic steatosis index (HSI) [6]. Hepatic fibrosis was assessed by NAFLD fibrosis score (NFS) [7], AST to platelet ratio index (APRI) [8], and FIB-4 index [9]. Comparisons of the variables before and after the pemafibrate treatment were analyzed by the Wilcoxon signed-rank tests. Pearson’s simple correlation coefficients were performed to determine the correlations between the parameters. P values of < 0.05 and < 0.1 were considered to be statistically significant, and to have tendency, respectively. The study was approved by the Ethics Committee of the National Center for Global Health and Medicine (NCGM-S-004344-00), and the study was performed in accordance with the Declaration of Helsinki.
| Results | ▴Top |
We performed a post hoc analysis of the data obtained from 134 patients including 82 males and 52 females who were included in the previous study [5]. At baseline, the mean ± standard deviation (SD) of age and body mass index (BMI) were 59.9 ± 15.4 and 27.6 ± SD kg/m2. Eighty-nine patients (66%) had diabetes.
Changes in the indexes for hepatic steatosis and fibrosis at 1 year after the start of pemafibrate were shown in Table 1. Pemafibrate significantly reduced HSI at 1 year after the start of pemafibrate. NFS did not show a significant change after 1 year. However, APRI was significantly reduced by pemafibrate after 1 year. The FIB-4 index was reported to be superior to other tested noninvasive markers of fibrosis in Japanese patients with NAFLD, with a high negative predictive value for excluding advanced fibrosis [9]. For a FIB-4 index value < 1.45, fibrosis could be excluded with 98% certainty [9]. Although FIB-4 index did not show a significant change in all patients, FIB-4 index significantly decreased in patients with baseline FIB-4 index ≥ 1.45 at 1 year after the start of pemafibrate.
 Click to view | Table 1. Changes in the Indexes for Hepatic Steatosis and Fibrosis at 1 Year After the Start of Pemafibrate |
Correlations between the baseline values and changes in the indexes for hepatic steatosis and fibrosis during the 1-year pemafibrate treatment were shown in Figure 1. HSI at baseline tended to be negatively correlated with change in HSI after 1 year. There was no significant correlation between NFS at baseline and change in this score after 1 year. APRI and FIB-4 index at baseline were significantly and negatively correlated with changes in APRI and FIB-4 index at 1 year after the start of pemafibrate.
