| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 3, June 2023, pages 149-156
Pharmacological and Endoscopic Interventions for Prophylaxis of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis
Emmanuel Palomera-Tejedaa, Mihir Prakash Shaha, d , Bashar M. Attarb, c, Hassam Shahb, Bharosa Sharmaa, Roberto Oleasa, Vikram Kotwalb, c, Seema Gandhib, Hemant Raj Mutnejab, c
aDepartment of Internal Medicine, John H. Stroger, Jr. Hospital of Cook County, Chicago, IL, USA
bDivision of Gastroenterology and Hepatology, John H. Stroger, Jr. Hospital of Cook County, Chicago, IL, USA
cDivision of Gastroenterology and Hepatology, Rush University Medical Center, Chicago, IL, USA
dCorresponding Author: Mihir Prakash Shah, Department of Internal Medicine, John H. Stroger, Jr. Hospital of Cook County, Chicago, IL 60612, USA
Manuscript submitted March 31, 2023, accepted May 15, 2023, published online June 11, 2023
Short title: Interventions for Prophylaxis of PEP
doi: https://doi.org/10.14740/gr1620
| Abstract | ▴Top |
Background: Post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) represents the most common serious complication after endoscopic retrograde cholangiopancreatography (ERCP). Rectal non-steroidal anti-inflammatory drugs (NSAIDs) and pancreatic duct stenting (PDS) are the prophylactic interventions with more evidence and efficacy; however, PEP still represents a significant source of morbidity, mortality, and economic burden. Chronic statin use has been proposed as a prophylactic method that could be cheap and relatively safe. However, the evidence is conflicting. We aimed to evaluate the impact of endoscopic and pharmacological interventions including chronic statin and aspirin use, on the development of PEP.
Methods: A retrospective cohort study evaluated consecutive patients undergoing ERCP at John H. Stroger, Jr. Hospital of Cook County in Chicago from January 2015 to March 2018. Univariate and multivariate analyses were performed using logistic regression.
Results: A total of 681 ERCPs were included in the study. Twelve (1.76%) developed PEP. Univariate, multivariate, and subgroup analyses did not show any association between chronic statin or aspirin use and PEP. PDS and rectal indomethacin were protective in patients undergoing pancreatic duct injection. Pancreatic duct injection, female sex, and younger age were associated with a higher risk. History of papillotomy was associated with lower risk only in the univariate analysis (all P values < 0.05).
Conclusion: Chronic use of statins and aspirin appears to add no additional benefit to prevent ERCP pancreatitis. Rectal NSAIDs, and PDS after appropriate patient selection continue to be the main prophylactic measures. The lower incidence at our center compared with the reported data can be explained by the high rates of rectal indomethacin and PDS, the use of noninvasive diagnostic modalities for patient selection, and the expertise of the endoscopists.
Keywords: Endoscopic retrograde cholangiopancreatography; Post-ERCP pancreatitis; Rectal NSAIDs; Pancreatic duct stenting; Chronic statin use; Chronic aspirin use; Retrospective cohort study
| Introduction | ▴Top |
Post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) is the most common serious adverse event associated with endoscopic retrograde cholangiopancreatography (ERCP). The management of PEP stands for an estimated healthcare cost of 200 million dollars annually in the United States and is also a significant reason for malpractice claims related to ERCP [1-3]. The incidence of PEP has been estimated to be 3-6% in average-risk patients with a mortality rate reported up to 3% [4-8]. The etiology of PEP is a multifactorial process involving chemical, enzymatic, hydrostatic, allergic, and microbiological factors with subsequent activation of pancreatic enzymes leading to autodigestion and local and systemic inflammation [9, 10]. Several studies have reported several risk factors for the development of PEP including history of acute pancreatitis, female sex, younger age, and pancreatic duct (PD) injection [11-14]. They seem to be independent, additive, and they are divided into operator-related, procedure-related, and patient-related factors [10, 15].
Significant efforts have been conducted to elucidate effective prophylactic measures to reduce the incidence of PEP [16]. The use of PD stents and rectal non-steroidal anti-inflammatory drugs (NSAIDs) have been successfully shown to be effective prophylactic interventions [17-20]. The current guidelines recommend rectal NSAIDs peri-procedurally in all patients and prophylactic pancreatic stenting in high-risk individuals [5, 21, 22]. Although non-rectally administered NSAIDs have failed to show any benefit for PEP prophylaxis, the effect of chronic use of aspirin on PEP incidence has been explored only in one observational study (Table 1) [5, 19, 23-28].
