| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Review
Volume 15, Number 6, December 2022, pages 308-313
Gastrocolic Fistula: An Extraordinary Gastrointestinal Fistula
Subhi Mansoura, Rozan Marjiyeh-Awwada, Safi Khurib, c
aDepartment of General Surgery, Rambam Health Care Campus, Haifa, Israel
bHepatoPancreatoBiliray (HPB) and Surgical Oncology Unit, Rambam Health Care Campus, Haifa, Israel
cCorresponding Author: Safi Khuri, HPB and Surgical Oncology Unit, General Surgery Department, Rambam Health Care Campus, Haa’leya Hashniya, Haifa 31096, Israel
Manuscript submitted October 5, 2022, accepted November 10, 2022, published online December 1, 2022
Short title: Gastrocolic Fistula
doi: https://doi.org/10.14740/gr1576
- Abstract
- Introduction
- Historical Perspectives and Classification Systems
- Etiological Causes
- Clinical Presentation
- Diagnosis
- Management
- Summary
- References
| Abstract | ▴Top |
Gastrocolic (GC) fistula, a rare gastrointestinal pathological condition, is defined as an abnormal connection between the stomach and the colon. Mostly, it involves the greater curvature of the stomach and the transverse part of the colon. Its precise incidence rate is unknown and largely differs between western and eastern nations. Etiological causes differ as well between the two worlds. Although several precipitating diseases are reported, nowadays, the most common causes are malignant diseases of the stomach (eastern countries) and colon (western world). Patients with GC fistulas usually present late and complain mainly of vomiting, diarrhea, and severe weight loss. This in turn leads to malnutrition, vitamin deficiencies and electrolyte disturbances. Being a rare condition, and usually forgotten, diagnosis is usually challenging to the treating physicians. Workup usually involves a combination of radiological and endoscopic tests. Long-term survival is unknown, and patients usually have poor prognosis. The aim of this review is to summarize the relevant articles in the English literature for this abnormal medical condition, with emphasis on the different etiologies, pathogenesis, clinical presentation, and management, in order to increase physicians’ awareness of such uncommon medical problem.
Keywords: Gastrocolic fistula; Uncommon fistula; Malignant disease; Benign ulcer; Barium enema; Surgical management
| Introduction | ▴Top |
Gastrocolic (GC) fistula is defined as an abnormal pathological communication between the epithelialized mucosal layer of the stomach and the large bowel [1]. It is very rare gastrointestinal medical condition, and its precise incidence is unknown and differs between the western and eastern nations. In western countries, its incidence following operations is about 0.4% [2]. It is a disease of females between ages 50s and 60s (female/male (F/M) ratio: 2:1). On the other hand, in eastern world, mainly Japan, it is more common in males with an M/F ratio of 5:2 [3]. Although any part of both organs can be involved, the abnormal fistulous connection usually develops between the greater curvature of the stomach and distal part of the transverse colon [4]. This is mainly due to the close proximity between the aforementioned organ parts, being separated only by the GC ligament. Sometimes, a third gastrointestinal organ may be involved as well. In spite of a variety of therapeutic approaches (invasive and noninvasive), patients usually suffer from poor prognosis, mainly due to late diagnosis and therefore late intervention [3]. Long-term survival is usually unknown due to a lack of reported long-term follow-up.
| Historical Perspectives and Classification Systems | ▴Top |
The first case of GC fistula was described by Albrecht von Haller in 1755 [5]. In this case, which was not reported to the English literature, the patient suffered from an advanced malignant tumor of the stomach. It is in 1920 that the first case of GC fistula was reported in the English literature, by Douglas Firth [6]. Benign gastric ulcer was the primary pathological disease causing this fistula. Worth mentioning, during the early years of the previous century, only malignant diseases were suspected to be the causative factors for GC fistulas. It is largely due to Douglas’s reported case, which has increased physicians’ awareness, especially during the second half of the previous century, for benign diseases and medical treatment as an etiology for GC fistulas.
