| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 16, Number 1, February 2023, pages 9-16
Endoscopic Ultrasound Predicts Risk of Occult Intra-Abdominal Metastases in Localized Gastric Cancer: A Validation Study
Fares Ayouba, e , Christopher G. Chapmanb, Heather Chenc, Namrata Setiac, Kevin Roggind, Uzma D. Siddiquib
aSection of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, TX 77030, USA
bCenter for Endoscopic Research and Therapeutics (CERT), The University of Chicago Medicine, Chicago, IL 60637, USA
cDepartment of Pathology, University of Chicago Medicine, IL 60637, USA
dDepartment of Surgery, University of Chicago Medicine, IL 60637, USA
eCorresponding Author: Fares Ayoub, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, TX 77030, USA
Manuscript submitted November 20, 2022, accepted January 9, 2023, published online February 28, 2023
Short title: Risk for Intra-Abdominal Metastases in GC
doi: https://doi.org/10.14740/gr1589
| Abstract | ▴Top |
Background: In gastric cancer (GC) patients without imaging evidence of distant metastasis, diagnostic staging laparoscopy (DSL) is recommended to detect radiographically occult peritoneal metastasis (M1). DSL carries a risk for morbidity and its cost-effectiveness is unclear. Use of endoscopic ultrasound (EUS) to improve patient selection for DSL has been proposed but not validated. We aimed to validate an EUS-based risk classification system predicting risk for M1 disease.
Methods: We retrospectively identified all GC patients without positron emission tomography (PET)/computed tomography (CT) evidence of distant metastasis who underwent staging EUS followed by DSL between 2010 and 2020. T1-2, N0 disease was EUS “low-risk”; T3-4 and/or N+ disease was “high-risk”.
Results: A total of 68 patients met inclusion criteria. DSL identified radiographically occult M1 disease in 17 patients (25%). Most patients had EUS T3 tumors (n = 59, 87%) and 48 (71%) patients were node-positive (N+). Five (7%) patients were classified EUS “low-risk” and 63 (93%) were classified “high-risk”. Of 63 “high-risk” patients, 17 (27%) had M1 disease. The ability of “low-risk” EUS to predict M0 disease at laparoscopy was 100% and DSL would have been avoided in five patients (7%). This stratification algorithm showed a sensitivity of 100% (95% confidence interval (CI): 80.5-100%) and a specificity of 9.8% (95% CI: 3.3-21.4%).
Conclusions: Use of an EUS-based risk classification system in GC patients without imaging evidence of metastasis helps identify a subset of patients at low-risk for laparoscopic M1 disease who may avoid DSL and proceed directly to neoadjuvant chemotherapy or resection with curative intent. Larger, prospective studies are needed to validate these findings.
Keywords: Gastric cancer; Endoscopic ultrasound; Diagnostic staging laparoscopy; Laparoscopy; Staging; Imaging; Metastasis
| Introduction | ▴Top |
Gastric cancer (GC) is the fifth most commonly diagnosed cancer in the world, affecting at least 1 million people worldwide in 2018 [1]. In the United States, an estimated 27,600 cases are expected to be diagnosed in 2020 with at least 11,010 deaths [2]. Outside of countries with established screening programs, GC is often detected at an advanced stage with a high fatality rate estimated to be around 75% [3]. At diagnosis, staging cross-sectional imaging with computed tomography (CT) and/or magnetic resonance imaging (MRI) is used to assess for metastatic disease and endoscopic ultrasound (EUS) is recommended for accurate staging including assessment for local lymph node involvement. Patients found to have non-metastatic, localized GC can be offered surgical resection with curative intent.
While the ability of cross-sectional imaging to ascertain distant metastases has improved in recent decades, both CT and MRI continue to lack adequate sensitivity to detect small metastatic deposits on the peritoneal or liver surface [4]. PET/CT is also increasingly used by some centers, but its use remains limited outside of identification of distant hematogenous metastasis due to its limited spatial resolution and high false-positive rate [5]. As such, both the US-based National Comprehensive Cancer Network (NCCN) [6] and European Society for Medical Oncology (ESMO) [7] guidelines recommend the selective use of diagnostic staging laparoscopy (DSL) with or without peritoneal washings to assess for radiographically occult metastatic disease (M1) which would preclude curative resection in those with locally advanced GC.