 Click to view | Table 1. Studies Evaluating the Utility of Statins and Aspirin as Prophylactic Method for PEP |
Statins have also been looked at as a potential prophylactic drug to prevent PEP based on the growing evidence that statins have pleiotropic anti-inflammatory properties and prior reports of risk and severity reduction for non-ERCP-related acute pancreatitis [29]. However, the results of the previous trials evaluating statins for prophylaxis of PEP are conflicting (Table 1).
In this study, the authors planned to evaluate the effect of chronic use of statin and aspirin as well as pancreatic duct stenting (PDS) on PEP among different patients with varying risk profiles.
| Materials and Methods | ▴Top |
The authors conducted a single-center retrospective cohort study at John H. Stroger, Jr. Hospital, a tertiary-level public hospital in Chicago, Illinois. The primary study endpoint was to assess the protective effect of chronic use of statins and PDS on PEP among different patients with varying risk profiles. The secondary outcome was to investigate the effect of chronic use of aspirin on the incidence of PEP, the effect of other risk factors on the incidence of PEP, and the association between these factors and severity of PEP. Our study was performed in accordance with the Institutional Review Board (IRB) guidelines for John H. Stroger Jr. Hospital of Cook County, under the category of retrospective chart review. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as the Helsinki Declaration.
Patient population
The author included consecutive patients > 18 years old undergoing ERCP in either inpatient or outpatient settings from January 2015 to March 2018. The author included patients with complete information related to the procedure and excluded patients with an established diagnosis of acute pancreatitis at the time of ERCP, patients with a history of hepaticojejunostomy, patients with a history of papillectomy, patients undergoing papillectomy during ERCP, and patients with intended PD cannulation. Patients with traumatic pancreatic injury and baseline elevation of lipase/amylase were also excluded from the study.
Data collection
Baseline characteristics of the eligible subjects, procedural data, and the post-procedure clinical course were obtained by three collaborators from the Electronic Medical Records (Cerner Corp) in the medication lists at the time of the procedure, progress notes, and procedure notes. Informed consent was waived given that no greater than minimal risk was involved. The patient identifiers were not included. The following epidemiological, clinical, and procedure-related information was collected: age, gender, race, indication of ERCP, inpatient or outpatient setting, PD injection, PD stenting, common bile duct (CBD) stenting, papillotomy, history of papillotomy, use of indomethacin peri-procedure, smoking, chronic use of statins (≥ 30 days), chronic use of aspirin (≥ 30 days), post-ERCP pancreatitis and severity. Due to the low reporting rate, the following data were not included: difficulty canulation (time of cannulation and the number of cannulation attempts), use of prophylactic intravenous fluids, and topical epinephrine. Prophylactic interventions were performed at the endoscopist’s discretion based on the patient’s risk profile. PEP was defined according to the revised Atlanta classification when at least two out of the three criteria were present: characteristic abdominal pain, amylase, or lipase elevation more than three times the normal upper limit, and/or imaging with findings of acute pancreatitis after ERCP [30]. The severity was classified according to the consensus criteria proposed by Cotton et al [31].
Methodology
Quantitative data were evaluated for normality graphically and by the Shapiro-Wilk test for samples n < 50 or the Kolmogorov-Smirnov test for samples n > 50. It was summarized using means and standard deviations (SDs) or medians and interquartile ranges. Qualitative data were described using frequencies and proportions. The analysis of basal characteristics was done as follows: differences between the groups were assessed using the Student’s t-test for normal quantitative variables, Mann-Whitney for non-normal quantitative variables, analysis of variance (ANOVA) for normal quantitative in the case of more than two groups, Kruskal-Wallis for non-normal quantitative data in the case of more than two groups, and Chi-square/Fisher’s exact test for qualitative variables. A univariate and multivariate analysis was performed to assess the predictors of the incidence of PEP, including statin use, PDS, and possible confounders. The multivariate regression analysis included the variables with a P-value less than 0.15 in the bivariate analysis, the already stablished prophylactic interventions (rectal NSAIDs and placement of a pancreatic stent), as well as our collected variables that are considered definite risk factors of PEP according to the 2014 ESGE guidelines [32] (suspected of Oddi dysfunction, female gender, previous acute pancreatitis, and pancreatic injection). The odds ratio (OR) and adjusted OR (aOR) with 95% confidence intervals (CIs) were calculated to investigate the frequency and strength of association. Both OR and aOR were calculated with binary logistic regression in the bivariate and multivariate analysis. A P-value (two-sided) of less than 0.05 was considered statistically significant for this study. All the statistical analyses were performed using the R statistical package and R studio [33, 34].