Several classification systems have been developed to classify GC fistulas depending on daily amount of secretions, mode of formation, or according to involved organs [7]. It is divided into low-output fistula when daily amount is less than 500 mL and high-output when daily amount is more than 500 mL. On the other hand, according to formation mode, it is divided into primary and secondary, when it develops spontaneously or following surgical resection of the stomach or percutaneous endoscopic gastrostomy (PEG) migration, respectively [8]. The most commonly used classification system, which divides GC fistulas into three types, depends on accompanied involved organs: internal (between the stomach and the colon: GC/cologastric depending on the primary organ of fistula origination), external (between the colon and the skin (colocutaneous fistula)) and complicated when a third organ other than the stomach and colon is also involved (such as gastropancreatocolic/gastrojejunocolic fistulas) [9, 10]. The latter type is the least common and the most difficult to manage.
| Etiological Causes | ▴Top |
As have been mentioned before, the etiological factors that can cause the development of GC fistulas have undergone many changes over the years during the last century. This in turn, increased physicians’ awareness in treating this rare group of patients. Three periods, in terms of causative factors can be noticed: 1) early 1990s, during which malignant tumors were thought to be the sole precipitating factors for GC fistulas; 2) late 1990s when benign diseases, especially benign gastric ulcers, were highly recognized and became the most common causes; and 3) early 2000s, which demonstrates dramatic decrease in reports of GC fistulas due to gastric ulcers. Nowadays, it is well known that malignant as well as benign gastrointestinal diseases can lead to GC fistula. In addition, some medical treatment may play a role in the etiology.
The most common cause of GC fistula in adults is malignant disease of the stomach or colon [11]. In the eastern world, gastric cancer is the most frequent cause, while carcinoma of the colon, especially the transverse part, is the most common in western countries. GC fistula due to malignant disease is usually rare and a late complication, mainly due to delayed tumor detection. This type of fistula may constitute a sign of advanced stage of the tumor due to direct invasion of adjacent organ and signals poor prognosis [3]. Other less common malignant tumors that can cause GC fistula include lymphoma of the stomach, Hodgkin lymphoma, carcinoid tumor of the colon, locally advanced tumors of the duodenum, pancreas, or biliary tree or metastatic lesions to the stomach [7, 12]. In his case, Chiang et al described a female patient with cervical cancer, who developed metastatic lesion to the stomach in the form of ulcer developing GC fistula [12]. Few cases of primary gastric lymphoma were reported as the cause of GC fistula [13-15]. In most of them, advanced stage non-Hodgkin large B-cell lymphoma is the most common lymphoma subtype. Lynch et al [16] described a case of long-term survival following surgical management of GC fistula due to carcinoid tumor of the transverse colon.
A long list of benign diseases causing GC fistula is already mentioned.
Crohn’s disease
Crohn’s disease is a well-known fistulous gastrointestinal disease, of which GC fistula is an extremely rare complication [17]. The most accepted theory for GC fistula development in patients with Crohn’s disease is the transmural involvement of a segment of the transverse colon with secondary involvement of the stomach (cologastric fistula). The first case of GC fistula due to Crohn’s disease was described by Bargan et al in 1937 [18]. Very few cases were reported to the English literature since then. Wu et al [19] described a case of young patient with Crohn’s disease who suffered from GC fistula and was missed by the initial workup which included upper and lower endoscopy as well as abdominal computed tomography (CT) scan. The patient was treated by surgical means.
Benign gastric ulcer disease
Benign gastric ulcer disease is also regarded as a potential cause for GC fistula. The first case was reported, as already mentioned, in 1920 [6]. Since then, several reports in the form of case reports and case series were published. Abeygunasekera et al [10] reported a case of complicated GC fistula (gastropancreaticocolic fistula) due to benign gastric ulcer that was treated by non-surgical measures. A case series of 14 patients [20], most of which were males in their 40s was reported. In this case series, diagnosis was made mostly by barium swallow or enema, and the patients were treated successfully either by surgical (eight patients) or by conservative (non-surgical/six patients) means. One case of mortality (due to line sepsis) was reported in the non-surgical group of patients. Worth mentioning, most of the reported case studies in the English literature were before the era of proton pump inhibitors (PPIs) management (before 1990); for later on, a decrease in reported cases was observed. This demonstrates a marked improvement in the treatment of patients with benign gastric ulcer.