While DSL spares patients with radiographically occult M1 disease unnecessary laparotomy, it is not without its costs and associated morbidity. A recent cost-effectiveness analysis found DSL not to be cost-effective unless it is used selectively where the procedure yield is expected to be high [8]. It remains unclear what factors may improve patient selection for DSL and which patients may proceed directly to curative laparotomy. Power et al have previously [9] reported on the value of EUS to risk stratify patients into “low-risk” and “high-risk” groups based on EUS TNM staging. In their 2009 study, the authors found that patients considered “low-risk” by EUS criteria (T1-2 tumor and N0 nodal status) had only a 4% chance of having radiographically occult M1 disease at DSL, with a negative predictive value of 96%. All other patients (T3-4 and/or N+) were considered “high-risk” and had a 25% risk of M1 disease at DSL. They proposed that following validation, their staging algorithm may allow those with “low-risk” disease to proceed directly to resection.
The aim of our study was to validate an EUS-based staging algorithm classifying GC patients without positron emission tomography (PET)/CT imaging evidence of metastasis as high or low risk for peritoneal metastasis on DSL.
| Materials and Methods | ▴Top |
The study included patients treated at the University of Chicago Medicine between 2010 and 2020 with pathologically confirmed gastric adenocarcinoma without PET/CT evidence of distant metastasis who had had staging EUS followed by DSL. Institutional review board approval was obtained from the University of Chicago IRB (IRB Study #11-0721-CR010). Data were collected by manual chart review and only patients with complete data were included.
All included patients had undergone cross-sectional imaging of the chest, abdomen, and pelvis (PET/CT with oral and intravenous contrast) and did not have findings suggestive of distant metastasis. Patients subsequently underwent esophagogastroduodenoscopy (EGD) with a forward-viewing endoscope to document the morphology and location of the tumor. This was followed by EUS in standard fashion using radial echoendoscopes from Olympus USA (Center Valley, PA) as indicated to obtain adequate images. Images were interpreted by experienced advanced endoscopists at the time of the procedure. TNM staging was based on the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual for GC [10]. Lesions were considered T1 if they invade up to the submucosa (layer 3), T2 if they invade up the muscularis propria and up to the subserosa (layer 4), T3 if they invade the serosa (layer 5), and T4 if they invade into adjacent structures. Lymph nodes were considered to be malignant if they were larger than 5 mm, hypoechoic, round, and well-delineated. As previously reported [9], EUS was used to stratify patients into “low-risk” or “high-risk” for laparoscopic M1 disease. Patients were considered to be “low-risk” if they were found to have T1-2, N0 disease, and considered “high-risk” if they were found to have T3-4 and/or node positive (N+) disease. N status was assessed at standard lower thoracic and abdominal EUS stations including the paraesophageal, lesser curvature, celiac trunk, mid gastric body and gastric antrum. Figure 1a, b shows EUS findings in a patient classified as “high-risk”.