| Results | ▴Top |
From January 2015 to March 2018, 739 ERCPs were performed at the gastroenterology department of our institution. The author excluded 58 ERCPs according to our prespecified inclusion and exclusion criteria (Fig. 1). Table 2 depicts the baseline characteristics of the population included in the study and the difference between the two main treatment groups: chronic statin intake and pancreatic duct stenting. A total of 681 ERCPs (53% females) were included for the analysis with a mean age of 54.6 (SD 16.1). Black and Hispanic patients were the most prevalent (35.3% and 33.9%, respectively), and inpatient ERCP was the most common setting (60.8%). The most common indications were choledocholithiasis and benign biliary obstruction. Only 79 (11.6%) of the cases underwent PD injection. Three hundred thirty-one patients underwent papillotomy, 306 had a history of papillotomy, and 401 underwent biliary stent placement (48.6%, 44.9%, and 58.9%, respectively). Regarding the consumption of chronic medications, 159 were chronic statin users, and 111 were chronic aspirin users (23.5% and 16.3%, respectively). Two-hundred twelve were active smokers (31.1%). The most prevalent prophylactic strategies used against PEP were rectal indomethacin (64.5%) and PDS (7.8%). It is important to mention that in the case of PD injection, the rate of PDS was 57%. Twelve patients (1.76%) were diagnosed with PEP. Five patients were diagnosed with mild PEP, three with moderate PEP, and four with severe PEP. Regarding the baseline characteristics between the different treatment groups, the mean age was significantly greater in the chronic statin user group, aspirin was more often used in the chronic statin group, and chronic statin use was more prevalent among black and Asian population (all P values ≤ 0.001). Gender, ERCP setting, indication, PD injection, PD stent placement, CBD stent placement, endoscopic sphincterotomy, history of endoscopy sphincterotomy, rectal indomethacin, and smoking were not statistically different between the chronic statin use and non-statin groups (all P values > 0.05). PDS was statistically significant more