Chronic relapsing pancreatitis and acute necrotizing pancreatitis
GC fistula may develop as a complication of chronic relapsing pancreatitis as well as acute necrotizing pancreatitis [21, 22]. Fistula formation is an uncommon yet reported complication following severe episode of necrotizing infected pancreatitis. Although any part of the gastrointestinal tract may be affected, the colon is the most common organ to be involved by a fistulous tract [23]. Multiple theories can explain this rare complication: 1) erosion theory: pancreatic enzymes erosion into adjacent organs wall, which in turn leads to degradation of both stomach and colonic walls and fistula formation; 2) ischemic theory: severe inflammatory process causing thrombosis of arterial supply and venous drainage, which in turn causes ischemic changes and possible fistula formation; 3) surgical theory: following surgical intervention (necrosectomy vs. drainage) with possible iatrogenic injury. Up to date, very few case reports (less than five) of GC fistulas following pancreatitis were reported.
Diverticulitis
Diverticulitis of the large bowel, especially at the splenic flexure, was mentioned as a cause for GC fistula [24]. Reviewing the current English literature reveals only one case report of diverticulitis causing GC fistula.
Medications
Medications, especially aspirin, steroid, non-steroidal anti-inflammatory drugs (NSAIDs) and adrenocorticotropic hormone (ACTH), are rare reported causes of GC fistulas. The relationship between the increased usages of these medications, mainly aspirin, has been studied extensively. In a case series including eight patients with radiologically proven GC fistula, aspirin-induced ulcer at the greater curve of the stomach was the cause in half of the patients (four patients) [25]. Other report described aspirin-induced ulcer resulting in GC fistula formation in a middle-aged female patient [26]. Abusers of other NSAIDs types and steroids have a higher risk for ulcer-induced GC fistula [27].
PEG
PEG placement is an effective technique, used nowadays for enteral feeding for patients with inability for self-oral feeding. Although regarded as a safe procedure, complications such as infection, leakage, free perforation, displacement, and GC fistulas are reported, with the latter being a very rare complication. Huang et al [28] described the case of GC fistula 2 months following PEG insertion, when patient complaint was the passage of undigested food by stool. PEG causing GC fistula has been reported in different articles as well [29, 30]. The mechanism includes either continuous irritation with chronic inflammation or direct invasion of the colon and fistula formation.
Gastric operations
Gastric operations have been regarded as a benign cause for GC fistulas. The history of the first case dates back to 1948 [31], when a complicated type of GC fistula (involving the stomach, jejunum and transverse colon) developed following surgical vagotomy done for gastric ulcer. During the last two decades, a dramatic increase in bariatric surgeries for the management of morbid obesity has been noticed. Since then, several reports for GC fistula mainly following laparoscopic sleeve gastrectomy, and to a lesser extent, following Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric band (LAGB) have been reported. Few cases (less than 10) of GC fistulas after sleeve gastrectomy have been described [32-34]. The most feared complication following this procedure is stapler line leakage, with an incidence rate of 1-7% [35]. Leak is usually classified as acute (within 1 week of index operation), early (1 - 6 weeks), late (6 - 12 weeks) and chronic (more than 12 weeks) [36]. GC fistula following sleeve gastrectomy develops usually in patients suffering from chronic leak. The suggested pathogenesis is as follow: chronic leak leads to a chronic inflammatory process and adhesions of the stomach to the colon, usually the transverse part, this in turn leads to continuous irritation and fistula formation. Up to date, only two cases of GC fistulas, as a late complication following RYGB have been reported in the English literature [37, 38]. The exact pathogenesis for this complication is unknown, yet it is believed that chronic marginal ulcer at the gastrojejunal anastomosis is the precipitating etiology. GC fistula may develop following LAGB [39], due to colonic wall penetration by the band.
Other rare causes
Extremely rare causes of GC fistulas include: tuberculosis, trauma, cytomegalovirus infection of the stomach, colitis and following coronary artery bypass with right gastroepiploic artery involvement [7, 40].
