| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 2, April 2023, pages 79-91
Outcomes and Complications of Radiological Gastrostomy vs. Percutaneous Endoscopic Gastrostomy for Enteral Feeding: An Updated Systematic Review and Meta-Analysis
Zohaib Ahmeda, j, k, Umair Iqbalb, j, Muhammad Azizc, Syeda Faiza Arifd, Joyce Badale, Umer Farooqf, Wade Lee-Smithg, Manesh Kumar Gangwania, Faisal Kamalh, Abdallah Kobeissyc, Asif Mahmooda, Ali Nawrasc, Harshit S. Kharab, Bradley D. Conferb, Douglas G. Adleri
aDepartment of Internal Medicine, University of Toledo, Toledo, OH, USA
bDivision of Gastroenterology and Hepatology, Geisinger Medical Center, Danville, PA, USA
cDivision of Gastroenterology and Hepatology, University of Toledo, Toledo, OH, USA
dDow University of Health Sciences, Karachi, Pakistan
eUniversity of Toledo College of Medicine and Life Sciences, Toledo, OH, USA
fDepartment of Internal Medicine, Rochester General Hospital, Rochester, NY, USA
gUniversity of Toledo Libraries, University of Toledo, Toledo, OH, USA
hDivision of Gastroenterology, University of California San Francisco, San Francisco, CA, USA
iCenter for Advanced Therapeutic Endoscopy (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO, USA
jZohaib Ahmed and Umair Iqbal contributed equally and shared the first authorship.
kCorresponding Author: Zohaib Ahmed, Department of Internal Medicine, University of Toledo Medical Center, Toledo, OH, USA
Manuscript submitted January 27, 2023, accepted March 9, 2023, published online April 28, 2023
Short title: PRG vs. PEG for Enteral Feeding
doi: https://doi.org/10.14740/gr1593
| Abstract | ▴Top |
Background: Percutaneous endoscopic gastrostomy (PEG) and percutaneous radiological gastrostomy (PRG) are commonly utilized to establish access to enteral nutrition. However, data comparing the outcomes of PEG vs. PRG are conflicting. Therefore, we aimed to conduct an updated systemic review and meta-analysis comparing PRG and PEG outcomes.
Methods: Medline, Embase, and Cochrane library databases were searched until February 24, 2023. Primary outcomes included 30-day mortality, tube leakage, tube dislodgement, perforation, and peritonitis. Secondary outcomes included bleeding, infectious complications, and aspiration pneumonia. All analyses were conducted using Comprehensive Meta-Analysis Software.
Results: The initial search revealed 872 studies. Of these, 43 of these studies met our inclusion criteria and were included in the final meta-analysis. Of 471,208 total patients, 194,399 received PRG and 276,809 received PEG. PRG was associated with higher odds of 30-day mortality when compared to PEG (odds ratio (OR): 1.205, 95% confidence interval (CI): 1.015 - 1.430, I2 = 55%). In addition, tube leakage and tube dislodgement were higher in the PRG group than in PEG (OR: 2.231, 95% CI: 1.184 - 4.2 and OR: 2.602, 95% CI: 1.911 - 3.541, respectively). Perforation, peritonitis, bleeding, and infectious complications were higher with PRG than PEG.
Conclusion: PEG is associated with lower 30-day mortality, tube leakage, and tube dislodgement rates than PRG.
Keywords: Percutaneous endoscopic gastrostomy; Percutaneous radiological gastrostomy; Enteral feeding
| Introduction | ▴Top |
Enteral feeding is a common strategy to maintain nutritional status when oral feeding is unfeasible, high risk, or requires supplementation. Gastrostomy feeding is favored over nasogastric tube feeding when medium and long-term enteral nutrition is indicated [1]. Enteral nutrition is superior to parenteral nutrition in terms of nutritional outcome, morbidity reduction, and gut function preservation [2]. Head and neck cancers, motor neuron disorders, cerebral vascular accidents, and malnutrition are the most common indications for gastrostomy tube placement [3]. The two most common placement techniques are percutaneous endoscopic gastrostomy (PEG) and percutaneous radiological gastrostomy (PRG) [4]. While PEG is the preferred approach at many centers, PRG and surgical gastrostomy are still frequently performed, especially in patients unable to undergo PEG [5, 6].
Current literature regarding adverse event rates of different gastrostomy tube placement approaches presents conflicting findings. Some studies have found that PRG leads to fewer adverse events than PEG [7], while some suggest that PEG is safer than PRG in select patients. Other studies have found no significant difference between the various approaches [8, 9]. Several systematic reviews and meta-analyses comparing PEG and PRG have previously been published [10-12]. However, these meta-analyses have certain drawbacks because they mainly focus on a single outcome or include only a small number of studies. Therefore, we aimed to conduct an updated systematic review of the literature and a comprehensive meta-analysis comparing PEG and PRG outcomes.
| Materials and Methods | ▴Top |
A comprehensive search strategy to identify reports of studies comparing radiologically and endoscopically guided placement of gastrostomy tubes was constructed using truncated keywords, phrases, and database subject headings developed in Embase by an experienced health sciences librarian (WL-S). This strategy was translated to MEDLINE (PubMed platform, NCBI), Cochrane Central Register of Controlled Trials, the Web of Science Core Collection, Korean Citation Index, SciELO (Web of Science platform, Clarivate) and the regional indexes of the Global Index Medicus (World Health Organization) with all searches performed on December 21, 2021 and subsequently updated on February 24, 2023 (Supplementary Material 1, www.gastrores.org). No publication date or language limits were used. All results were exported to EndNote 20 citation management software (Clarivate, Philadelphia, PA, USA) and duplicates were removed by successive iterations of EndNote’s duplicate detection algorithms and manual inspection.
