| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 5, October 2022, pages 232-239
Diagnostic Accuracy of Elastography and Liver Disease: A Meta-Analysis
Preeti Malika, n, o , Shreejith Pillaib, n, Kriti Agarwalc, Salwa Abdelwahedd, Renu Bhandarie, Abhishek Singhf, Anusha Chidharlag, Kajal Patelh, Priyanka Singhi, Pritika Manaktalaj, Rizwan Rabbanik, Thoyaja Koritalal, Sachin Guptam
aDepartment of Pathology, Montefiore Medical Center, Bronx, NY, USA
bDepartment of Internal Medicine, Henry Ford Health System, Detroit, MI, USA
cDepartment of Internal Medicine, Hackensack Meridian Health Palisades Medical Center, North Bergen, NJ, USA
dDepartment of Family and Community Medicine, University of Missouri, Kansas City, MO, USA
eDepartment of Internal Medicine, Manipal College of Medical Sciences, Pokhara, Nepal
fDepartment of Internal Medicine, Mount Sinai Morningside, New York, NY, USA
gDepartment of Hematology Oncology, University of Kansas Medical Center, Kansas City, KS, USA
hDepartment of Internal Medicine, Smt Kashibai Navale Medical College, Nahre, Maharashtra, India
iApex Heart and Vascular Center, Passaic, NJ, USA
jDepartment of Internal Medicine, Canton Medical Education Foundation/NEOMED, Canton, OH, USA
kDepartment of Internal Medicine, Temple University Hospital, Philadelphia, PA, USA
lDepartment of Internal Medicine, Mayo Clinic Health System, Mankato, MN, USA
mDepartment of Internal Medicine, Reading Hospital, West Reading, PA, USA
nPreeti Malik and Shreejith Pillai contributed equally to this article as first authors.
oCorresponding Author: Preeti Malik, Department of Pathology, Montefiore Medical Center, Bronx, NY 10467, USA
Manuscript submitted July 18, 2022, accepted September 19, 2022, published online October 19, 2022
Short title: Elastography and Liver Disease
doi: https://doi.org/10.14740/gr1557
| Abstract | ▴Top |
Background: Ultrasound-based transient elastography (TE) is a non-invasive alternative to liver biopsy for the staging of hepatic fibrosis due to various chronic liver diseases. This meta-analysis aims to assess the diagnostic accuracy of TE for detecting liver cirrhosis (F4) and severe fibrosis (F3) in patients with chronic liver diseases, in comparison to the gold standard liver biopsy.
Methods: A systematic search was performed using PubMed search engine following Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines from inception to May 2021. The meta-analysis studies evaluating the diagnostic accuracy of TE for severe fibrosis and cirrhosis were identified. We conducted a meta-meta-analysis to generate pooled estimates of the sensitivity, specificity, and diagnostic odds ratios (ORs) for F3 and F4 fibrosis stage.
Results: We included five studies with a total of 124 sub-studies and 20,341 patients in our analysis. Three studies have reported the diagnostic accuracy of TE in detecting F3/severe fibrosis stage and found 81.9% pooled sensitivity (95% confidence interval (CI): 79.9-83.7%; P < 0.001) (I2 = 0%), 84.7% pooled specificity (95% CI: 81.3-87.6%) (I2 = 81%; P = 0.02). All five studies reported the diagnostic accuracy of TE in detecting F4/liver cirrhosis stage. We found 84.8% pooled sensitivity (95% CI: 81.4-87.7%) (I2 = 86.4%; P < 0.001), 87.5% pooled specificity (95% CI: 85.4-89.3%) (I2 = 90%; P < 0.001) and pooled diagnostic OR (41.8; 95% CI: 3.9 - 56.5) (I2 = 87%; P < 0.001).
Conclusions: Ultrasound-based TE has excellent diagnostic accuracy for identifying cirrhosis and liver fibrosis stages 3. Future studies should focus on estimating the diagnostic accuracy of other fibrosis stages in chronic liver disease patients. This will eventually decrease the risk associated with invasive liver biopsy.
Keywords: Transient elastography; Liver cirrhosis; Liver fibrosis; Diagnostic accuracy; Liver biopsy
| Introduction | ▴Top |
Liver cirrhosis is one of the most common causes of death worldwide. In Latin America, 2.7% of deaths are due to liver cirrhosis [1]. It is the end stage of progressive liver fibrosis, where the morphology of hepatocytes is distorted. The etiology of liver cirrhosis can be a toxic, infectious, allergic, autoimmune, or vascular process or an inborn error of metabolism [2, 3]. Among these causes, alcoholic liver disease is the most common cause [3]. Globally around 10.6 million prevalent cases of decompensated cirrhosis and about 112 million prevalent cases of compensated cirrhosis [4] are identified in 2017. In the USA, 0.15% of the population is estimated to have liver cirrhosis [5].