GC fistula has been described also in the pediatric age group of patients, with causes being inadvertent ingestion of foreign body and corrosive acid material. A case of multiple magnets [41] ingestion causing GC fistula was reported. An endoscopic trial for magnets removal failed and the child was consequently treated by surgical means. Ingestion of bathroom floor cleaning acid was the cause in another case of a 14-year-old girl [42], treated conservatively with spontaneous fistula healing after 3 months. The pathogenesis for the aforementioned causes in children is unknown.
| Clinical Presentation | ▴Top |
Patients with GC fistulas can present with any gastrointestinal symptom, of which the most common is the clinical triad of vomiting, diarrhea, and weight loss [5, 6, 11]. This in turn may cause severe malnutrition. The vomitus is usually of fecal odor or contents, while the diarrhea is usually acidic due to the rapid passage of gastric acidic contents to the large bowel. Although diarrhea has been mentioned as one of the clinical triad, its presence is highly variable and depends on several fistula-related factors such as: fistula orifice diameter, length of the fistulous tract and the presence/absence of obstruction [43]. Hence, some patients may present with constipation rather than diarrhea. About 30% of patients with GC fistula reported gastrointestinal bleeding in the form of melena, hematochezia, or hematemesis [10]. Other less common documented symptoms include abdominal pain, nausea, fecal halitosis, dizziness, and general weakness.
The previously mentioned clinical symptoms may result in malnutrition and anorexia, dehydration and electrolyte disturbances, metabolic disorders, vitamins deficiency, anemia, sepsis, acute kidney injury and in severe forms multi-organ failure.
| Diagnosis | ▴Top |
Diagnosis of GC fistula is very challenging to the treating physicians, and high index of suspicion is warranted for early diagnosis and treatment. Usually, a combination of tests including radiological exams and upper/lower endoscopy is needed.
An abdomino-pelvic CT scan with oral contrast is a very good diagnostic model, yet it lacks high rates of sensitivity and specificity [11, 44]. This radiological exam is essential as it can identify the underlying cause and extent of the disease (in the case of malignancy or inflammatory process) and length of the fistulous tract. Endoscopy, either lower or upper, should not be regarded as the initial tests during work up for GC fistula, as these tests are highly dependent on the diameter of the fistula orifice as small orifices are easily missed [45]. On the contrary, gastroscopy and colonoscopy are crucial to exclude an underlying gastric or colonic malignancy as predisposing diseases, respectively.
The diagnostic method of choice for GC fistula is barium enema. The diagnostic accuracy rate for such modality is up to 95% [46]. Barium swallow is another reliable diagnostic tool, yet its diagnostic rates are very low at 25% only (Fig. 1).
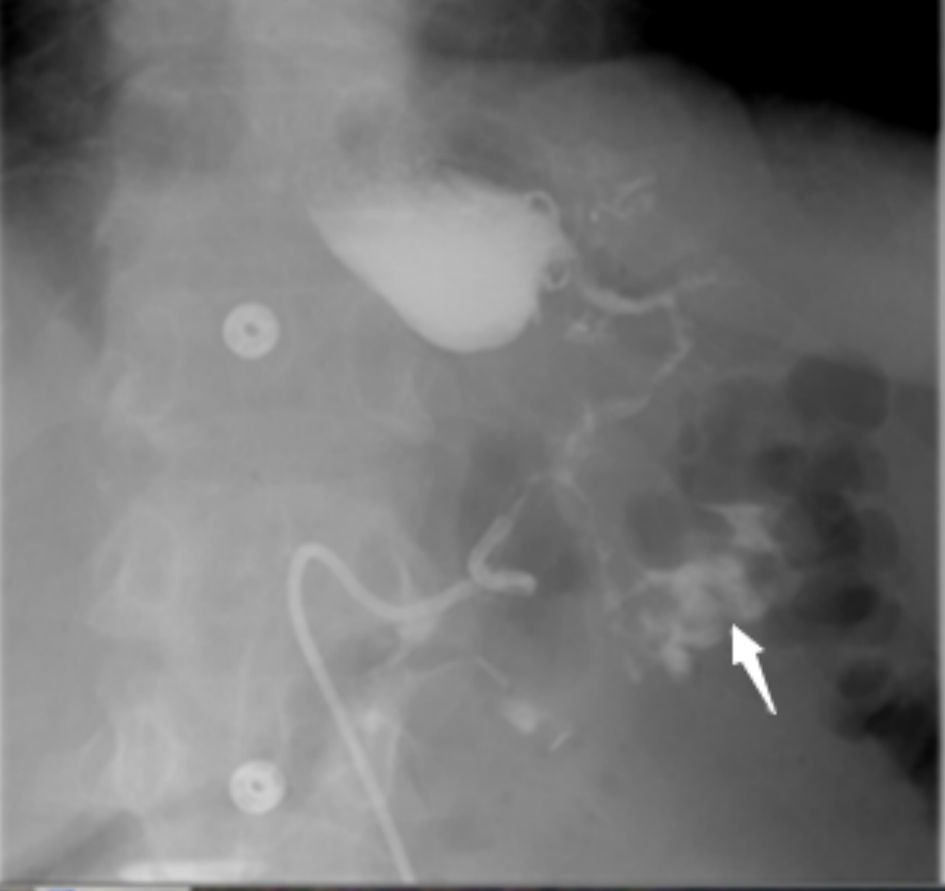 Click for large image | Figure 1. Barium swallow test demonstrates leakage of contrast from the upper part of the stomach with communication (fistula) to the transverse colon and contrast material filling the colon (arrow). |
| Management | ▴Top |