Screening and data collection
The studies were screened by two independent reviewers (ZA and UI). The initial screening was based on titles and abstracts, with the full-text screening of relevant publications following. Next, two independent reviewers extracted the data (ZA and JB). Any discrepancies in study selection and data extraction were resolved through mutual discussion and consensus between the authors. Finally, data on demographics (age, gender), indications for the procedure, and outcomes (30-day mortality, tube leakage, tube dislodgement, perforation, peritonitis, bleeding, infections, and aspiration pneumonia) were collected and summarized using Microsoft Excel (Microsoft, Redmond, Washington, USA).
Data synthesis and statistical analysis
Statistical analysis was conducted utilizing Comprehensive Meta-analysis Software. We used both fixed and random-effects model for this meta-analysis, with point estimates, variance, and weights for each study based on study size and the number of events. When there was significant statistical heterogeneity, we used the random effects model. In outcomes with low or no statistical heterogeneity, we used the fixed effects model. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for all the outcomes. An I2 test was used to evaluate the heterogeneity of the studies. An I2 value of 0-25% represented insignificant heterogeneity, and > 75% represented significant heterogeneity. To assess the robustness of our study results, we performed a sensitivity analysis after removing one study at a time. This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. The PRISMA checklist is provided in Supplementary Material 2 (www.gastrores.org).
Quality assessment
Methodological Index for Non-Randomized Studies (MINORS) was used to assess study quality [14]. Non-comparative studies are graded on eight MINORS criteria, with each item ranging from 0 to 2 (0 if not reported; 1 if reported but inadequate; 2 if reported and adequate). A global score of 16 for non-comparative studies and 24 for comparative studies is considered ideal. Two authors (ZA and UF) independently carried out the quality assessment, and disagreements were handled by a third reviewer (JB).
Bias assessment
The risk of bias within each individual study was determined using the MINORS scale for cohort studies and the Cochrane risk of bias tool for randomized controlled trials (RCTs) [14, 15]. Publication bias was assessed qualitatively by funnel plot visualization and quantitatively by Egger’s regression analysis.
| Results | ▴Top |
The initial search revealed 872 studies. Of these, 43 studies including 471,208 patients met our inclusion criteria and were included in the final meta-analysis [6, 16-57]. There was one RCT, five non-randomized prospective studies, and 37 retrospective studies. Figure 1 elaborates our systematic literature search process. A total of 194,350 patients underwent PRG placement, and 276,741 patients underwent PEG placement. Baseline characteristics, including patient demographics and indications for gastrostomy tube placement, are reported in Table 1. The quality and bias assessment of studies is summarized in Table 2 and 3. There was no publication bias as assessed by the funnel plot diagram and Egger’s regression test (two-tailed P-value = 0.72259) (Fig. 2).
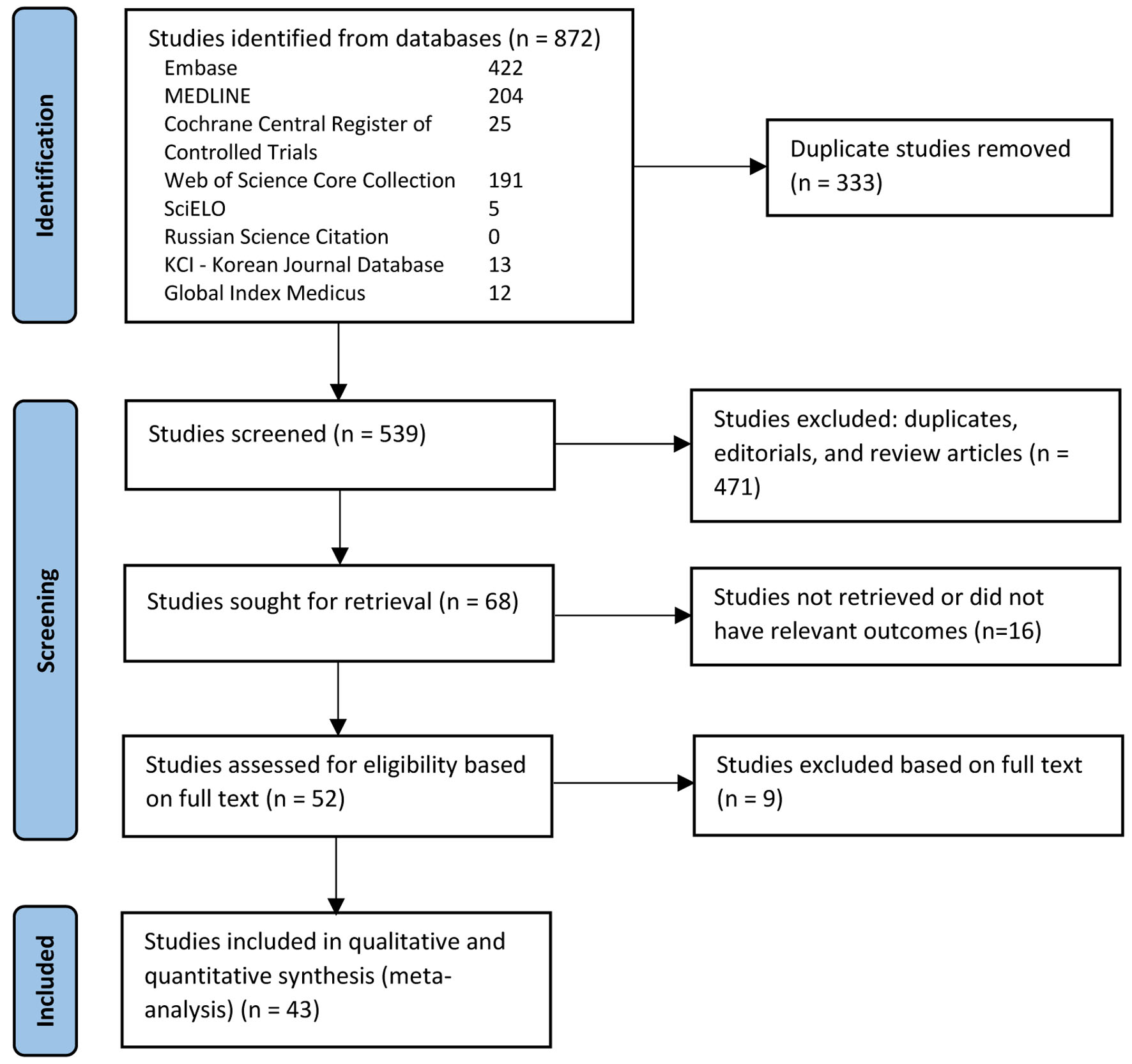 Click for large image | Figure 1. PRISMA flow diagram of the literature review process. |
 Click to view | Table 1. Study Characteristics |
Quality and bias assessment
The quality assessment of studies is summarized in Table 2, and the bias assessment is summarized in Table 3. There was no publication bias as assessed by the funnel plot diagram and Egger’s regression test (two-tailed P-value = 0.72259) (Fig. 2).