used in the following groups: patients admitted to the hospital, PD injection, patients without a history of papillotomy, patients without undergoing biliary stenting, and patients without rectal indomethacin use (all P values < 0.05). Age, gender, ethnicity, smoking, chronic statin use, chronic aspirin use, and indication of ERCP were not statistically different between the PDS and non-PDS groups (all P values > 0.05).
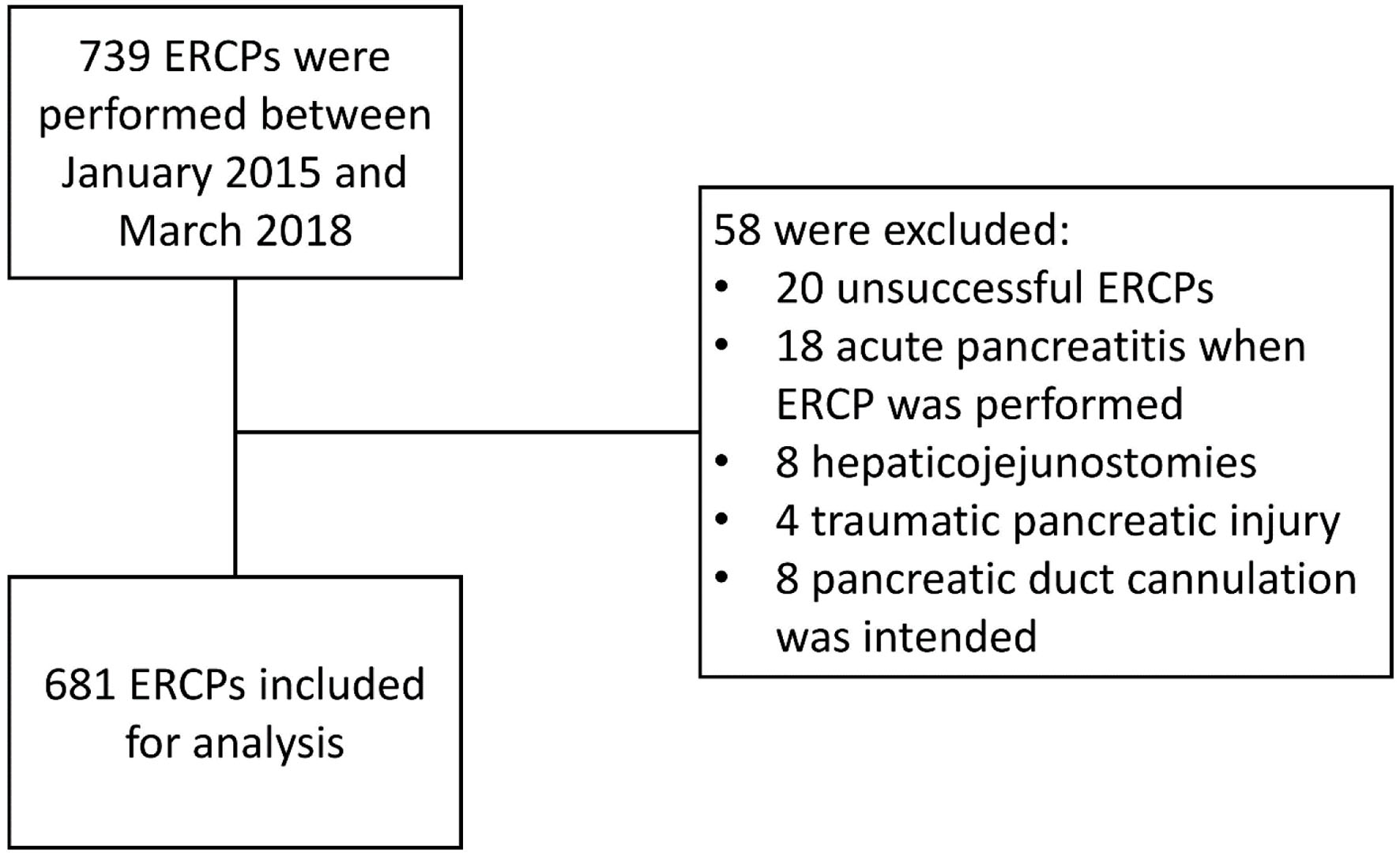 Click for large image | Figure 1. Patient selection flowchart. ERCP: endoscopic retrograde cholangiopancreatography. |
 Click to view | Table 2. Baseline Characteristics and Differences Between Treatment Groups |
The author conducted a univariate and multivariate analysis to explore the association of the independent variables with PEP development. The results are described in Table 3. Post-ERCP pancreatitis frequency was 2.1% and 0.62% in the non-statin and statin groups, respectively; it did not reach statistical significance (P = 0.24). On univariate analysis, young age, female sex, PD injection, and patient without a history of papillotomy were found to be significantly associated with the development of PEP (all P values < 0.05). A logistic regression model was fitted. In the logistic regression model, female gender (P = 0.047, aOR 0.18 (0.02 - 0.92)) and PD injection (P < 0.001, aOR 21.4 (5.28 - 93.6)) were found to be associated with increased risk of PEP. PDS was found to be protective against PEP (P = 0.03, OR (0.07 (0.003 - 0.54)). The authors conducted subgroup analyses for the main risk factors: female gender (n = 365) and PD injection (n = 79) groups. The analyses revealed that young age remained a significant risk factor for PEP (P = 0.007, OR 0.91 (0.84 - 0.96)) in patients undergoing pancreatic duct injection. Pancreatic duct stenting (P = 0.04 OR 0.11 (0.06 - 0.67)) and rectal indomethacin (P = 0.08, OR 0.91 (0.01 - 0.43)) were protective factors in these specific groups. In the female gender group, PD injection remained a strong predictor of PEP (P < 0.001 OR 6.11 (0.84 - 0.96)). The rest of the included variables were not statistically significant.