There are structural consequences of chronic liver diseases. The persistent continued inflammation of hepatocytes stimulates a complex activation process that includes excess synthesis and deposition of type 1 collagen by activated hepatic stellate cell (HSC), among others extracellular matrix and increased cell proliferation leading to fibrogenic response [6]. Fibrosis can be reversed if detected early enough, and the underlying liver disease that caused the development of fibrosis can be cured or treated. If fibrosis is left untreated, it can lead to cirrhosis and liver cancer [7]. Accurate assessment of the severity of liver fibrosis and a reliable diagnosis of cirrhosis are important steps for the management of patients with chronic liver diseases, as they provide information that guides therapeutic decisions [8, 9].
Liver biopsy is the gold standard for the staging of fibrosis and diagnosis of cirrhosis; however, diagnostic accuracy is correlated with the length of the biopsy specimen [10]. Liver biopsy remains a costly and invasive procedure that requires physicians and pathologists to be sufficiently trained, in order to obtain adequate and representative results which limits the use of liver biopsy for mass screening [11]. Sampling errors and risk of complications are other limitations for the use of liver biopsy. Recently introduced transient elastography (TE) is a non-invasive test to determine the staging of hepatic fibrosis and stiffening of the liver due to scarring. It allows examination of 100 times more significant volume of liver tissue as compared to a liver sample obtained through liver biopsy. Different shear wave-based elastography methods are available, including TE, point shear wave elastography, and two-dimensional shear wave elastography (2D-SWE). The most extensively evaluated elastography method for liver stiffness is TE (FibroScan; Echosens, Paris, France) [11, 12].
It involves using a transducer on the end of a US probe, which transmits 50 MHz pressure waves, and the resultant “shear wave” velocity is measured. This shear wave velocity correlates with liver stiffness and helps estimate the stage of liver fibrosis [5]. It is also used to predict the complications caused by cirrhosis like portal hypertension and has excellent patient acceptance [5, 13]. It can monitor dynamic changes of liver fibrosis, especially in hepatitis C and hepatitis B, during antiviral or anti-fibrotic treatment [14, 15]. Some studies have evaluated 2D-SWE in patients infected with chronic hepatitis B (n = 226 and n = 303) and revealed diagnostic accuracies according to area under receiver operating characteristic (AUROC) levels of 88-92%, 93-95%, and 95-98% for significant fibrosis, severe fibrosis, and liver cirrhosis, respectively [16, 17]. In one study, 2D-SWE was compared to TE, and the diagnostic accuracies were significantly superior to TE for all fibrosis stages [16].
| Materials and Methods | ▴Top |
Literature search strategy
In this meta-meta-analysis, we aim to evaluate the diagnostic accuracy of TE in diagnosing liver fibrosis stage 3 and 4 in patients with chronic liver disease compared to liver biopsy (gold standard). A systematic search was performed following Preferred Reporting Items for Systematic reviews and Meta-Analyze (PRISMA) guidelines [18] from inception to May 2021. The meta-analysis studies were searched using PubMed with keywords ((“point shear wave elastography” (title/abstract) OR “transient elastography” (title/abstract) OR “Fibroscan” (title/abstract) OR “transient sonoelastography” (title/abstract) OR “two dimensional shear wave elastography” (title/abstract)) AND (“non-alcoholic fatty liver disease” (title/abstract) OR “nonalcoholic steatohepatitis” (title/abstract) OR “fatty liver” (title/abstract) OR “liver fibrosis” (title/abstract) OR “cirrhosis” (title/abstract) OR “chronic liver disease” (title/abstract) OR “hepatitis” (title/abstract)) (Fig. 1).