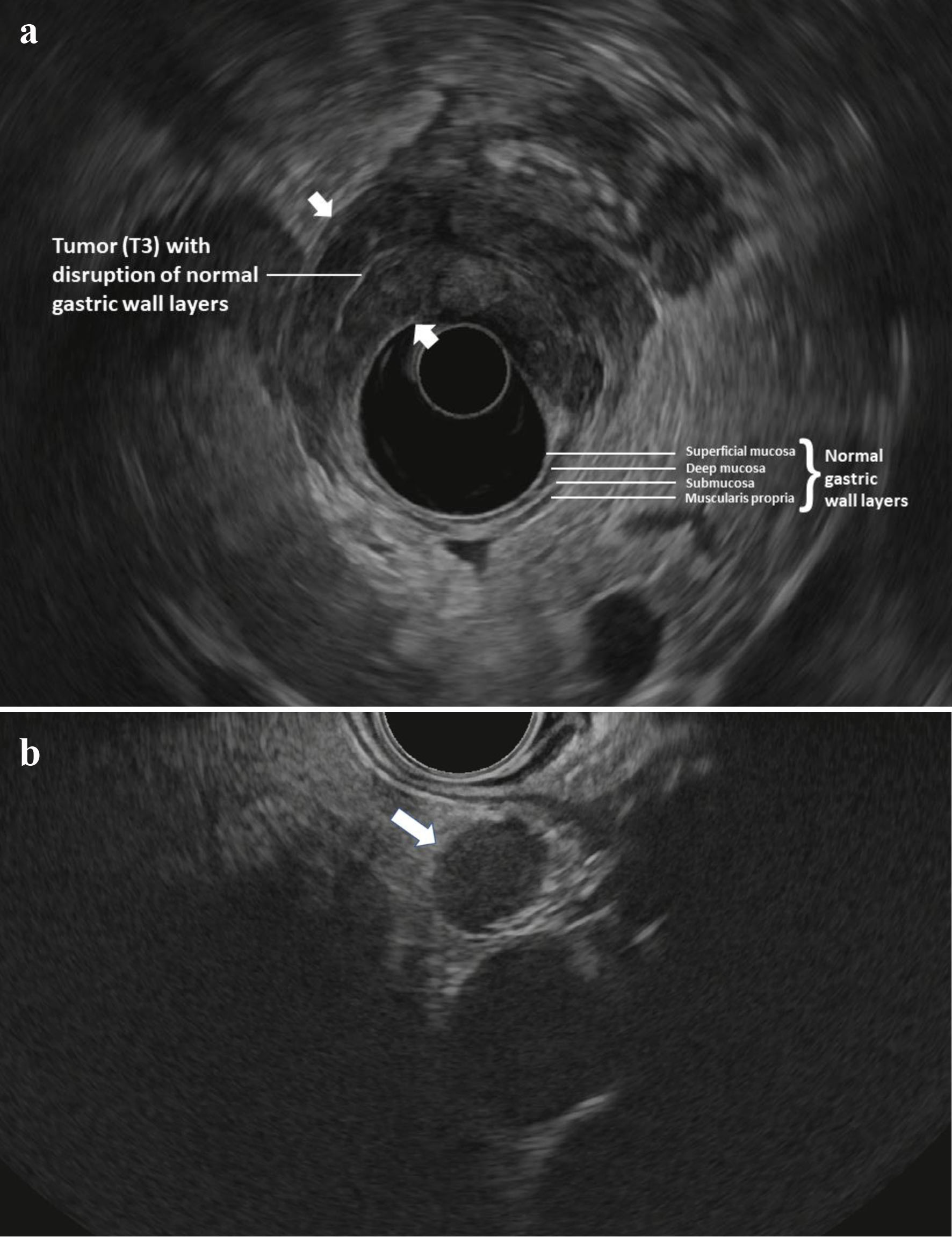 Click for large image | Figure 1. (a) Radial EUS image of a T3 antral tumor showing normal gastric layers on EUS and the large tumor (between arrowheads) disrupting the normal layers and invading through the muscularis propria and into the serosa. This patient was classified as “high-risk” and ultimately was found to have M1 disease on DSL. (b) Linear EUS image of an N+ tumor showing a malignant appearing lymph node (arrow). EUS: endoscopic ultrasound; DSL: diagnostic staging laparoscopy. |
All patients underwent DSL. This was performed in a standard fashion for all patients by placing a 5-mm port through the abdominal wall under direct visualization, followed by insufflation of the peritoneal cavity to 15 mm Hg using CO2 gas followed by complete visualization of the stomach, liver, and entire peritoneal surface. Additional ports were placed in the right and left upper quadrants if required. Peritoneal fluid washings were obtained from the left upper quadrant, right upper quadrant, and pelvis after the instillation of 500 mL of sterile saline into the peritoneal cavity. M1 disease was defined as the presence of pathologically confirmed metastases and/or positive cytology (Fig. 2) not apparent on pre-laparoscopic staging imaging.