 Click to view | Table 2. Quality Assessment of Non-Randomized Studies via Methodological Index for Non-Randomized Studies (MINORS) |
 Click to view | Table 3. Risk of Bias Assessment of the Included RCTs |
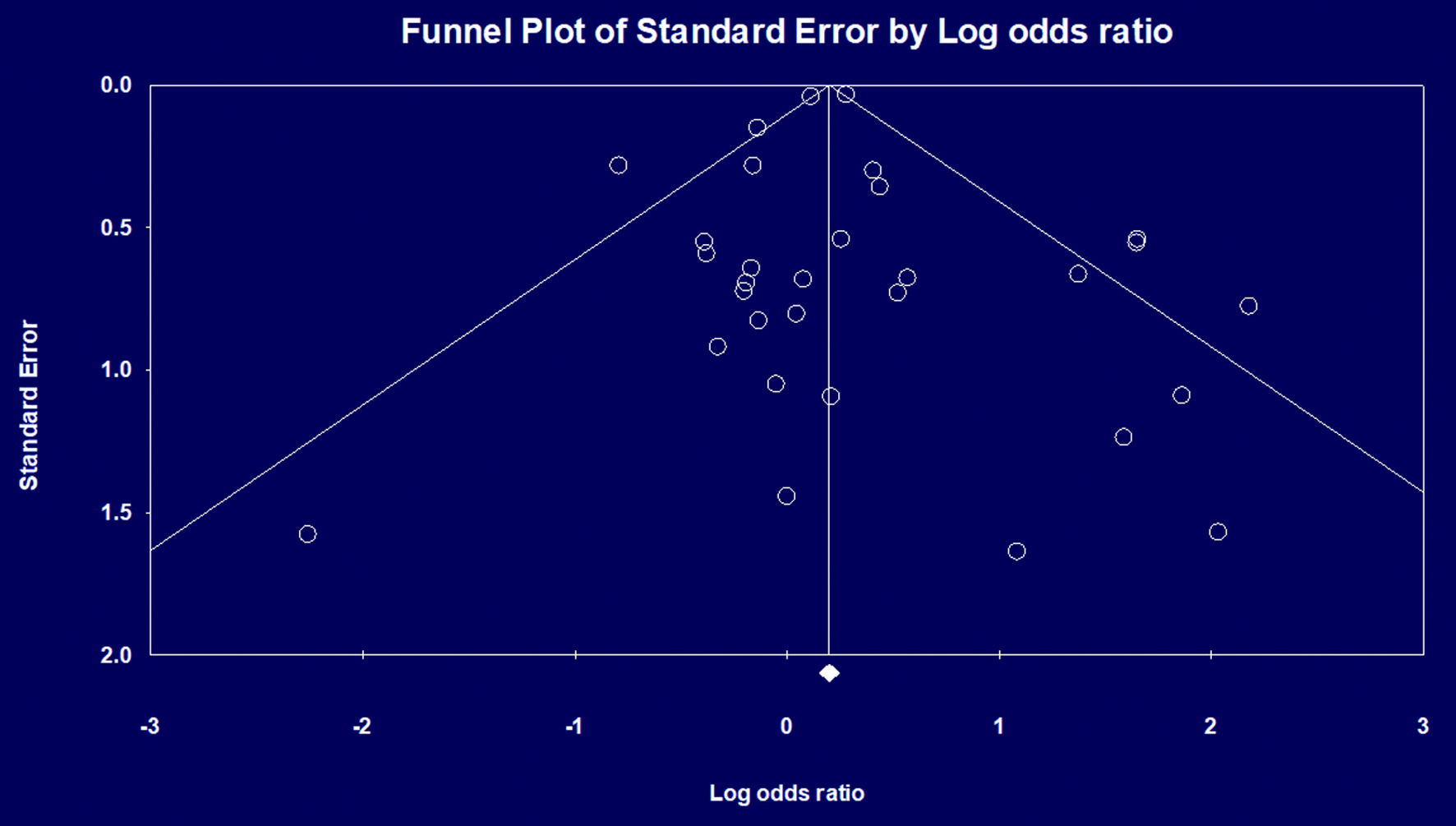 Click for large image | Figure 2. Publication bias assessed by funnel plot diagram. |
Primary outcomes
30-day mortality
PRG was associated with higher odds of 30-day mortality when compared to PEG (OR: 1.205, 95% CI: 1.015 - 1.430, I2 = 55%) on primary analysis (Fig. 3a). Sensitivity analysis revealed consistent results, with PRG being associated with a higher risk of 30-day mortality when compared to PEG placement. We performed a subgroup analysis after removing the three studies with the largest sample sizes, which confirmed consistent results without their influence (OR = 1.352, 95% CI = 1.001 - 1.826).