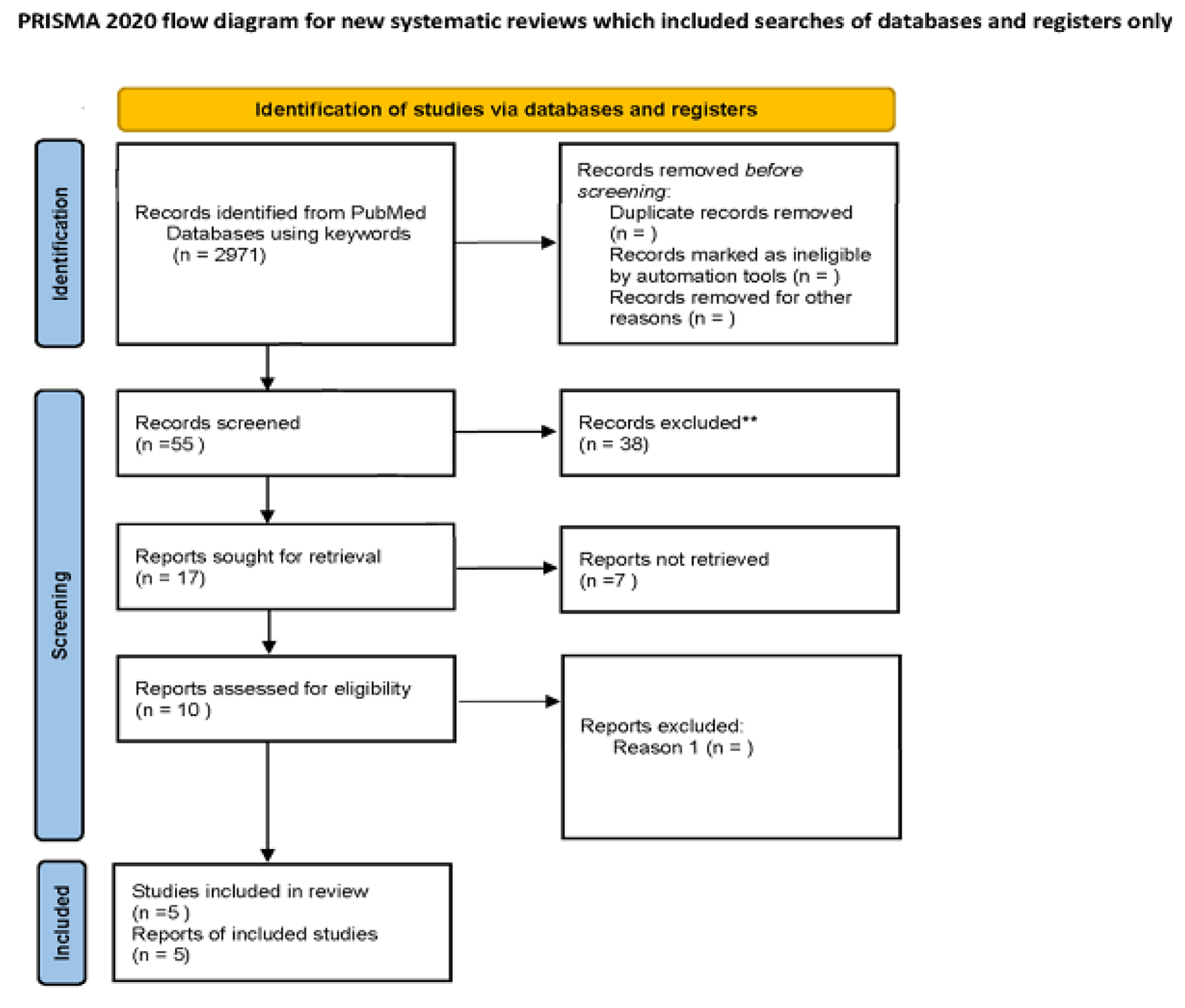 Click for large image | Figure 1. Flow diagram of literature search and study selection process of included studies. |
Study selection and data extraction
Abstracts and full-length articles for meta-analysis studies which have availability of data were reviewed and data were collected for quantitative analysis. Kriti Agarwal and Anusha Chidharla independently screened all of the identified studies and assessed full texts to determine eligibility. Any disagreement was resolved through consensus with Preeti Malik.
We have included meta-analysis studies which have evaluated the diagnostic accuracy of TE in detecting fibrosis stage 3 and 4 according to METAVIR or other systems which can be transformed to METAVIR compared with gold standard biopsy in chronic liver disease patients. Studies published in non-English language, animal studies, randomized clinical trials, non-full text and those comparing different elastography methods without comparing with liver biopsy were excluded.
The following data variables were extracted: author’s name, study year, sample size, studies included in the meta-analysis, type of chronic liver disease, and type of elastography as described in Table 1 [19-23].
 Click to view | Table 1. Characteristics of Studies Included |
Statistical analysis
We used the Review Manager version 5.3 software (https://training.cochrane.org/online-learning/core-software/revman) and Open METAXL for analysis. We performed a random effects model irrespective of heterogeneity to estimate the pooled sensitivity, specificity and diagnostic odds ratio (OR) and their respective 95% confidence interval (95% CI). I2 values of 25%, 50%, and 75% represented low, medium, and high heterogeneity. P < 0.05 was considered statistically significant. The Newcastle-Ottawa Scale (NOS) scale was used to estimate the risk of bias among studies. We calculated the true positive, false positive, true negative and false negative of each study based on the reported sensitivity and specificity.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
| Results | ▴Top |
We screened 55 publications, out of which 10 full-text articles were assessed for eligibility using inclusion and exclusion criteria. Five meta-analysis studies were excluded because they were not in English and did not compare the diagnostic accuracy of elastography with biopsy. After detailed assessment, as of May 20, 2021, a total of five meta-analysis studies were selected to evaluate the diagnostic accuracy of TE. The flow diagram of the search result Is shown in Figure 1.