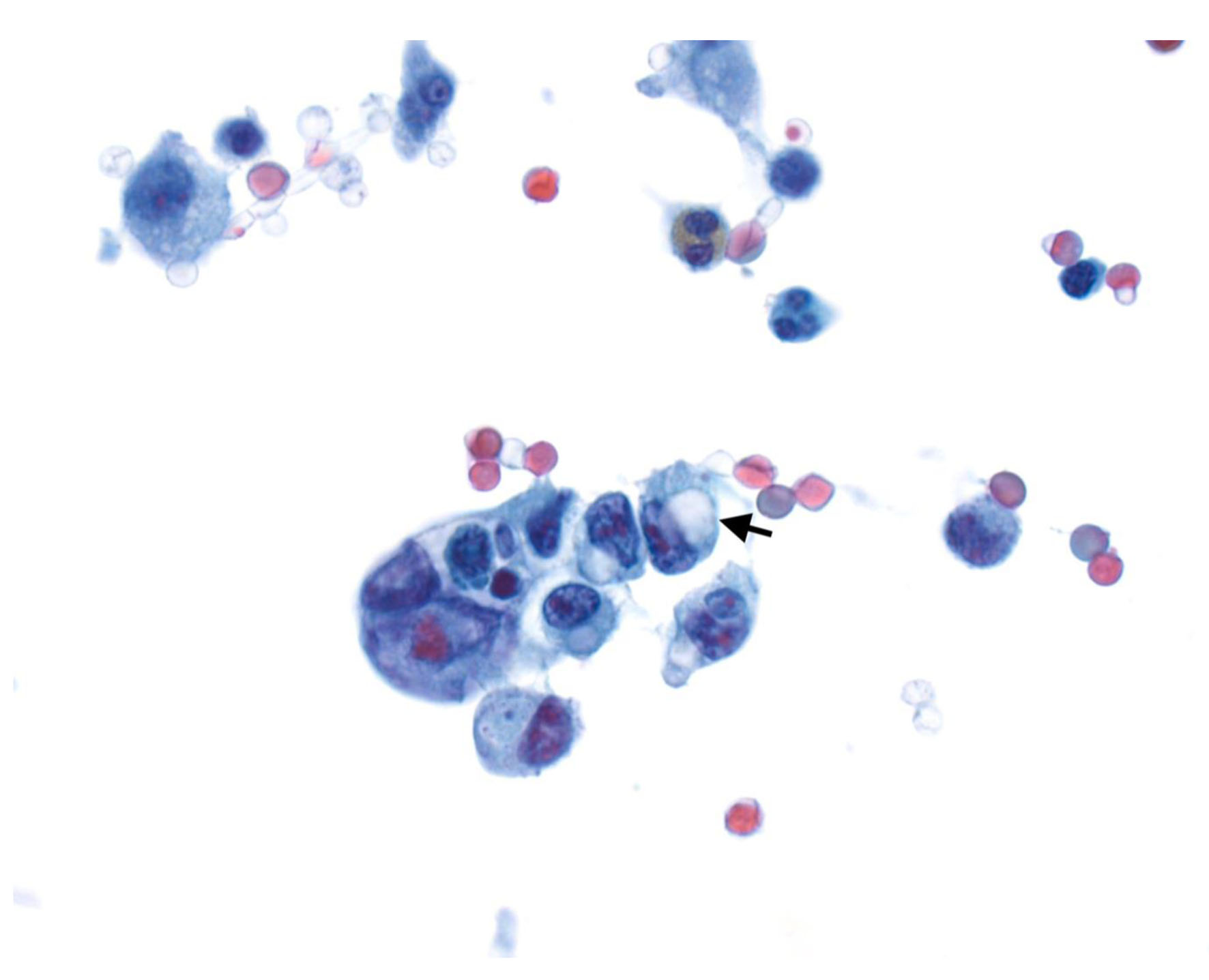 Click for large image | Figure 2. Cytospin from peritoneal fluid shows a cluster of tumor cells with focal signet ring cell differentiation (arrow) (Papinocolou stain, × 600). |
Sensitivity was calculated in standard fashion as (true positive/(true positive + false negative)) and specificity as (true negative/(true negative + false positive)). Pooled sensitivity and specificity analysis was performed by combining data from this study and data from the original Power et al study to generate pooled sensitivity and specifity estimates. Comparisons of categorical variables were performed using Pearson’s Chi-square or Fisher’s exact test, as appropriate. Continuous parametric variables were compared with Student’s t-test and non-parametric variables with the Mann-Whitney U test. A P-value < 0.05 was considered statistically significant and all tests were two-tailed. Stata version 15.0 (Stata Corp., College Station, TX) was used for all statistical analyses.