 Click to view | Table 3. Relationship Between Independent Variables and PEP |
| Discussion | ▴Top |
Despite the mechanistic evidence supporting the suppressive effects of statin medications on pancreatic inflammation, the current study failed to support risk reduction in PEP through statins. Although earlier reports, including a 2012 meta-analysis of large randomized controlled trials, suggested a lower incidence of acute pancreatitis (non-ERCP-related) among statin users compared with patients without statin use [29, 35-37], the evidence among patients undergoing ERCP is conflicting. Two retrospective cohort studies [24, 25] showed a possible benefit of chronic statin use as a prophylactic agent for PEP but growing evidence, including a prospective multicentric observational trial of 1,150 patients, suggests otherwise (Table 1) [23]. The univariate analysis of our study showed a protective tendency against the development of PEP in regular statin users, but it did not reach statistical significance and was not maintained during the multivariate analysis when other variables were considered. Our report is consistent with subsequent studies that reported a lack of benefit of chronic use of statins as a prophylactic method for PEP. Similarly, chronic use of aspirin was not associated with decreased incidence of PEP in any of our analyses. Even though the dose of chronic aspirin for cardiovascular prevention is dosed significantly lower compared with the recommended one for full anti-inflammatory effect, which could potentially explain this result, our study adds to previous reports pointing to the lack of benefit of both chronic aspirin use and anti-inflammatory dose of non-rectally administrated NSAIDs [3, 23].
Within our institution, indomethacin is the only rectal NSAID used in our patient population. It was associated with a reduction of PEP in the group of patients who received PD injections. The protective effect was not seen in the general sample; however, our study was not powered or intended to detect any difference in the incidence of PEP with the use of indomethacin in patients with regular risk.
PDS represents the primary preventative strategy implanted by endoscopists, and it is also a formal recommendation within the American Society of Gastrointestinal Endoscopy (ASGE) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines [32, 38]. In line with these recommendations, our results showed that PDS is highly protective among patients undergoing PD injection with OR 0.08 (95% CI 0.01 - 0.43); the protective effect was also present in the general sample after controlling the rest of the variables in the multivariate analysis.
Concerning other statistically significant risk factors, young age was associated with the development of PEP and history of prior endoscopic papillotomy was a protective factor in the univariate analysis, while PD injection and female gender were significant associations in both univariate and multivariate analyses. PD injection was the strongest independent factor for the development of PEP and was present in each of the analyses, including subgroup analyses. These findings are consistent with previously reported risk factors in several early studies [10, 15]. Smoking has been associated with the development of chronic pancreatitis which has been reported to be a potential protective factor for PEP; however, our analysis is also in line with earlier reports pointing to the lack of association [23].
The incidence of PEP in the present study was 1.76% in the general sample and 8.9% among patients undergoing PD injection, the strongest identified risk factor. This result differs from the findings previously reported in multiple studies, with an incidence of PEP varying from 3% to 15% [3]. This contrast may be explained by several factors. Our institution seems to implement preventative interventions (rectal NSAIDs, PDS) at a higher rate than what is reported in the literature. Secondly, patients selected for ERCP have already been evaluated by advanced imaging such as endoscopic ultrasound (EUS) or magnetic resonance cholangiopancreatography (MRCP), or risk stratified by their laboratories. Lastly, the number of ERCP completed annually at our institution (> 200) and the adequate skillset of endoscopists may likely play a role in the low incidence of PEP found in the present study.
Strengths and limitations
The present study has several strengths. It included data from a large sample of consecutive patients undergoing ERCP with significant diversity in race, age, and indications. A multivariate analysis using a comprehensive database was performed to include possible confounders. The main limitation is the study’s retrospective nature and the data collection process since it is impossible to assure the accuracy of many of the variables like circumstances during the performance of ERCP, drug adherence, or time of drug administration, which can modify the anti-inflammatory effect of statins. The study also had a limited number of patients who developed PEP among statin users, which likely represents a lack of statistical power to detect any risk modification. The power was also limited to detect any effect on the severity of PEP, mortality, and local or systemic complications due to the same reason. Moreover, most of the available ERCPs reports at our institution are not explicit about the difficulty of ERCPs and time needed for the cannulation; they are known significant risk factors and could not be included in the calculations.
Conclusion
Chronic use of statins and aspirin appear to add no additional benefit to prevent ERCP pancreatitis. Routine use of rectal NSAIDs, especially in high-risk patients, and pancreatic stenting after appropriate patient selection are the main therapeutic tools to decrease the incidence of PEP. The effectivity of statins as a prophylactic intervention against PEP demonstrated by the early studies is greatly limited by the lack of generalizability, especially in the light of the availability of better-established prophylactic measures as rectal indomethacin or PD stents. Well-designed prospective studies are needed to explore the optimal patient selection, as well as stent caliber and length, before performing PDS as a prophylactic intervention.