Stage F3
Three out of the five meta-analysis studies were included in the meta-meta-analysis to evaluate the diagnostic accuracy of TE for staging liver fibrosis F ≥ 3 (severe fibrosis). We found 81.9% pooled sensitivity (95% CI: 79.9-83.7%; P < 0.001) (I2 = 0%) and 84.7% pooled specificity (95% CI: 81.3-87.6%) (I2 = 81%; P = 0.02) (Fig. 2a, b).
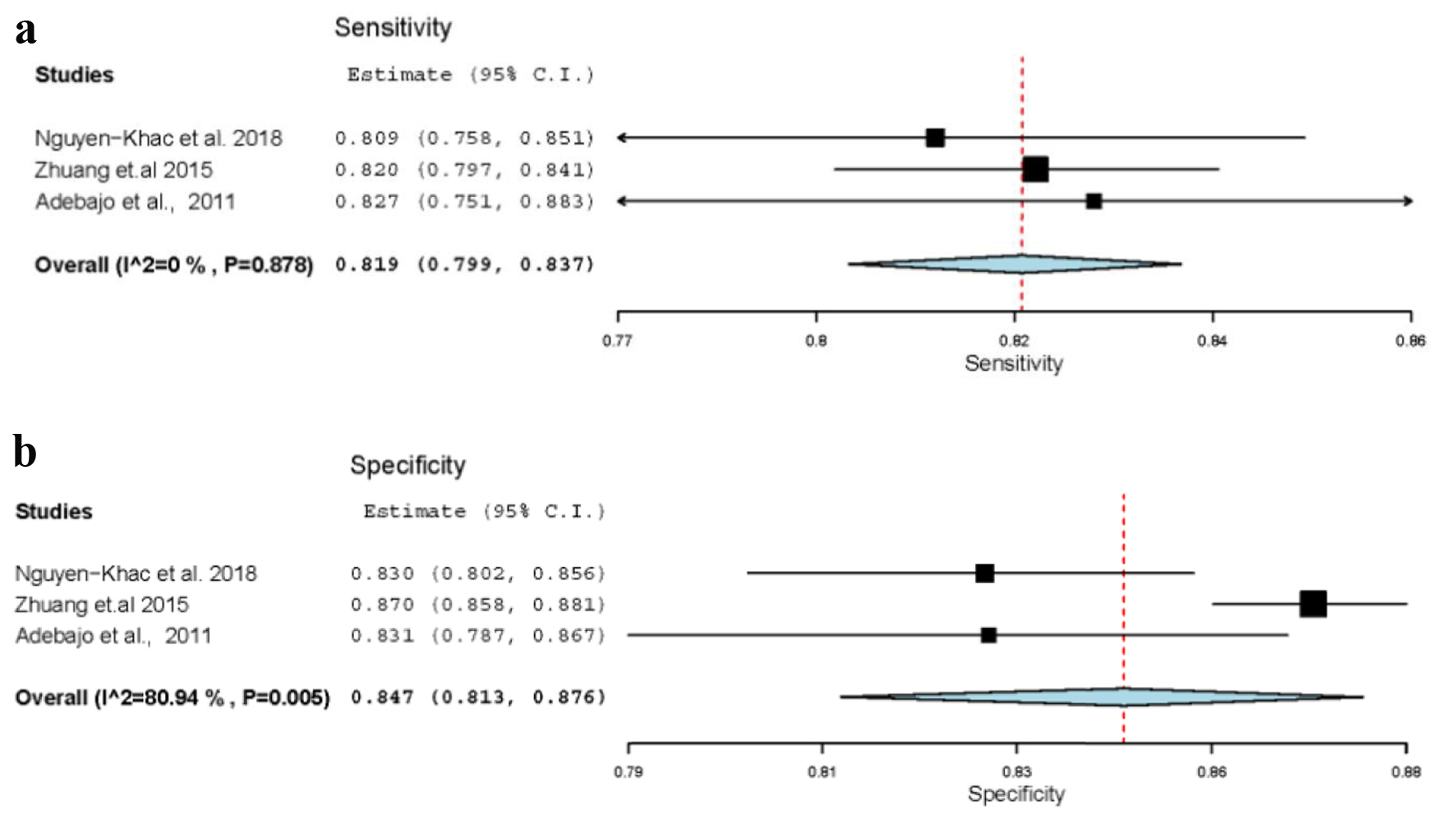 Click for large image | Figure 2. (a) Sensitivity of stage F3. (b) Specificity of stage F3. |
Stage F4: sensitivity, specificity and diagnostic odds ratio
Five meta-analysis studies have reported the performance of transient elastography in detecting fibrosis stage 4/liver cirrhosis. We found 84.8 % pooled sensitivity (95% CI: 81.4-87.7%) (I2 = 86.4%; P < 0.001), 87.5% pooled specificity (95% CI: 85.4-89.3%) (I2 = 90%; P < 0.001) and pooled diagnostic OR (41.8; 95% CI: 3.9 - 56.5) (I2 = 87%; P < 0.001) (Fig. 3a-c).