| Results | ▴Top |
Sixty-eight patients met our inclusion criteria. Diagnostic laparoscopy identified radiographically occult metastatic disease in 17 patients (25%). Sixteen patients had positive cytology (M1 cyt+), five of which also had visible peritoneal/organ metastasis, and only one patient had visible peritoneal metastasis with negative cytology (M1 cyt-).
Patient and tumor characteristics are outlined in Table 1. Overall, 42 patients (62%) were male, and the median age was 64 years with a range of 16 - 87 years. The majority of patients (n = 34, 50%) were Caucasian and 28% (n = 19) were African American. Tumors were most commonly located in the gastric body (37%) and antrum (31%), with a majority (n = 53, 78%) demonstrating poorly differentiated histology. No significant differences in baseline patient characteristics, tumor location or histology were noted between laparoscopic M0 and M1 disease patients.
 Click to view | Table 1. Patient and Tumor Characteristics |
Morphologically, an ulcerated tumor was noted in 47 patients (69%) without differences between M0 vs. M1 patients (Table 2). Significantly more laparoscopic M1 patients demonstrated a linitis plastica appearance (as noted by the endoscopist and defined as a diffusely thickened gastric wall with a limited ability to insufflate the stomach during EGD) (47% vs. 18%, P = 0.015) and circumferential gastric involvement (as noted by the endoscopist and defined as circumferential involvement of the affected gastric segment by tumor as noted on EGD/EUS) (65% vs. 33%, P = 0.023). On EUS, the majority of patients had EUS T3 tumors (n = 59, 87%) and 48 patients had N+ disease (71%). No patients were noted to have EUS evidence of metastases, and only two patients (4%) were found to have ascites on EUS and both had M0 disease on laparoscopy. Five (7%) patients were classified as EUS “low-risk” for M1 disease (T1-T2 and N0) with the remainder (n = 63, 93%) classified “high-risk” (T3-T4 and/or N+).
 Click to view | Table 2. Endoscopic Characteristics |
At DSL, a total of 17 (25%) patients were found to have M1 disease (Table 3). Six (9%) had visible metastatic deposits, four along the peritoneum, one omental, and one with diaphragmatic caking. Seven patients (10%) had ascites. All except one patient underwent diagnostic peritoneal lavage and 16 patients (24%) were found to have positive cytology. Of the 63 “high-risk” by EUS patients, 17 (27%) had laparoscopic M1 disease (Table 4). No patients identified as “low-risk” by EUS staging had laparoscopic M1 disease. Thus, the ability of “low-risk” EUS status to identify M0 disease at laparoscopy was 100%, and DSL would have been avoided in five patients (7%). The test characteristics of this stratification algorithm showed sensitivity of 100% (95% confidence interval (CI): 80.5-100%), specificity of 9.8% (95% CI: 3.3-21.4%), a positive predictive value of 26.9% (95% CI: 25.2-28.8%), and a negative predictive value of 100%.