Acknowledgments
None to declare.
Financial disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was waived.
Author Contributions
Emmanuel Palomera-Tejeda: conception and design of the work, analysis and interpretation of the work, drafting and revising the work. Mihir Prakash Shah: data acquisition, interpretation of the work, drafting and revising the work. Bashar M. Attar: conception and design of the work, revising the work and final approval of the version to be published. Hassam Shah: conception and design of the work, data acquisition, analysis and interpretation of the work. Bharosa Sharma and Roberto Oleas: data acquisition. Vikram Kotwal, Seema Gandhi and Hemant Raj Mutneja: revising the work, and final approval of the version to be published.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ANOVA: analysis of variance; ASGE: American Society of Gastrointestinal Endoscopy; CBD: common bile duct; CI: confidence interval; ERCP: endoscopic retrograde cholangiopancreatography; ESGE: European Society of Gastrointestinal Endoscopy; EUS: endoscopic ultrasound; MRCP: magnetic resonance cholangiopancreatography; NSAID: non-steroidal anti-inflammatory drug; OR: odds ratio; PDS: pancreatic duct stenting; PEP: post-ERCP pancreatitis
| References | ▴Top |
- Cotton PB. Analysis of 59 ERCP lawsuits; mainly about indications. Gastrointest Endosc. 2006;63(3):378-382.
doi pubmed - Trap R, Adamsen S, Hart-Hansen O, Henriksen M. Severe and fatal complications after diagnostic and therapeutic ERCP: a prospective series of claims to insurance covering public hospitals. Endoscopy. 1999;31(2):125-130.
doi pubmed - Elmunzer BJ. Reducing the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Endosc. 2017;29(7):749-757.
doi pubmed - Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, Khashab MA, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81(1):143-149.e149.
doi pubmed - Facciorusso A, Crino SF, Tacelli M, Antonini F, Fantin A, Barresi L. Chronic use of statins and risk of post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15(2):195-202.
doi pubmed - Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104(1):31-40.
doi pubmed - Zhou W, Li Y, Zhang Q, Li X, Meng W, Zhang L, Zhang H, et al. Risk factors for postendoscopic retrograde cholangiopancreatography pancreatitis: a retrospective analysis of 7,168 cases. Pancreatology. 2011;11(4):399-405.
doi pubmed - Mutneja HR, Vohra I, Go A, Bhurwal A, Katiyar V, Palomera Tejeda E, Thapa Chhetri K, et al. Temporal trends and mortality of post-ERCP pancreatitis in the United States: a nationwide analysis. Endoscopy. 2021;53(4):357-366.
doi pubmed - Tryliskyy Y, Bryce GJ. Post-ERCP pancreatitis: Pathophysiology, early identification and risk stratification. Adv Clin Exp Med. 2018;27(1):149-154.
doi pubmed - Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54(4):425-434.
doi pubmed - George S, Kulkarni AA, Stevens G, Forsmark CE, Draganov P. Role of osmolality of contrast media in the development of post-ERCP pancreatitis: a metanalysis. Dig Dis Sci. 2004;49(3):503-508.
doi pubmed - Tarnasky P, Cunningham J, Cotton P, Hoffman B, Palesch Y, Freeman J, Curry N, et al. Pancreatic sphincter hypertension increases the risk of post-ERCP pancreatitis. Endoscopy. 1997;29(4):252-257.
doi pubmed - Luo H, Wang X, Zhang R, Liang S, Kang X, Zhang X, Lou Q, et al. Rectal Indomethacin and Spraying of Duodenal Papilla With Epinephrine Increases Risk of Pancreatitis Following Endoscopic Retrograde Cholangiopancreatography. Clin Gastroenterol Hepatol. 2019;17(8):1597-1606.e1595.
doi pubmed - Johnson GK, Geenen JE, Johanson JF, Sherman S, Hogan WJ, Cass O. Evaluation of post-ERCP pancreatitis: potential causes noted during controlled study of differing contrast media. Midwest Pancreaticobiliary Study Group. Gastrointest Endosc. 1997;46(3):217-222.
doi pubmed - Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335(13):909-918.