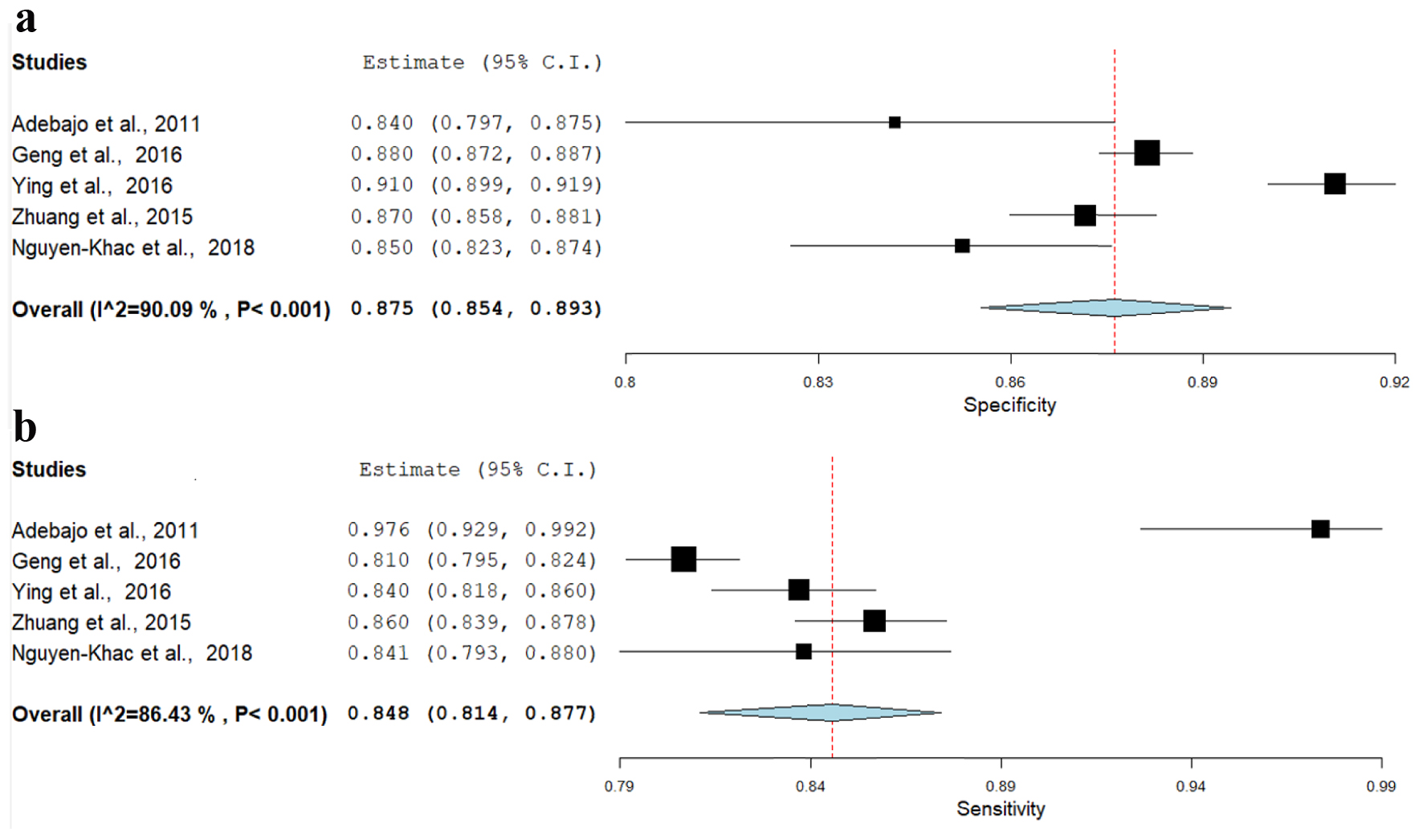 Click for large image | Figure 3. (a) Sensitivity of stage F4. (b) Specificity of stage F4. (c) Diagnostic odds ratio of stage F4. |
| Discussion | ▴Top |
This meta-meta-analysis evaluated diagnostic accuracy of TE in detecting F4 stage of liver fibrosis compared to gold standard liver biopsy in patients with chronic liver disease. We included five studies with a total of 124 sub-studies and 20,341 patients in our analysis. Three studies have reported the diagnostic accuracy of TE in detecting F3/severe fibrosis stage and found 81.9% pooled sensitivity (95% CI: 79.9-83.7%; P < 0.001) (I2 = 0%) and 84.7% pooled specificity (95% CI: 81.3-87.6%) (I2 = 81%; P = 0.02). All five studies which were included in our meta-meta-analysis reported the diagnostic accuracy of TE in detecting F4/liver cirrhosis stage. We found 84.8% pooled sensitivity (95% CI: 81.4-87.7%) (I2 = 86.4%; P < 0.001), 87.5% pooled specificity (95% CI: 85.4-89.3%) (I2 = 90%; P < 0.001) and pooled diagnostic OR (41.8; 95% CI: 3.9 - 56.5) (I2 = 87%; P < 0.001). Our findings indicate that TE has high diagnostic performance for detecting liver fibrosis stages 3 and 4. This supports the use of TE as an alternative to invasive methods like liver biopsy for assessing advanced stages of chronic liver disease and avoiding its complications/limitations.