 Click to view | Table 3. Diagnostic Laparoscopy Characteristics |
 Click to view | Table 4. Endoscopic Ultrasound High-Risk and Low-Risk Subdivision and Laparoscopic Identification of Occult M1 Disease |
Pooled analysis
In a total of 94 GC patients without PET/CT evidence of metastasis identified in the original study by Power et al [9], 68 patients were identified as EUS “high-risk”, and of those 17 (25%) had laparoscopic M1 disease. Of the remaining 26 EUS “low-risk” patients, only one (4%) had laparoscopic M1 disease. We pooled data from the Power et al study with ours to generate pooled sensitivity and specificity with 95% CIs. Overall, pooled test characteristics from the combined 168 patients showed a sensitivity of 97.1% (95% CI: 85-99.9%), a specificity of 23.6% (95% CI: 16.5-31.9%) and an overall test accuracy of 39.5% (95% CI: 31.9-47.5%). A total of 31 patients (18.4%) would have avoided DSL and proceeded directly to laparotomy, only one of which would be noted to have intra-peritoneal metastasis (from the original Power et al cohort).
Sensitivity analysis
Given some concerns about the operator dependency of EUS in the determination of EUS N stage in the literature, we performed a sensitivity analysis using only patient T status without their N status to determine their risk group. Our findings were nearly identical, since only one patient who had a T2 N1 tumor moved into a “low risk” category, but was found to have M0 disease at DSL.
| Discussion | ▴Top |
In this retrospective analysis of 68 GC patients without PET/CT evidence of distant metastases, we validated an EUS-based, risk stratification algorithm classifying patients into “low-risk” and “high-risk” categories for radiographically occult M1 disease on DSL. The stratification algorithm showed a sensitivity of 100% and patients stratified as “low-risk” on EUS (T1-T2 and N0) had a 0% chance of having M1 disease on DSL.
To our knowledge, the pilot study by Power et al and our current validation are the only analyses studying EUS as a risk stratification tool to improve patient selection for DSL. Power et al studied 94 patients without radiographic evidence of metastasis and found 19% to have occult M1 disease at laparoscopy, similar to our observed findings. A minority of patients (n = 26, 28%) in their study had “low-risk” findings on EUS (T1-2 and N0) and only one of those (4%) had M1 disease at DSL. EUS “high-risk” patients (T3-4, N+, or both) were found to have M1 disease in 25% of cases (similar to our analysis where we found 27%). These findings, now validated by our study, suggest that this algorithm is best used to identify those patients at “low-risk” for M1 disease on DSL, allowing such a select group to avoid DSL and proceed directly to neoadjuvant therapy or curative resection. Indeed, when we pooled our patient-level data with data from the original Power et al study, we found that from the combined cohort of 168 patients, a total of 31 patients (18.4%) would have avoided DSL and proceeded directly to laparotomy, only one of which would be noted to have intra-peritoneal metastasis (from the original Power et al cohort).
Up to 41% of all GC patients harbor occult intra-abdominal metastatic disease, despite no identifiable metastatic disease on preoperative cross-sectional imaging (CT or MRI), PET scans and/or EUS [11]. Identification of positive cytology has been found to be the strongest independent preoperative predictor of GC recurrence and mortality highlighting the importance of DSL with peritoneal lavage and cytology [12]. Despite the importance of cytology, we have previously shown that M1 disease may be missed without visual inspection making both cytological examination and visual inspection critical aspects of successful DSL [13]. As such, DSL has been advocated by both US and European guidelines [6, 7] to help patients avert the morbidity of non-therapeutic laparotomy. National-level US data suggest up to one-third of patients experience a complication following total gastrectomy for gastric malignancy and up to 5% die within 30 days [14]. While these rates may be lower in non-therapeutic laparotomies and at expert centers, some data suggest a proportion of patients with noted occult metastases at laparotomy may still undergo palliative gastrectomy or bypass in the same session with subsequent morbidity and mortality [11].
DSL has been shown to have excellent sensitivity at detecting intra-abdominal metastatic disease; Ramos et al performed a meta-analysis of five studies with 240 participants and found an overall pooled sensitivity of 84.6% and specificity of 100% [15]. However, despite the known benefits of DSL and data on the futility of attempting gastrectomy in those with intra-abdominal metastases [16, 17], its use in GC patients has been limited. In a Canadian study of 2,399 GC patients by Coburn et al, only 12.8% underwent DSL [18]. Even lower rates were noted in a 2011 US population study of 6,388 GC patients aged 65 and above with only 7.9% of patients undergoing DSL [11]. However, rates of DSL in the above US study doubled between 1998 and 2005 indicating increasing uptake of the procedure by US-based surgeons.