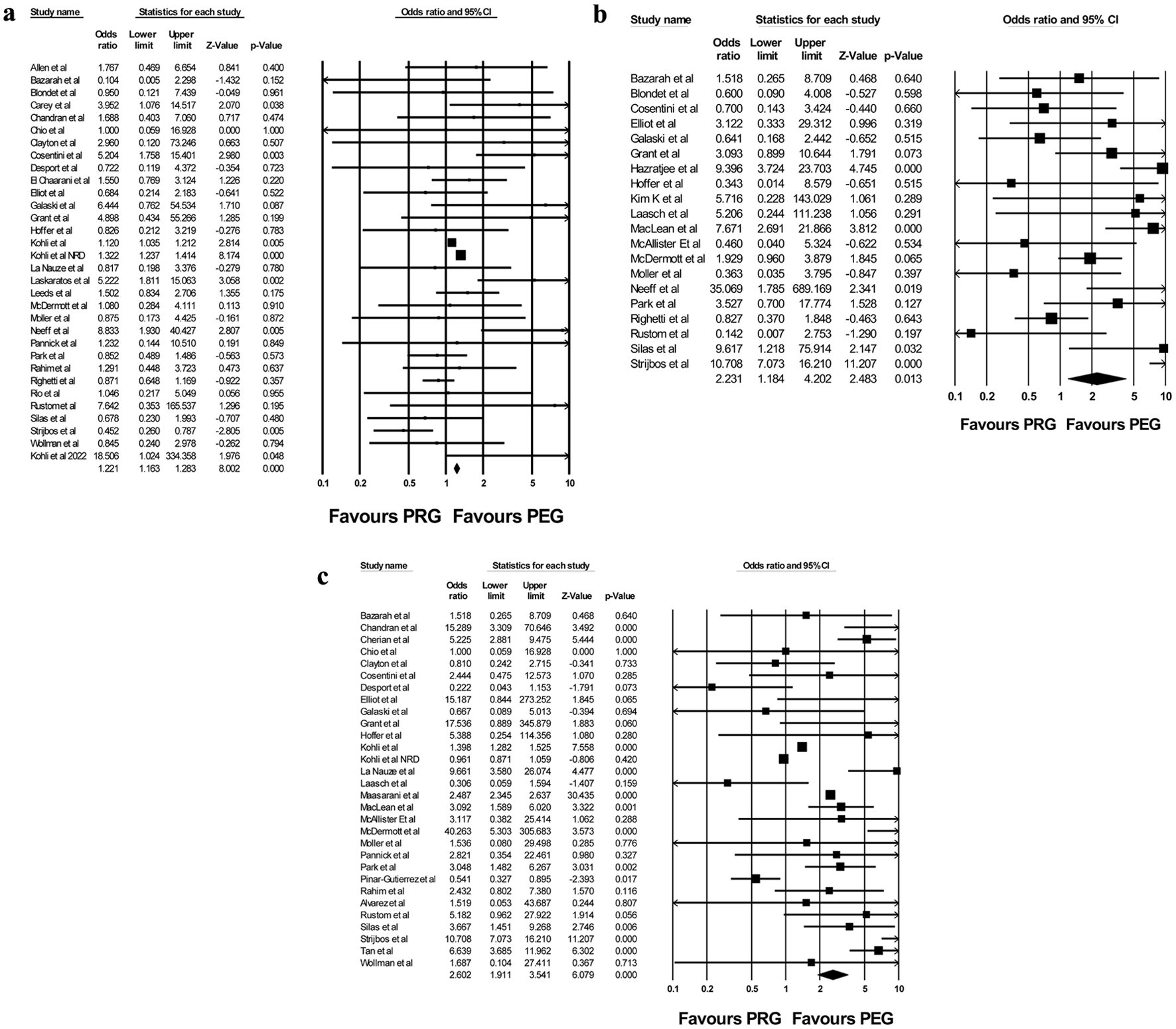 Click for large image | Figure 3. Forest plot of (a) 30-day mortality, (b) tube leakage, and (c) tube dislodgement between PRG and PEG. PEG: percutaneous endoscopic gastrostomy; PRG: percutaneous radiological gastrostomy. |
Tube leakage
PRG was associated with higher odds of tube leakage when compared to PEG (OR: 2.231, 95% CI: 1.184 - 4.2, I2 = 76%) on primary analysis (Fig. 3b). Sensitivity analysis revealed consistent results, with PRG associated with higher odds of leakage compared to PEG.
Tube dislodgement
PRG was associated with higher odds of tube dislodgement when compared to PEG (OR: 2.602, 95% CI: 1.911 - 3.541, I2 = 94%) on primary analysis (Fig. 3c). Sensitivity analysis confirmed these results. On subgroup analysis without the three largest studies, results were again consistent (OR = 2.927, 95% CI = 1.755 - 4.882).
Perforation
PRG was associated with higher odds of perforation when compared to PEG (OR: 1.758, 95% CI: 1.45 - 2.31, I2: 33.13%) on primary analysis. However, this did not achieve statistical significance in the sensitivity analysis (OR: 1.306, 95% CI: 0.971 - 1.755).