There are few studies supporting our results such as Qi et al’s study which found that sensitivity, specificity, and diagnostic OR of TE at F4 in chronic hepatitis B (CHB) infection was 78%, 84%, and 14.44, respectively [24]. Similarly, Zhang et al’s study used TE for predicting a negative predictive value in advanced fibrosis and cirrhosis in CHB were 92.4% and 98.7%, respectively [25]. Meta-analysis by Talwalkar et al included nine studies and showed a pooled estimate for sensitivity 87% and for specificity 91% in patients with stage 4 fibrosis suggesting good performance of TE which is similar to our findings [26]. Tsochatzis et al included 40 studies and found that the sensitivity and specificity were dependent on the degree of fibrosis [27]. They found that for F2 stage disease, the sensitivity and specificity were 79% and 78%, respectively, whereas for cirrhosis they were 83% and 89%. They also determined that accuracy of TE as evaluated by post-test biopsy was 78% for F2 stage disease and 88% for cirrhosis. Our study also points towards similar results by showing higher diagnostic accuracy in detecting fibrosis at the F4 stage compared to F3 stage.
The meta-analysis of Stebbing et al included 22 studies and found that the sensitivity was 71.9%, specificity was 82.4% for significant fibrosis (≥ F2) and they were 84.5% and 94.7%, respectively, for cirrhosis [28]. Friedrich-Rust et al performed a meta-analysis that assessed the overall performance of TE for diagnosing liver fibrosis and they also analyzed what factors influence the accuracy [29]. They included 50 studies and found that the mean AUROC curve varied depending on the severity of the fibrosis; the AUROC for significant fibrosis (F ≥ 2) was 0.84, for severe fibrosis (F ≥ 3) was 0.89, and for cirrhosis (F ≥ 4) was 0.94. Factors that influenced AUROC were underlying liver disease, scoring system used, and country. Another systematic review and meta-analysis was done by Adebajo et al, comparing TE with liver biopsy for the detection of significant fibrosis (five studies) and cirrhosis (five studies) in patients with recurrent hepatitis C virus (HCV) after liver transplant. The results yielded excellent TE estimates of the sensitivity and specificity for detecting cirrhosis (F ≥ 4) and good estimates for detecting significant fibrosis (F ≥ 2) [23].
Elastography mainly aims at imaging the stiffness of the liver. The various types of elastography include quasi-static method elastography, vibro-acoustography, and TE. TE uses the acoustic force; therefore, it might slightly displace the tissue from its focal point [30]. A study done by Burriel et al has mentioned that TE has an overall 12% chance of being a cost-effective intervention for the European and Asian population [31]. TE is non-invasive and easy to use, and can be done repeatedly [32]. However, there are several limitations of TE which include minimal anatomic orientation, limited depth of penetration, and patient positioning requirements [33, 34]. Shear wave propagation is also attenuated by fluid and adipose tissue [33, 35]. Hence, these limitations may result in failed examinations in patients who are obese, who have anatomic distortions, ascites, and elevated central venous pressures [36]. Factors associated with unreliable results included body mass index (BMI) > 30 kg/m2, age > 52 years, female sex, operator inexperience, and type 2 diabetes mellitus [33].
Even though liver biopsy is the standard gold test, there are disadvantages associated like hemorrhage and blood transfusion due to bleeding and mortality risk [37]. In addition, it also depends on the pathologist; the chance of error rate in disease staging is 20% [38].