Management of GC fistula is highly variable and depends mainly on the underlying pathological disease. As most patients present initially with vomiting, diarrhea, dehydration, malnutrition, and electrolyte disturbances, we recommend the step wise approach. Initial supportive measures including rehydration with intravenous fluids, electrolyte disturbances and acute kidney injury correction, vitamin supplements, and nutritional support rich in protein and carbohydrates, usually by means of total parenteral nutrition (TPN) is highly advisable for few days [47].
In general, the mainstay of treatment is surgical resection of the fistulous tract alone or along with the involved organs.
In the case of malignant disease of the stomach or colon, a complete systemic workup is recommended by chest and abdominopelvic CT scan and positron emission tomography (PET) scan. Surgical resection of the fistula along with the involved organs is the therapeutic option of choice, even for palliative measures in patients with metastatic disease (stage IV disease). As these patients are already debilitated due to diarrhea and malnutrition, they will not tolerate the systemic side effects of chemo-radiotherapy.
Recently, endoscopic management using various endoscopic devices has become an accepted initial therapy, especially for benign diseases causing GC fistula. Available endoscopic devices include loops, hemoclips, over the scope clip (OTSC) system, fibrin glue injection and endoscopic stents [48]. The success rate for the aforementioned devices depends on fistula-related factors, such as fistula orifice diameter and consistency of the surrounding tissues. Large orifices and chronic fibrotic surrounding tissues are negative predictive factors for successful endoscopic management, mainly by loops or hemoclips. Endoscopic injection of fibrin glue into the fistulous tract have been reported with variable success rates [49]. In his case, Monkemuller et al [48] report a successful endoscopic closure of a GC fistula, following complex upper gastrointestinal surgery, by OTSC system. A successful endoscopic closure of GC fistula following PEG insertion by OTSC system has also been reported in another case report [50]. A case series including three patients with complicated GC fistula (gastrocolocutaneous fistula) following endoscopic gastrostomy was reported by Nunes et al [51]. Patients were treated conservatively with spontaneous fistula closure in all patients, followed by combined endoscopic and laparoscopic fistula resection and gastrostomy in two patients. Sometimes, more than one endoscopic trial is needed to achieve complete closure of the fistula. Most of the reported cases and case series for benign diseases were treated by surgical resection. Although two-stage procedures, with resection of the fistula along with the involved organs and end colostomy was the preferred operation, nowadays, in the absence of severe ongoing inflammatory process, a single-stage procedure is highly recommended [45].
Worth mentioning, all cases of GC fistula following bariatric procedures were treated by surgical means, except one [52], which was treated successfully by endoscopic measures and primary closure of the fistulous orifices by hemoclips.
Some authors recommend the use of somatostatin analogue (octreotide) as an adjunct, to decrease the gastrointestinal secretions. Although the relationship between this medication and pancreatic fistulas have already been studied, its potential use and success rates in treating GC fistulas are largely unknown up to date [20].