doi pubmed - Leerhoy B, Elmunzer BJ. How to Avoid Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Gastrointest Endosc Clin N Am. 2018;28(4):439-454.
doi pubmed - Luo H, Zhao L, Leung J, Zhang R, Liu Z, Wang X, Wang B, et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387(10035):2293-2301.
doi pubmed - Shen C, Shi Y, Liang T, Su P. Rectal NSAIDs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in unselected patients: Systematic review and meta-analysis. Dig Endosc. 2017;29(3):281-290.
doi pubmed - Patai A, Solymosi N, Mohacsi L, Patai AV. Indomethacin and diclofenac in the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis of prospective controlled trials. Gastrointest Endosc. 2017;85(6):1144-1156.e1141.
doi pubmed - Nicolas-Perez D, Castilla-Rodriguez I, Gimeno-Garcia AZ, Romero-Garcia R, Nunez-Diaz V, Quintero E. Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a cost-effectiveness analysis. Pancreas. 2015;44(2):204-210.
doi pubmed - Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366(15):1414-1422.
doi pubmed pmc - Njei B, McCarty TR, Muniraj T, Sharma P, Jamidar PA, Aslanian HR, Varadarajulu S, et al. Comparative effectiveness of pharmacologic and endoscopic interventions for prevention of post-ERCP pancreatitis: a network meta-analysis. Endosc Int Open. 2020;8(1):E29-E40.
doi pubmed pmc - Cardenas-Jaen K, Archibugi L, Poropat G, Korpela T, Maisonneuve P, Aparicio JR, Udd M, et al. Chronic use of statins and acetylsalicylic acid and incidence of post-endoscopic retrograde cholangiopancreatography acute pancreatitis: A multicenter, prospective, cohort study. Dig Endosc. 2021;33(4):639-647.
doi pubmed - Mahamid M, Watad A, Bragazzi NL, Wengrower D, Wolff J, Livovsky D, Amital H, et al. Chronic use of statins and their effect on prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Front Pharmacol. 2018;9:704.
doi pubmed pmc - Hadi YB, Naqvi SF, Abdelqader A, Kupec J, Nasr J. Reduced risk of post ERCP pancreatitis in statin users. BMC Gastroenterol. 2020;20(1):125.
doi pubmed pmc - Martinez-Moneo E, Cardenas-Jaen K, Fernandez-Laso AB, Millastre-Bocos J, Torralba-Gallego A, Martin-Arriero S, Alfaro-Almajano E, et al. Statin consumption and risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreatology. 2020;20(5):801-805.
doi pubmed - Hakuta R, Nakai Y, Hamada T, Suzuki Y, Inokuma A, Oyama H, Kanai S, et al. Regular Statin Use and Incidence of Postendoscopic Retrograde Cholangiopancreatography Pancreatitis. J Clin Gastroenterol. 2020;54(10):905-910.
doi pubmed - Facciorusso A, Buccino VR, Tonti P, Sacco R. Statin use does not decrease the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis. Expert Rev Gastroenterol Hepatol. 2020;14(7):511-513.
doi pubmed - Preiss D, Tikkanen MJ, Welsh P, Ford I, Lovato LC, Elam MB, LaRosa JC, et al. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308(8):804-811.
doi pubmed - Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262(3):751-764.
doi pubmed - Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383-393.
doi pubmed - Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46(9):799-815.
doi pubmed - RStudio Team. RStudio: Integrated Development for R. RStudio. Published online. 2020.
- Team RC. R: a language and environment for statistical computing. Published online. 2013.
- de-Madaria E. Statins for the Prevention of Acute Pancreatitis. Am J Gastroenterol. 2017;112(12):1765-1767.
doi pubmed - Thisted H, Jacobsen J, Munk EM, Norgaard B, Friis S, McLaughlin JK, Sorensen HT, et al. Statins and the risk of acute pancreatitis: a population-based case-control study. Aliment Pharmacol Ther. 2006;23(1):185-190.
doi pubmed - Poropat G, Archibugi L, Korpela T, Cardenas-Jaen K, de-Madaria E, Capurso G. Statin use is not associated with an increased risk of acute pancreatitis-A meta-analysis of observational studies. United European Gastroenterol J. 2018;6(8):1206-1214.
doi pubmed pmc - Asge Standards of Practice Committee, Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, et al. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85(1):32-47.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