Peritonitis
PRG was associated with higher odds of peritonitis when compared to PEG (OR: 1.369, 95% CI: 1.81 - 1.586, I2 = 0%) on primary analysis. However, this did not achieve statistical significance in sensitivity analysis (OR: 1.066, 95% CI: 0.653 - 1.742).
Secondary outcomes
Bleeding
PRG was associated with a greater risk of bleeding than PEG (OR: 1.376, 95% CI: 1.257 - 1.507, I2 = 72%). However, this did not achieve statistical significance on sensitivity analysis (OR: 0.883, 95% CI: 0.74 - 1.051).
Infectious complications
PRG was associated with higher odds of infectious complications than PEG (OR: 1.193, 95% CI: 1.136 - 1.253, I2 = 23.3%). However, this did not achieve statistical significance on sensitivity analysis (OR: 1.124, 95% CI: 0.996 - 1.268).
Aspiration pneumonia
There was no significant difference in risk of aspiration pneumonia when comparing PRG with PEG (OR: 0.931, 95% CI: 0.654 -1.324, I2 = 15%). Sensitivity analysis confirmed these results.
| Discussion | ▴Top |
This systematic review and meta-analysis comparing PRG and PEG outcomes found higher odds of 30-day mortality, tube leakage, and tube dislodgement with PRG compared to PEG. Rates of bleeding, perforation, infectious complications, and peritonitis were significantly higher with PRG than PEG, but these results did not achieve statistical significance in a sensitivity analysis.
The odds of 30-day mortality after PRG was significantly higher than PEG, which is consistent with the results of previously published meta-analyses. Strijbos et al [12] reported a 30-day mortality rate of 7% for PEG vs. 11% for PRG in their 2017 meta-analysis of 2,027 patients. Lim et al also reported a lower 30-day mortality rate of 5.5% for PEG vs. 10.5% for PRG (OR: 0.6, CI 0.38 - 0.94, P = 0.026) in their 2016 meta-analysis of 2,183 patients [11]. The lower 30-day mortality rate in the PEG group could be attributed to several factors. First, prophylactic antibiotics are widely used and recommended among patients undergoing PEG placement [58], while present data on the use of antibiotics before PRG are conflicting. Clements et al reported no significant difference with antibiotic prophylaxis in early peristomal infection rate in their retrospective analysis [59]. In contrast, in an RCT, Ingraham et al reported early infection rate was 11.8% in the placebo arm and 0.0% in the antibiotic arm [60]. Currently, prophylactic antibiotics are not widely recommended for patients undergoing PRG, as the procedure avoids the oropharyngeal bacterial contamination of the endoscope as it traverses through the mouth and pharynx that occurs during PEG [61]. Second, PEG and PRG often use gastrostomy tubes of different sizes. PRG tubes are thinner in diameter (10 - 14 French) than PEG tubes (20 French), which could affect rates of tube blockage and result in aspiration pneumonia, increasing risk of respiratory decompensation and mortality [62]. In addition, because PRG tubes are secured using a balloon retention system, they are typically less secure than PEG tubes, resulting in greater risk of peristomal leakage and tube displacement, potentially leading to peritonitis and bowel perforation [63]. Furthermore, the two groups differed in terms of indication for gastrostomy tube placement. PEG patients had a higher prevalence of neurological disorders, whereas PRG patients had a higher prevalence of head and neck malignancy. These distinctions could have influenced our study’s findings.
Tube-related complications, tube leakage, and tube dislodgement were more common among patients undergoing PRG than PEG. Strijbos et al reported the rate of tube-related complications to be 16% for PRG vs. 6% for PEG [12]. Failure of equipment, particularly balloon failure, has been identified as a primary cause of tube dislodgment in PRG [22]. Additionally, the smaller diameter of PRG tubes compared to PEG tubes may increase the chance of blockage, as well as risk of tube dislodgement. Contrary to our results, a meta-analysis by Wollman et al reported a higher rate of tube-related complications in PEG patients (16%) than PRG patients (12%) (P ≤ 0.001) [10].
To assess whether any single study had a dominant effect on the meta-analysis, we excluded one study at a time and analyzed its impact on the primary summary estimate. We found that after removing certain studies from the analysis one at a time, some results were no longer significant. For example, overall results indicated that PRG is associated with a higher rate of infections than PEG. However, this was not significantly different based on our sensitivity analysis, which is consistent with previous studies.
Despite being associated with higher 30-day mortality rates and tube-related complications, PRG is still an important option for patients requiring gastrostomy tubes. In contrast to PEG, PRG can be placed without requiring sedation. PRG can also be used in patients with severe esophageal stenosis or malignant esophageal and oropharyngeal cancers that are poor candidates for PEG [64].
We compared our study to the meta-analysis by Strijbos et al [12] published in 2018. The limitations of their meta-analysis included a low number of studies, failure to include all available studies that met inclusion criteria, and lack of reporting on all outcomes. Our meta-analysis has the following strengths: systematic literature search with well-defined inclusion criteria, inclusion of all available studies in the current literature, careful exclusion of redundant studies, high-quality studies with detailed data extraction, and rigorous study quality evaluation. Our pooled rates are calculated from 471,091 patients, a very robust figure. However, our study has some limitations, many of which are inherent to any meta-analysis. Most of the studies included are retrospective, which likely contributed to selection bias. Although 30-day mortality is high with PRG, it should be highlighted that the underlying comorbid illnesses that required the patient to have a gastrostomy tube were likely the cause of mortality, rather than procedural complications. Because we were unable to account for comorbidities, it is plausible that individuals who received PRG were sicker than those who received PEG, which could explain the higher mortality rate. Furthermore, because our meta-analysis identified studies from various databases, there is a chance of patient overlap. However, our sensitivity analysis for each outcome bolsters our meta-analysis findings. Nonetheless, our study represents the best estimate of PRG and PEG clinical outcomes currently available in the literature.