In some cases, the fistula healed spontaneously with supportive management only [42], or by acid reducing agents (PPIs/ H2 blockers) when the underlying pathological disease was benign gastric ulcer [53].
| Summary | ▴Top |
The term GC fistula is a very old medical term, which dates back to more than 100 years. Its history has experienced dramatic changes in terms of etiology, diagnosis as well as management. Although GC fistula is a well-studied medical disease, it still poses great challenge to the treating medical team. This is mainly due to it being uncommon and highly forgotten. Patients usually present late and have poor prognosis. Several pathological diseases have been raised and proved as etiological precipitating factors, with malignant diseases of the stomach and colon being the most common. Of the available tests for diagnosis, barium enema has the highest diagnostic rates, yet a combination of radiological and endoscopic exams is needed for a complete workup. Management should be headed by a multidisciplinary team that includes surgeons, gastroenterologists, radiologist, nutritionists, and oncologists (in the case of malignant disease) in a stepwise manner. Supportive management is the initial therapeutic step followed by conservative, endoscopic or surgical management, depending on the etiological medical cause.
Acknowledgments
None to declare.
Financial Disclosure
This is a self-funding article and none to be declared.
Conflict of Interest
The authors have no conflict of interest to declare.
Author Contributions
S. Mansour and R. Marjiyeh-Awwad collected the data and designed the review. S. Khuri wrote and approved the final paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
GC: gastrocolic; CT: computed tomography
| References | ▴Top |
- Lim CH, Kim SW, Kim JS, Cho YK, Park JM, Lee IS, Choi MG, et al. Successful palliation of a gastrocolic fistula secondary to gastric cancer by insertion of a covered colonic stent. Gastrointest Endosc. 2011;73(6):1314-1317.
doi pubmed - Marshall SF, Knud-Hansen J. Gastrojejunocolic and gastrocolic fistulas. Ann Surg. 1957;145(5):770-782.
doi pubmed - Matsuo S, Eto T, Ohara O, Miyazaki J, Tsunoda T, Kanematsu T. Gastrocolic fistula originating from transverse colon cancer: report of a case and review of the Japanese literature. Surg Today. 1994;24(12):1085-1089.
doi pubmed - Mallaiah L, Brozinsky S, Fruchter G, Siraj Uddin M. Malignant gastrocolic fistula case report and review of the literature. Am J Proctol Gastroenterol Colon Rectal Surg. 1980;31(11):35442557.
- Haller A. Opuscula pathologica, 1755. Voorheeve N (Ed.), Die klinische und radiologische diagnose der fistula gastrocolica. Deutsch Arch Klein Med. 1912;106:294-308.
doi - Firth D. A case of gastrocolic fistula. The Lancet. 1920;195(5046):1061.
doi - Stamatakos M, Karaiskos I, Pateras I, Alexiou I, Stefanaki C, Kontzoglou K. Gastrocolic fistulae; From Haller till nowadays. Int J Surg. 2012;10(3):129-133.
doi pubmed - Clark FD. Gastrocolic fistula secondary to right gastroepiploic-coronary artery bypass. Can J Surg. 2005;48(5):417-418.
- Ruffolo C, Angriman I, Scarpa M, D'Odorico A, Polese L, Barollo M, Bertin M, et al. A gastrocolic fistula in Crohn's disease. Dig Dis Sci. 2005;50(5):933-934.
doi pubmed - Abeygunasekera S, Freiman J, Engelman J, Glenn D, Craig P. Gastropancreaticocolic fistula: complication of a benign ulcer. Gastrointest Endosc. 2004;59(3):450-452.
doi - Forshaw MJ, Dastur JK, Murali K, Parker MC. Long-term survival from gastrocolic fistula secondary to adenocarcinoma of the transverse colon. World J Surg Oncol. 2005;3(1):9.
doi pubmed - Chiang JM, Wang JY. Gastrocolic fistula due to a metastatic marginal ulcer from carcinoma of the cervix. Int J Gynaecol Obstet. 1994;47(2):173-174.
doi - Yokoyama Y, Kashyap S, Ewing E, Bloch R. Gastrosplenocolic fistula secondary to non-Hodgkin B-cell lymphoma. J Surg Case Rep. 2020;2020(1):rjz376.
doi pubmed - Leong W, Xu M, Ni L, Su J, Yang D. A gastro-colic fistula secondary to high-grade B-cell gastric lymphoma in a patient with AIDS: a case report. J Int Med Res. 2021;49(4):3000605211006602.
doi pubmed - Buyukberber M, Gulsen MT, Sevinc A, Koruk M, Sari I. Gastrocolic fistula secondary to gastric diffuse large B-cell lymphoma in a patient with pulmonary tuberculosis. J Natl Med Assoc. 2009;101(1):81-83.
doi - Lynch RC, Boese HL. Carcinoid tumor of transverse colon complicated by gastrocolic fistula: survival following resection. Surgery. 1955;38(3):600-604.