Any discussion of an algorithm invoking EUS to stratify patients for DSL must take into consideration its diagnostic accuracy. In a 2015 Cochrane review of EUS T and N staging in 50 studies and a total of 4,397 GC patients, authors found excellent sensitivity and specificity in discriminating T1 to T2 from T3 to T4 gastric carcinomas as compared to pathology evaluation (as the reference standard), at 86% and 90%, respectively [19]. Similarly, sensitivity and specificity for the metastatic involvement of lymph nodes (N-stage) were 83% and 67%, respectively. These findings suggest that despite the operator-dependent nature of many ultrasound-based imaging modalities, an algorithm using EUS staging criteria to determine who undergoes DSL can have sufficient sensitivity and specificity to be used broadly.
While DSL is recommended by society guidelines and its uptake in the US is increasing, its cost-effectiveness has recently been scrutinized in an analysis by Li et al [8]. In their analysis of GC patients without imaging evidence of metastasis, they found that preoperative DSL required an investment of $107,012 per quality-adjusted life year (QALY) and would only meet cost-effectiveness benchmarks compared to a direct-to-surgery approach if the probability of occult metastases was greater than 31.5% or when using a preoperative imaging modality with a sensitivity for occult metastases greater than 86.3%. The pooled sensitivity of “low-risk” EUS stage, as validated in our analysis, at 97.1% would theoretically allow DSL to meet standard cost-effectiveness thresholds under most scenarios, further supporting the use of such a stratification algorithm. In fact, an estimated $3,890 (range $2,723 - $6,223) would be saved per “low-risk” patient identified on EUS by foregeoing DSL (using 2016 US dollars and only including direct costs at Medicare reimbursement levels including provider, facility, and anesthesiology components of payment) [8].
While our primary aim was to validate the EUS-based, risk stratification algorithm initially proposed by Power et al, we also performed analyses to identify any endoscopic features that may predict M1 disease status. We found significantly more laparoscopic M1 patients demonstrated a linitis plastica appearance (47% vs. 18%, P = 0.015) and circumferential gastric involvement (65% vs. 33%, P = 0.023). Linitis plastica has been associated with an advanced cancer stage and found to be a poor marker for survival [20]. The association of linitis plastica with M1 disease has previously been reported, with one study finding laparoscopic M1 disease in 77% of all linitis plastica patients at laparoscopy [21]. As above, circumferential gastric involvement was also found to be a significant predictor of M1 disease. This is likely a surrogate indicator of advanced tumor size, which is known to be associated with a poor prognosis [22]. Taking into account the subjective nature of “linitis plastica”, if validated, both of these factors may be incorporated in future risk assessment scores that include both endoscopic and ultrasonographic criteria, possibly enhancing the sensitivity and specificity of the final algorithm.
Our analysis may be limited by its retrospective nature, although we included all GC patients without PET/CT evidence of metastases at our institution, limiting the scope of possible selection bias. In fact, external validation using a retrospective approach is not thought to be inferior to a prospective one as highlighted by Altman et al in their highly cited reference article on model validation methods “What do we mean by validating a prognostic model?” [23]. Optimally, a trial randomizing patients to DSL versus a direct-to-surgery approach based on EUS findings would provide the best data to support such an approach. Our study provides additional data that may facilitate such a future study. Our center being a tertiary referral facility may have skewed GC cases to a slightly more advanced stage; only about 9% of our patients had T1-T2 disease perhaps limiting the generalization of our findings. However, such a low proportion of early-stage disease is more likely explained by the delayed nature of GC diagnosis in countries without an established screening program such as the United States.
In conclusion, we found that the use of an EUS-based risk classification system in GC patients without PET/CT evidence of metastasis may help identify a subset at low-risk for laparoscopic M1 disease with excellent sensitivity. Such patients may potentially avoid DSL and proceed directly to neoadjuvant chemotherapy or resection with curative intent. More prospective data are warranted to validate these criteria.