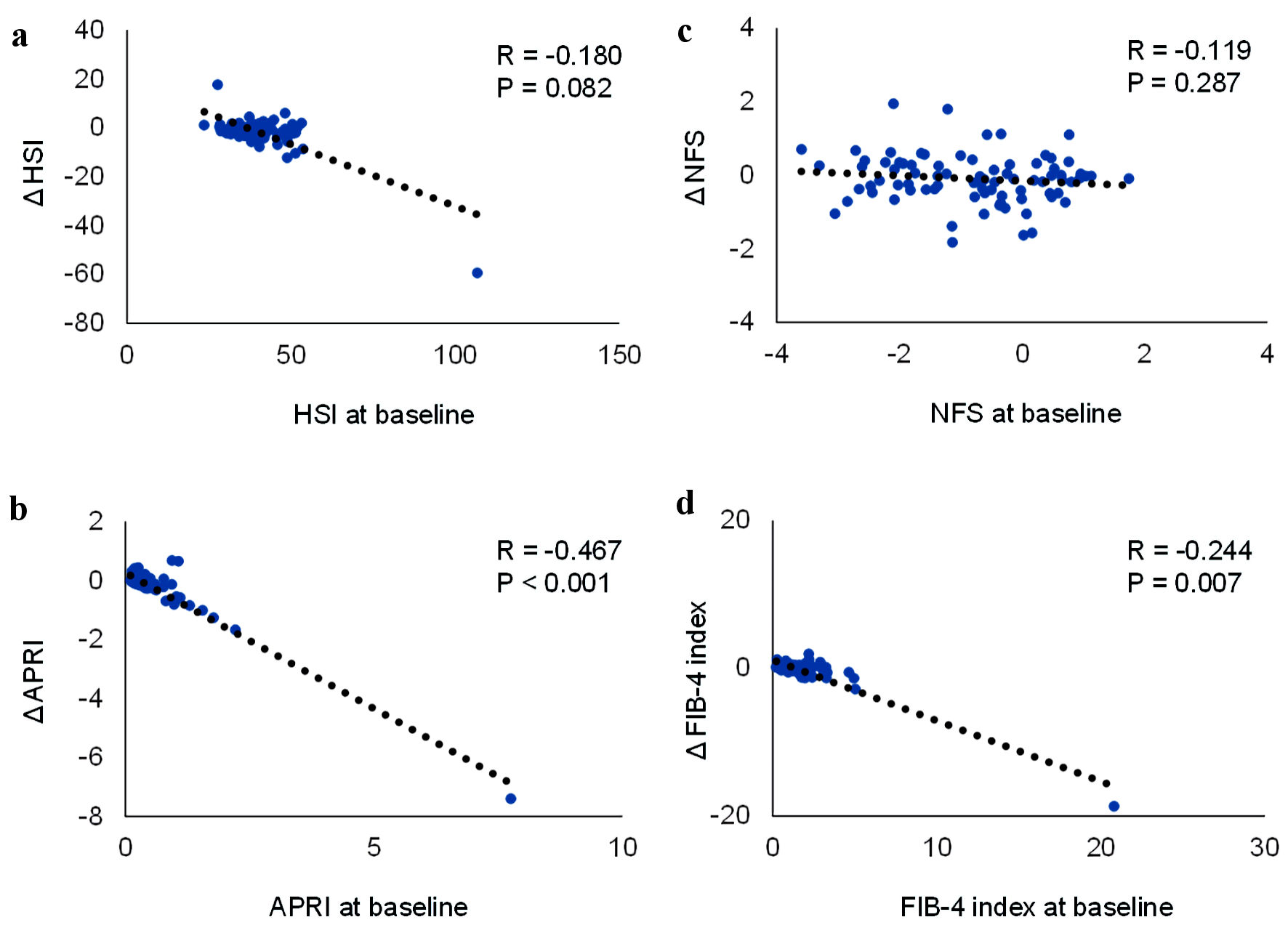 Click for large image | Figure 1. Correlations between the baseline values and the changes of hepatic steatosis index (HSI), nonalcoholic fatty liver disease (NAFLD) fibrosis score (NFS), aspartate aminotransferase (AST) to platelet ratio index (APRI) and FIB-4 index during the 1-year pemafibrate treatment. |
| Discussion | ▴Top |
Our study showed that pemafibrate significantly improved the indexes for hepatic fibrosis which was evaluated by APRI and FIB-4 index. The improvements in APRI and FIB-4 index were significantly correlated with baseline APRI and FIB-4 index values. However, an improvement in NFS by pemafibrate was not obtained. FIB-4 index was calculated using the following formula: (age × AST)/(platelet count × √ALT) [9]. APRI was calculated with the formula: AST/upper limit of normal range of AST/platelet count × 100 [8]. NFS was calculated as -1.675 + 0.037 × age + 0.094 × BMI + 1.13 × diabetes mellitus (DM) (yes = 1, no = 0) + 0.99 × AST/ALT ratio - 0.013 × platelet count - 0.66 × albumin [7]. The changes in FIB-4 index and APRI are determined by changes in serum AST and ALT levels and platelet count. Serum ALT significantly decreased (from 42 ± 45 to 31 ± 31 IU/L; P < 0.001), and AST tended to decrease (from 38 ± 21 to 29 ± 21 IU/L; P = 0.078), and platelet count significantly increased (from 23.8 ± 6.1 to 25.5 ± 6.3 × 104/mL; P < 0.001), which contributed to an improvement in FIB-4 index and APRI. Further, changes in AST (r = -0.954; P < 0.001), ALT (r = -0.723; P < 0.001) and platelet count (r = -0.282; P = 0.001) were significantly and inversely correlated with those at baseline, which can explain a significant correlation of changes in FIB-4 index and APRI with baseline values. Serum albumin (from 4.3 ± 0.4 to 4.4 ± 0.4 g/dL; P < 0.001) and platelet count included in NFS significantly increased, however, BMI (from 27.6 ± 4.6 to 27.6 ± 4.7 kg/m2; P = 0.803) and AST/ALT (from 1.08 ± 1.46 to 1.17 ± 0.64; P = 0.426) did not show significant changes. In addition, since the change in NFS greatly depends on the presence or absence of diabetes, it may be difficult to evaluate by NFS in mixed population of diabetic and non-diabetic patients. These factors may explain no change in NFS by pemafibrate. Our previous study showed that an improvement in liver function such as AST and albumin was significantly associated with reduction in TG and TG-rich lipoproteins, suggesting that an improvement of FIB-4 index and APRI was also induced due to reduction of TG and TG-rich lipoproteins by pemafibrate. Pemafibrate was reported to improve liver fibrosis assessed by magnetic resonance elastography (MRE) or Fibroscan-aspartate aminotransferase (FAST) score [10, 11], which agreed with our study.