TE measures the shear wave speed through the liver which reflects liver stiffness and not actual amount of fibrosis in the liver. Hence, conditions which increase the stiffness of the liver independent of fibrosis will result in an increased liver stiffness measurement (LSM) and will result in a falsely high estimate of liver fibrosis. LSMs are falsely high in acute hepatitis during alanine transaminase (ALT) flares, hepatic congestion and cholestasis, leading to variability of optimal cut-off levels for the diagnosis of fibrosis and cirrhosis in different etiologies of liver disease. Also, there are differences in the optimal cut-off values reported in different studies [39].
Among the technical limitations, the inclusion of non-parenchymal tissue, such as gallbladder, blood vessels, and bile ducts, alters measurement velocity. Another one is the depth of measurement, as the 2 - 7 cm is ideal for measurement [40]. A study done by Lesmana et al at the F2 stage shows the lower sensitivity (60.3%) and specificity (63.6%) of TE [41].
The tissue response to a known mechanical stimulus forms the principal basis for elastography. This stimulus can be static, quasistatic, or dynamic depending on the tissue being studied. Dynamic stimulus-based techniques like US-based shear wave elastography and magnetic resonance (MR) elastography which typically use vibrations in the range of 20 - 500 Hz and study the properties of the waves produced by the vibrations propagating through the tissues. Chronic liver disease leads to the accumulation of collagen fibers leading to fibrosis and resulting in increased liver parenchymal stiffness. As there is faster propagation of mechanical waves through stiffer tissue which resists deformation, this change in this mechanical property helps differentiate normal liver parenchyma from fibrotic liver and cirrhosis.
Limitations of the study
There were not enough meta-analysis studies done to evaluate the diagnostic performance for F0-F2 fibrosis stages. Hence, we could not provide strong evidence of diagnostic accuracy of fibrosis stage F0-F2. Additionally, meta-analyses included in the study have included various chronic liver disease which might explain the heterogeneity in the results. Among the technical limitations of elastography is the inclusion of non-parenchymal tissue, such as gallbladder, blood vessels, and bile ducts, alters measurement velocity. Another one is the depth of measurement, as the 2 - 7 cm is ideal for measurement [40]. A study done by Lesmana et al at the F2 stage shows the lower sensitivity (60.3%) and specificity (63.6%) of TE [41]. However, more studies or meta-analyses are required before any definitive conclusion can be drawn. Despite the limitations our meta-meta-analysis shows higher diagnostic accuracy of TE in detecting liver cirrhosis and severe fibrosis compared to liver biopsy.
Future directions of liver elastography
Liver elastography is an emerging and dynamic research modality which can be used repeatedly over a period of time due to its non-invasive nature. It has a prognostic significance in determining long-term survival in patients with chronic hepatitis. Further studies are being undertaken to determine prefibrosis liver stiffness which could be beneficial with earlier diagnosis and treatment in high-risk individuals [42]. There is evidence in patients with hemochromatosis for use of TE with serum ferritin algorithms to accurately determine severe fibrosis [43]. There is also active research for the use of elastography in characterization of liver tumors [44, 45].
Conclusion
Novel elastography is an emerging new technique for the better diagnosis and management of the patients with chronic liver disease. Our study focused on the accuracy of TE in diagnosing F3 and F4 stages of liver fibrosis and we found TE showed promising results in detecting advanced stages of liver fibrosis comparable to gold standard liver biopsy. Given the limitations of biopsy like sampling error, invasiveness and associated complications, elastography has recently emerged as the modality of choice to accurately determine liver fibrosis, before it leads to development of cirrhosis and also monitoring of the conditions like HCV, hepatitis B virus (HBV), and nonalcoholic fatty liver disease (NAFLD). But the study of TE has its limitations and technical challenges like lack of standardization of diagnostic threshold across manufacturers, operator and patient dependency, different fibro scan cut off for different chronic liver disease and limited diagnostic accuracy of the F0-F2 stage.
Additional studies are required to determine standardization and to assist with early diagnosis and prompt treatment. Use of elastography is being incorporated into decision making for quantification of the extent of fibrosis and treatment of liver disorders to prevent progression. There is need for collaboration of manufacturers, national societies, and researchers to work together in overcoming the limitations. Hence, these non-invasive techniques can be used for early detection of advancing liver disease.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
The data used in this study are deidentified and collected from the studies published online thus informed consent or IRB approval was not needed for this study.