- Pichney LS, Fantry GT, Graham SM. Gastrocolic and duodenocolic fistulas in Crohn's disease. J Clin Gastroenterol. 1992;15(3):205-211.
doi pubmed - Logio T, Chaiken B, Roth J, Newman E, Siegel T. The management of Crohn's colitis with colonogastric fistula. Report of a case. Dis Colon Rectum. 1987;30(9):699-704.
doi pubmed - Wu S, Zhuang H, Zhao JY, Wang YF. Gastrocolic fistula in Crohn's disease detected by oral agent contrast-enhanced ultrasound: A case report of a novel ultrasound modality. World J Gastroenterol. 2020;26(17):2119-2125.
doi pubmed - Shaik AS, Singh B, Haffejee AA. Gastrocolic fistula as a complication of benign gastric ulcer. Afr Med J. 1999;89:1011-1014.
- Hansen CP, Christensen A, Thagaard CS, Lanng C. Gastrocolic fistula resulting from chronic pancreatitis. South Med J. 1989;82(10):1309-1310.
doi pubmed - Andrew D, Shyam K, Johny J, Beaty S. Elderly male patient with gastrocolic fistula following severe acute necrotising pancreatitis. BMJ Case Rep. 2021;14(1):e240426.
doi pubmed - Jiang W, Tong Z, Yang D, Ke L, Shen X, Zhou J, Li G, et al. Gastrointestinal fistulas in acute pancreatitis with infected pancreatic or peripancreatic necrosis: a 4-year single-center experience. Medicine (Baltimore). 2016;95(14):e3318.
doi pubmed - Ghahremani GG, Olsen J. Gastrocolic fistula secondary to diverticulitis of the splenic flexure: report of a case. Dis Colon Rectum. 1974;17(1):98-99.
doi pubmed - Levine MS, Kelly MR, Laufer I, Rubesin SE, Herlinger H. Gastrocolic fistulas: the increasing role of aspirin. Radiology. 1993;187(2):359-361.
doi pubmed - Gutnik SH, Willmott D, Ziebarth J. Gastrocolic fistula-secondary to aspirin abuse. S D J Med. 1993;46(10):358-360.
- Coughlin GP, Willing RL, Hamilton DW. Gastro-colic fistula complicating benign gastric ulcer in analgesic abusers. Aust N Z J Med. 1979;9(3):314-315.
doi pubmed - Huang SY, Levine MS, Raper SE. Gastrocolic fistula with migration of feeding tube into transverse colon as a complication of percutaneous endoscopic gastrostomy. AJR Am J Roentgenol. 2005;184(3 Suppl):S65-66.
doi pubmed - Calleja Aguayo E, Delgado Alvira R, Elias Pollina J, Gonzalez Esgueda A, Esteban Ibarz JA. [The PEG: why wait?]. Cir Pediatr. 2010;23(1):24-27.
- Murphy S, Pulliam TJ, Lindsay J. Delayed gastrocolic fistula following percutaneous endoscopic gastrostomy (PEG). J Am Geriatr Soc. 1991;39(5):532-533.
doi pubmed - Key JA. Gastro-colic fistula after vagotomy for stomal ulcer. Br Med J. 1949;2(4619):138.
doi pubmed - Nguyen D, Dip F, Hendricks L, Lo Menzo E, Szomstein S, Rosenthal R. The surgical management of complex fistulas after sleeve gastrectomy. Obes Surg. 2016;26(2):245-250.
doi pubmed - Bhasker AG, Khalifa H, Sood A, Lakdawala M. Management of gastro-colic fistula after laparoscopic sleeve gastrectomy. Asian J Endosc Surg. 2014;7(4):314-316.