Acknowledgments
None to declare.
Financial Disclosure
No grants or funding was used to support this work.
Conflict of Interest
The authors have no conflict of interest to disclose relevant to this manuscript.
Informed Consent
This was a retrospective study and informed consent was waived per the approval of the institutional review board given the deidentified nature of the retrospective analysis.
Author Contributions
Fares Ayoub contributed to the study conception, data collection, and statistical analysis, and drafted and revised the manuscript. Uzma D. Siddiqui contributed to the study conception, and drafted and revised the manuscript. Christopher G. Chapman, Heather Chen, Namrata Setia, and Keven Roggin drafted and revised the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CI: confidence interval; DSL: diagnostic staging laparoscopy; EUS: endoscopic ultrasound; GC: gastric cancer; PET: positron emission tomography
| References | ▴Top |
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
doi pubmed - Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250-260.
doi pubmed - Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, Law C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer. 2012;15(Suppl 1):S3-18.
doi pubmed - Akin EA, Qazi ZN, Osman M, Zeman RK. Clinical impact of FDG PET/CT in alimentary tract malignancies: an updated review. Abdom Radiol (NY). 2020;45(4):1018-1035.
doi pubmed - Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(10):1286-1312.
doi pubmed - Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, Committee EG. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-v49.
doi pubmed - Li K, Cannon JGD, Jiang SY, Sambare TD, Owens DK, Bendavid E, Poultsides GA. Diagnostic staging laparoscopy in gastric cancer treatment: A cost-effectiveness analysis. J Surg Oncol. 2018;117(6):1288-1296.
doi pubmed - Power DG, Schattner MA, Gerdes H, Brenner B, Markowitz AJ, Capanu M, Coit DG, et al. Endoscopic ultrasound can improve the selection for laparoscopy in patients with localized gastric cancer. J Am Coll Surg. 2009;208(2):173-178.
doi pubmed - Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077-3079.
doi pubmed - Karanicolas PJ, Elkin EB, Jacks LM, Atoria CL, Strong VE, Brennan MF, Coit DG. Staging laparoscopy in the management of gastric cancer: a population-based analysis. J Am Coll Surg. 2011;213(5):644-651.e641.
doi pubmed - Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12(5):347-353.
doi pubmed - Mezhir JJ, Posner MC, Roggin KK. Prospective clinical trial of diagnostic peritoneal lavage to detect positive peritoneal cytology in patients with gastric cancer. J Surg Oncol. 2013;107(8):794-798.
doi pubmed - Bartlett EK, Roses RE, Kelz RR, Drebin JA, Fraker DL, Karakousis GC. Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of Surgeons National Surgical Quality Improvement Program database. Surgery. 2014;156(2):298-304.
doi pubmed - Ramos RF, Scalon FM, Scalon MM, Dias DI. Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur J Surg Oncol. 2016;42(9):1315-1321.
doi pubmed - Sarela AI, Miner TJ, Karpeh MS, Coit DG, Jaques DP, Brennan MF. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg. 2006;243(2):189-195.
doi pubmed - Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309-318.
doi pubmed - Coburn NG, Lourenco LG, Rossi SE, Gunraj N, Mahar AL, Helyer LK, Law C, et al. Management of gastric cancer in Ontario. J Surg Oncol. 2010;102(1):54-63.
doi pubmed - Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2015(2):CD009944.
doi - Blackham AU, Swords DS, Levine EA, Fino NF, Squires MH, Poultsides G, Fields RC, et al. Is Linitis Plastica a Contraindication for Surgical Resection: A Multi-Institution Study of the U.S. Gastric Cancer Collaborative. Ann Surg Oncol. 2016;23(4):1203-1211.
doi pubmed - Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Yamamura Y, Fujiwara M, Hibi K, et al. Detection of disseminated cancer cells in linitis plastica-type gastric carcinoma. Jpn J Clin Oncol. 2004;34(9):525-531.
doi pubmed - Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Oro S, Tatebe S, et al. Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg. 2006;192(3):296-300.
doi pubmed - Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453-473.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