Our study further showed that pemafibrate improved hepatic steatosis. HSI was calculated as 8 × (ALT/AST) + BMI + (2, if DM) + (2, if female) [6]. ALT/AST included in HSI significantly decreased from 1.23 ± 0.93 to 0.98 ± 0.47 (P = 0.001), which may contribute to reduction in HSI. Furthermore, the change in ALT/AST was significantly and inversely correlated with that at baseline (r = -0.866; P < 0.001), which may explain a significant correlation of change in HSI with HSI baseline value. However, the previous study reported that pemafibrate did not decrease liver fat content but had significant reduction in MRE-based liver stiffness at week 72 [10]. In another study, no significant reduction (P = 0.266) in controlled attenuation parameter (CAP) which has high diagnostic accuracy for detecting hepatic steatosis [12], was detected using Fibroscan at 48 weeks after pemafibrate administration.
In patients with hypertriglyceridemia, insulin resistance enhances the expression and activity of hormone-sensitive lipase (HSL) in adipose tissue which catalyzes the hydrolysis of TG into free FA (FFA) [4]. Increased serum FFA enters the liver, leading to overproduction of TG-rich lipoprotein, very low-density lipoprotein (VLDL). Insulin resistance is also associated with reduced VLDL degradation. Increased hepatic VLDL may induce inflammation and oxidative stress which develop and progress hepatic steatosis and fibrosis in such patients [4]. Pemafibrate reduces hepatic VLDL production by reducing FFA entry to liver and hepatic apo C-III production, which may contribute to an improvement in hepatic steatosis and fibrosis [4]. However, it remains unknown whether pemafibrate reduces liver disease progression and has a role in treatment of MASLD. To elucidate the effect of pemafibrate on the development and progression of hepatic steatosis and fibrosis, further studies using multiple modalities to evaluate hepatic steatosis and fibrosis and a greater number of patients should be performed.
Limitations of the study need to be addressed. This is a cross-sectional study, limiting inferences of causality and its direction. Although we did not change treatments for diabetes and hypertension and other lipid-lowering drugs intentionally during the study period, we cannot deny that such treatment might influence on the change of liver function. Furthermore, the parameters were not evaluated in 134 participants due to missing data. It weakens the scientific impact of this study.
In conclusion, the 1-year pemafibrate treatment improved hepatic steatosis and fibrosis indexes in patients with hypertriglyceridemia.
Acknowledgments
We thank the staff of the Division of Research Support, National Center for Global Health and Medicine Kohnodai Hospital.
Financial Disclosure
Authors have no financial disclosures to report.
Conflict of Interest
The authors declare that they have no conflict of interest concerning this article.
Informed Consent
Not applicable.
Author Contributions
HY designed the research, and MH, HK, HA collected and analyzed data. HY wrote the paper, and all authors approved the final version of paper.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238.
doi pubmed pmc - Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2023.
doi pubmed - Solis Herruzo JA, Garcia Ruiz I, Perez Carreras M, Munoz Yague MT. Non-alcoholic fatty liver disease. From insulin resistance to mitochondrial dysfunction. Rev Esp Enferm Dig. 2006;98(11):844-874.
doi pubmed - Yanai H, Adachi H, Hakoshima M, Katsuyama H. Molecular biological and clinical understanding of the statin residual cardiovascular disease risk and peroxisome proliferator-activated receptor alpha agonists and ezetimibe for its treatment. Int J Mol Sci. 2022;23(7):3418.
doi pubmed pmc - Yanai H, Katsuyama H, Hakoshima M. Effects of a novel selective peroxisome proliferator-activated receptor alpha modulator, pemafibrate, on metabolic parameters: a retrospective longitudinal study. Biomedicines. 2022;10(2):401.
doi pubmed pmc - Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503-508.
doi pubmed - Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846-854.
doi pubmed - Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726-736.
doi pubmed - Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2.
doi pubmed pmc - Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54(10):1263-1277.
doi pubmed pmc - Morishita A, Oura K, Takuma K, Nakahara M, Tadokoro T, Fujita K, Tani J, et al. Pemafibrate improves liver dysfunction and non-invasive surrogates for liver fibrosis in patients with non-alcoholic fatty liver disease with hypertriglyceridemia: a multicenter study. Hepatol Int. 2023;17(3):606-614.
doi pubmed pmc - de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60(5):1026-1031.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