doi pubmed - Garofalo F, Atlas H, Pescarus R. Laparoscopic treatment of gastrocolic fistula: a rare complication post-sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(9):1761-1763.
doi pubmed - Deitel M, Gagner M, Erickson AL, Crosby RD. Third International Summit: Current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7(6):749-759.
doi pubmed - Rosenthal RJ, International Sleeve Gastrectomy Expert P, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, et al. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8-19.
doi pubmed - Gehle DB, Pullatt RC, Elias PS. Gastrojejunocolic fistula: case report of a rare late complication of laparoscopic Roux-en-Y gastric bypass and review of the literature. Int J Surg Case Rep. 2021;84:106152.
doi pubmed - Schulman AR, Vaidya A, Tavakkoli A, Jajoo K, Ryou M. Making the connection. N Engl J Med. 2017;376(4):e5.
doi pubmed - Povoa AA, Soares C, Esteves J, Gandra A, Maciel R, Cardoso JM, Gandra L, et al. Simultaneous gastric and colic laparoscopic adjustable gastric band migration. Complication of bariatric surgery. Obes Surg. 2010;20(6):796-800.
doi pubmed - Myadam R, Zafar Y, Kalantri P. Hidden tunnel: a case of duodenal-colonic fistula caused by cytomegalovirus. Cureus. 2020;12(6):e8425.
doi - Ali A, Alhindi S. A child with a gastrocolic fistula after ingesting magnets: an unusual complication. Cureus. 2020;12(7):e9336.
doi - AR DC, Jehangir S. Gastrocolic fistula in a child following corrosive acid ingestion. BMJ Case Rep. 2017;2017:bcr2016217330.
- Mathewson C. Preliminary colostomy in the management of gastrocolic and gastrojejunocolic fistulae. Ann Surg. 1941;114(6):1004-1010.
doi pubmed - Lee LS, Foo CS, Chen CM, Poh CC. Gastrocolic fistula: a rare complication of gastric carcinoma. Singapore Med J. 2009;50(8):e274-276.
- Kumar GK, Razzaque MA, Naidu VG, Barbour EM. Gastrocolic fistulae in benign peptic ulcer disease. Ann Surg. 1976;184(2):236-240.
doi pubmed - Thoeny RH, Hodgson JR, Scudamore HH. The roentgenologic diagnosis of gastrocolic and gastrojejunocolic fistulas. Am J Roentgenol Radium Ther Nucl Med. 1960;83:876-881.
- Hager J, Gassner I. Gastrocolic fistula in a 7 week old: a rare complication after gastric perforation. J Pediatr Surg. 1994;29(12):1597-1598.
doi - Monkemuller K, Peter S, Alkurdi B, Ramesh J, Popa D, Wilcox CM. Endoscopic closure of a gastrocolic fistula using the over-the-scope-clip-system. World J Gastrointest Endosc. 2013;5(8):402-406.
doi pubmed - Kumar N, Larsen MC, Thompson CC. Endoscopic Management of Gastrointestinal Fistulae. Gastroenterol Hepatol (N Y). 2014;10(8):495-452.
- Ligresti D, Barbuscio I, Granata A, Martino A, Amata M, Volpes R, Traina M. Endoscopic closure of gastrocolocutaneous fistula following percutaneous endoscopic gastrostomy, by OverStitch Endoscopic Suturing System. Endoscopy. 2019;51(12):E384-E385.
doi pubmed - Nunes G, Paiva de Oliveira G, Cruz J, Santos CA, Fonseca J. Long-term gastrocolocutaneous fistula after endoscopic gastrostomy: how concerned should we be? GE Port J Gastroenterol. 2019;26(6):441-447.
doi pubmed - Safi Khuri, Yasin Kamel, Kinan Halon, Wisam Abboud, Bishara Bishara. Endoscopic management of gastro-colic fistula following leak from laparoscopic sleeve gastrectomy. J Clin Rev Case Rep. 2017;2(2).
- Morgan MD, Kapila H. Closure of a gastrocolic fistula after treatment with cimetidine. Postgrad Med J. 1981;57(669):463-465.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