In summary, PRG is associated with higher 30-day mortality and gastrostomy tube-related complications than PEG. Additional studies, particularly large RCTs, are warranted.
| Supplementary Material | ▴Top |
Suppl 1. Search strategy.
Suppl 2. PRISMA checklist.
Acknowledgments
This research was originally presented at the American College of Gastroenterology 2022 Annual Scientific Meeting in Charlotte, NC on October 25, 2022.
Financial Disclosure
No funding was received for the preparation of this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Zohaib Ahmed contributed to study planning and conduction, data collection and interpretation, manuscript drafting and revision. Umair Iqbal contributed to study planning, statistical analysis, manuscript drafting and revision. Muhammad Aziz contributed to statistical analysis, manuscript drafting. Syeda Faiza Arif, Joyce Badal, and Umer Farooq contributed to data collection, manuscript drafting. Faisal Kamal contributed to data interpretation, manuscript drafting and revision. Wade Lee-Smith contributed to study search strategy, data collection, manuscript drafting. Asif Mahmood contributed to manuscript revision. Abdallah Kobeissy, Harshit S. Khara, Ali Nawras, Bradley Confer, and Douglas G. Adler contributed to study design and conception, and critical manuscript revision.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ALS: amyotrophic lateral sclerosis; CI: confidence interval; CVA: cerebrovascular accident; HNC: head and neck cancers; MINORS: Methodological Index for Non-Randomized Studies; OR: odds ratio; PEG: percutaneous endoscopic gastrostomy; PRG: percutaneous radiological gastrostomy
| References | ▴Top |
- Cullen S. Gastrostomy tube feeding in adults: the risks, benefits and alternatives. Proc Nutr Soc. 2011;70(3):293-298.
doi - McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24(3):305-315.
doi - Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, Kurtz A, Farkas DT. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol. 2014;20(24):7739-7751.
doi pubmed pmc - Yuan Y, Zhao Y, Xie T, Hu Y. Percutaneous endoscopic gastrostomy versus percutaneous radiological gastrostomy for swallowing disturbances. Cochrane Database Syst Rev. 2016;2(2):CD009198.
doi pubmed pmc - Bankhead RR, Fisher CA, Rolandelli RH. Gastrostomy tube placement outcomes: comparison of surgical, endoscopic, and laparoscopic methods. Nutr Clin Pract. 2005;20(6):607-612.
doi - Park SK, Kim JY, Koh SJ, Lee YJ, Jang HJ, Park SJ, Small I, et al. Complications of percutaneous endoscopic and radiologic gastrostomy tube insertion: a KASID (Korean Association for the Study of Intestinal Diseases) study. Surg Endosc. 2019;33(3):750-756.
doi - Yang B, Shi X. Percutaneous endoscopic gastrostomy versus fluoroscopic gastrostomy in amyotrophic lateral sclerosis (ALS) sufferers with nutritional impairment: A meta-analysis of current studies. Oncotarget. 2017;8(60):102244-102253.
doi pubmed pmc - Galletti R, Finocchiaro E, Repici A, Saracco G, Zanardi M. Comparison of complication rates between endoscopic and fluoroscopic percutaneous gastrostomies. Nutrition. 2001;17(11-12):967-968.
doi - Bravo JG, Ide E, Kondo A, de Moura DT, de Moura ET, Sakai P, Bernardo WM, et al. Percutaneous endoscopic versus surgical gastrostomy in patients with benign and malignant diseases: a systematic review and meta-analysis. Clinics (Sao Paulo). 2016;71(3):169-178.
doi pubmed pmc - Wollman B, D'Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995;197(3):699-704.
doi - Lim JH, Choi SH, Lee C, Seo JY, Kang HY, Yang JI, Chung SJ, et al. Thirty-day mortality after percutaneous gastrostomy by endoscopic versus radiologic placement: a systematic review and meta-analysis. Intest Res. 2016;14(4):333-342.
doi pubmed pmc - Strijbos D, Keszthelyi D, Bogie RMM, Gilissen LPL, Lacko M, Hoeijmakers JGJ, van der Leij C, et al. A systematic review and meta-analysis on outcomes and complications of percutaneous endoscopic versus radiologic gastrostomy for enteral feeding. J Clin Gastroenterol. 2018;52(9):753-764.
doi - Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
doi pubmed pmc - Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716.
doi - Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
doi pubmed pmc - Allen JA, Chen R, Ajroud-Driss S, Sufit RL, Heller S, Siddique T, Wolfe L. Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: a retrospective study of complications and outcome. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(4):308-314.
doi - Alvarez-Alvarez M, Rodriguez-Lopez P, Velasco-Guardado A, Lopez-Alburquerque T. [Gastrostomy tubes in patients with amyotrophic lateral sclerosis: indications, safety and experience in a tertiary care centre]. Rev Neurol. 2022;75(2):41-44.
doi - Bazarah SM, Al-Rawas M, Akbar H, Qari Y. Percutaneous gastrostomy and gastrojejunostomy: radiological and endoscopic approach. Ann Saudi Med. 2002;22(1-2):38-42.
doi - Blondet A, Lebigot J, Nicolas G, Boursier J, Person B, Laccoureye L, Aube C. Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. J Vasc Interv Radiol. 2010;21(4):527-533.
doi - Carey E, Parsa SS, Huettl E, Sorbi D. Complications of radiologically and endoscopically-placed feeding gastrostomy tubes. Gastrointestinal Endoscopy. 2004;59(5):AB157-AB157.
doi - Vidhya C, Phoebe D, Dhina C, Jayne S, Robert F. Percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy: A comparison of outcomes at an Australian teaching hospital. Journal of Parenteral and Enteral Nutrition. 2017;41(2):272-273.
- Cherian P, Blake C, Appleyard M, Clouston J, Mott N. Outcomes of radiologically inserted gastrostomy versus percutaneous endoscopic gastrostomy. J Med Imaging Radiat Oncol. 2019;63(5):610-616.
doi - Chio A, Galletti R, Finocchiaro C, Righi D, Ruffino MA, Calvo A, Di Vito N, et al. Percutaneous radiological gastrostomy: a safe and effective method of nutritional tube placement in advanced ALS. J Neurol Neurosurg Psychiatry. 2004;75(4):645-647.
doi pubmed pmc - Clayton S, DeClue C, Lewis T, Rodriguez A, Kolkhorst K, Syed R, Kumar A, et al. Radiologic versus endoscopic placement of gastrostomy tube: comparison of indications and outcomes at a tertiary referral center. South Med J. 2019;112(1):39-44.
doi - Cosentini EP, Sautner T, Gnant M, Winkelbauer F, Teleky B, Jakesz R. Outcomes of surgical, percutaneous endoscopic, and percutaneous radiologic gastrostomies. Arch Surg. 1998;133(10):1076-1083.
doi - Desport JC, Mabrouk T, Bouillet P, Perna A, Preux PM, Couratier P. Complications and survival following radiologically and endoscopically-guided gastrostomy in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(2):88-93.
doi - ElChaarani B, Gal C, Lukolic I, Walsh J, Lee D. A comparison of complication rates between GI percutaneous endoscopic gastrostomy (PEG) and IR percutaneous radiologic gastrostomy (PRG) tube placement. American Journal of Gastroenterology. 2014;109:S54-S55.
doi - Elliott LA, Sheridan MB, Denyer M, Chapman AH. PEG — is the E necessary? A comparison of percutaneous and endoscopic gastrostomy. Clin Radiol. 1996;51(5):341-344.
doi - Galaski A, Peng WW, Ellis M, Darling P, Common A, Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol. 2009;23(2):109-114.
doi pubmed pmc - Grant DG, Bradley PT, Pothier DD, Bailey D, Caldera S, Baldwin DL, Birchall MA. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009;34(2):103-112.
doi - Hazratjee N, Agito M, Vanama RC, Lopez R, Rizk MK, Vargo JJ, Chahal P. Percutaneous gastrostomy tube insertion by gastroenterologists, surgical endoscopists, and interventional radiologists: Which is safer? Gastrointestinal Endoscopy. 2012;75(4):AB287.
doi - Hoffer EK, Cosgrove JM, Levin DQ, Herskowitz MM, Sclafani SJ. Radiologic gastrojejunostomy and percutaneous endoscopic gastrostomy: a prospective, randomized comparison. J Vasc Interv Radiol. 1999;10(4):413-420.
doi - Kim KO, Kim KH, Lee SH, Jang BI, Kim TN. Clinical comparison between percutaneous endoscopic and radiologic gastrostomy. Journal of Gastroenterology and Hepatology (Australia). 2014;29:291.
doi - Kohli DR, Kennedy KF, Desai M, Sharma P. Safety of endoscopic gastrostomy tube placement compared with radiologic or surgical gastrostomy: nationwide inpatient assessment. Gastrointest Endosc. 2021;93(5):1077-1085.e1071.
doi - Kohli DR, Kennedy KF, Desai M, Sharma P. Comparative safety of endoscopic vs radiological gastrostomy tube placement: outcomes from a large, nationwide veterans affairs database. Am J Gastroenterol. 2021;116(12):2367-2373.
doi - Kohli DR, Smith C, Chaudhry O, Desai M, DePaolis D, Sharma P. Direct percutaneous endoscopic gastrostomy versus radiological gastrostomy in patients unable to undergo transoral endoscopic pull gastrostomy. Dig Dis Sci. 2023;68(3):852-859.
doi - La Nauze RJ, Collins K, Lyon S, Bailey M, Kemp W, Nyulasi I, Roberts SK. Outcomes of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy tube insertion at a tertiary hospital. e-SPEN Journal. 2012;7(4):e144-e148.
doi - Laasch HU, Wilbraham L, Bullen K, Marriott A, Lawrance JA, Johnson RJ, Lee SH, et al. Gastrostomy insertion: comparing the options—PEG, RIG or PIG? Clin Radiol. 2003;58(5):398-405.
doi - Laskaratos FM, Walker M, Gowribalan J, Wojciechowska V, Jenkins A. A comparison of outcomes for PEG and RIG insertion in a district general hospital. Clinical Nutrition Supplement. 2012;7(1):254-255.
doi - Leeds JS, McAlindon ME, Grant J, Robson HE, Lee FK, Sanders DS. Survival analysis after gastrostomy: a single-centre, observational study comparing radiological and endoscopic insertion. Eur J Gastroenterol Hepatol. 2010;22(5):591-596.
doi - Maasarani S, Khalid SI, Creighton C, Manatis-Lornell AJ, Wiegmann AL, Terranella SL, Skertich NJ, et al. Outcomes following percutaneous endoscopic gastrostomy versus fluoroscopic procedures in the Medicare population. Surg Open Sci. 2021;3:2-7.
doi pubmed pmc - MacLean AA, Alvarez NR, Davies JD, Lopez PP, Pizano LR. Complications of percutaneous endoscopic and fluoroscopic gastrostomy tube insertion procedures in 378 patients. Gastroenterol Nurs. 2007;30(5):337-341.
doi - McAllister P, MacIver C, Wales C, McMahon J, Devine JC, McHattie G, Makubate B. Gastrostomy insertion in head and neck cancer patients: a 3 year review of insertion method and complication rates. Br J Oral Maxillofac Surg. 2013;51(8):714-718.
doi - McDermott CJ, Stavroulakis T. Gastrostomy in amyotrophic lateral sclerosis: effects of non-invasive ventilation - Authors' reply. Lancet Neurol. 2015;14(12):1153.
doi - Moller P, Lindberg CG, Zilling T. Gastrostomy by various techniques: evaluation of indications, outcome, and complications. Scandinavian Journal of Gastroenterology. 1999;34(10):1050-1054.
doi - Neeff M, Crowder VL, McIvor NP, Chaplin JM, Morton RP. Comparison of the use of endoscopic and radiologic gastrostomy in a single head and neck cancer unit. ANZ J Surg. 2003;73(8):590-593.
doi - Pannick S, Hicks L, Kim J, Velji Z, Colucci K, Wright A, Howson W. Radiologically vs endoscopically-placed gastrostomy feeding tubes: An audit of current practice and clinical outcomes in a large, multi-site UK NHS trust. Endoscopy. 2019;51(4):S85.
doi - Pinar-Gutierrez A, Serrano-Aguayo P, Garcia-Rey S, Vazquez-Gutierrez R, Gonzalez-Navarro I, Tatay-Dominguez D, Garrancho-Dominguez P, et al. Percutaneous Radiology Gastrostomy (PRG)-associated complications at a tertiary hospital over the last 25 years. Nutrients. 2022;14(22):4838.
doi pubmed pmc - Abd Rahim T, Sharratt C, Tinker M, Buchanan T, Bowling T. Gastrostomy insertions: Is it all about choosing the right patients? A comparison between percutaneous endoscopic gastrostomy (PEG) and radiologically inserted gastrostomy (RIG) indications, complications and mortality rates. Gut. 2014;63:A269-A270.
doi - Morris S, Righetti J, Fotoohi M, La Selva D, Kozarek RA. . Is IR gastrostomy placement safer than PEG? A retrospective review of 461 patients from 2014-2019. Gastrointestinal Endoscopy. 2020;91(6):AB624.
doi - Rio A, Ellis C, Shaw C, Willey E, Ampong MA, Wijesekera L, Rittman T, et al. Nutritional factors associated with survival following enteral tube feeding in patients with motor neurone disease. J Hum Nutr Diet. 2010;23(4):408-415.
doi - Rustom IK, Jebreel A, Tayyab M, England RJ, Stafford ND. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol. 2006;120(6):463-466.
doi - Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD, Bettmann MA, et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005;56(1):84-90.
doi - Strijbos D, Keszthelyi D, Gilissen LPL, Lacko M, Hoeijmakers JGJ, van der Leij C, de Ridder RJJ, et al. Percutaneous endoscopic versus radiologic gastrostomy for enteral feeding: a retrospective analysis on outcomes and complications. Endosc Int Open. 2019;7(11):E1487-E1495.
doi pubmed pmc - Tan G, Larion S, Crisona FJ, Harrell S, Rassameehiran S, Bhagatwala J, Rao SS. Is percutaneous endoscopic (PEG) versus percutaneous radiologic gastrostomy (PRG) safer for enteral nutrition: a comparative assessment. Gastrointestinal Endoscopy. 2019;89(6):AB495.
doi - Vashi P, Edwin P, Gupta D, Fulp CJ, Popiel B. Outcomes of endoscopic versus radiologic percutaneous gastrostomy tube placement in adult oncology patients. Gastrointestinal Endoscopy. 2015;81(5):AB234-AB235.
doi - Wollman B, D'Agostino HB. Percutaneous radiologic and endoscopic gastrostomy: a 3-year institutional analysis of procedure performance. AJR Am J Roentgenol. 1997;169(6):1551-1553.
doi - Lipp A, Lusardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev. 2013;2013(11):CD005571.
doi pubmed pmc - Clements W, Shvarts Y, Koukounaras J, Phan TD, Goh GS, Joseph T, Kuang R, et al. Radiologically Inserted Gastrostomy (RIG) at a tertiary center: periprocedural safety including rationalization of antibiotic prophylaxis. Journal of Clinical Interventional Radiology ISVIR. 2021;5(01):11-15.
doi - Ingraham CR, Johnson GE, Albrecht EL, Padia SA, Monroe EJ, Perry BC, Dobrow EM, et al. Value of antibiotic prophylaxis for percutaneous gastrostomy: a double-blind randomized trial. J Vasc Interv Radiol. 2018;29(1):55-61.e52.
doi - Karthikumar B, Keshava SN, Moses V, Chiramel GK, Ahmed M, Mammen S. Percutaneous gastrostomy placement by intervention radiology: Techniques and outcome. Indian J Radiol Imaging. 2018;28(2):225-231.
doi pubmed pmc - Lee C, Im JP, Kim JW, Kim SE, Ryu DY, Cha JM, Kim EY, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: a multicenter, retrospective study. Surg Endosc. 2013;27(10):3806-3815.
doi - Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20(26):8505-8524.
doi pubmed pmc - Ogino H, Akiho H. Usefulness of percutaneous endoscopic gastrostomy for supportive therapy of advanced aerodigestive cancer. World J Gastrointest Pathophysiol. 2013;4(4):119-125.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