Author Contributions
Conceptualization: Preeti Malik, Shreejith Pillai, and Kriti Agarwal. Methodology: Preeti Malik and Kriti Agarwal. Acquisition of data: Anusha Chidharla, Kriti Agarwal and Preeti Malik. Formal analysis and investigation: Preeti Malik. Writing original draft preparation: Preeti Malik, Shreejith Pillai, Kriti Agarwal, Salwa Abdelwahed, Renu Bhandari, Abhishek Singh, Anusha Chidharla, Kajal Patel, Priyanka Singh, and Pritika Manaktala. Writing review, critical feedback, and editing: Rizwan Rabbani, Thoyaja Koritala, and Sachin Gupta. Resources and supervision: Thoyaja Koritala and Sachin Gupta.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Garcia-Compean D, Villarreal-Perez JZ, Cavazos MEO, Lavalle-Gonzalez FJ, Borjas-Almaguer OD, Del Cueto-Aguilera AN, Gonzalez-Gonzalez JA, et al. Prevalence of liver fibrosis in an unselected general population with high prevalence of obesity and diabetes mellitus. Time for screening? Ann Hepatol. 2020;19(3):258-264.
doi pubmed - Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31(5):395-414.
doi pubmed - Wiegand J, Berg T. The etiology, diagnosis and prevention of liver cirrhosis: part 1 of a series on liver cirrhosis. Dtsch Arztebl Int. 2013;110(6):85-91.
doi pubmed - Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245-266.
doi - Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20(45):16820-16830.
doi pubmed - Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364(1-2):33-60.
doi pubmed - Servin-Abad L, Schiff ER. The treatment of hepatic fibrosis: reversal of the underlying disease process. Gastroenterol Hepatol (N Y). 2006;2(11):819-825.
- Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787-799.
doi pubmed - O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134(6):1764-1776.
doi pubmed - Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449-1457.
doi pubmed - European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264.
doi pubmed - Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34(2):169-184.
doi pubmed - Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol. 2012;18(2):163-173.
doi pubmed - Kim SU, Park JY, Kim DY, Ahn SH, Choi EH, Seok JY, Lee JM, et al. Non-invasive assessment of changes in liver fibrosis via liver stiffness measurement in patients with chronic hepatitis B: impact of antiviral treatment on fibrosis regression. Hepatol Int. 2010;4(4):673-680.
doi pubmed - Ogawa E, Furusyo N, Toyoda K, Takeoka H, Maeda S, Hayashi J. The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha-2b and ribavirin. Antiviral Res. 2009;83(2):127-134.
doi pubmed - Zeng J, Liu GJ, Huang ZP, Zheng J, Wu T, Zheng RQ, Lu MD. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur Radiol. 2014;24(10):2572-2581.
doi pubmed - Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269(3):910-918.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Geng XX, Huang RG, Lin JM, Jiang N, Yang XX. Transient elastography in clinical detection of liver cirrhosis: A systematic review and meta-analysis. Saudi J Gastroenterol. 2016;22(4):294-303.
doi pubmed - Ying HY, Lu LG, Jing DD, Ni XS. Accuracy of transient elastography in the assessment of chronic hepatitis C-related liver cirrhosis. Clin Invest Med. 2016;39(5):E150-E160.
doi pubmed - Nguyen-Khac E, Thiele M, Voican C, Nahon P, Moreno C, Boursier J, Mueller S, et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(9):614-625.
doi - Li Y, Huang YS, Wang ZZ, Yang ZR, Sun F, Zhan SY, Liu XE, et al. Systematic review with meta-analysis: the diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2016;43(4):458-469.
doi pubmed - Adebajo CO, Talwalkar JA, Poterucha JJ, Kim WR, Charlton MR. Ultrasound-based transient elastography for the detection of hepatic fibrosis in patients with recurrent hepatitis C virus after liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2012;18(3):323-331.
doi pubmed - Qi X, An M, Wu T, Jiang D, Peng M, Wang W, Wang J, et al. Transient Elastography for Significant Liver Fibrosis and Cirrhosis in Chronic Hepatitis B: A Meta-Analysis. Can J Gastroenterol Hepatol. 2018;2018:3406789.
doi pubmed - Zhang GL, Zhao QY, Lin CS, Hu ZX, Zhang T, Gao ZL. Transient elastography and ultrasonography: optimal evaluation of liver fibrosis and cirrhosis in patients with chronic hepatitis B concurrent with nonalcoholic fatty liver disease. Biomed Res Int. 2019;2019:3951574.
doi pubmed - Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214-1220.
doi pubmed - Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54(4):650-659.
doi pubmed - Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44(3):214-219.
doi pubmed - Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960-974.
doi pubmed - Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94(5):487-495.
doi pubmed - Serra-Burriel M, Graupera I, Toran P, Thiele M, Roulot D, Wai-Sun Wong V, Neil Guha I, et al. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71(6):1141-1151.
doi pubmed - Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol. 2010;25(11):1726-1731.
doi pubmed - Castera L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828-835.
doi pubmed - Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56(7):968-973.
doi pubmed - Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52(2):206-210.
doi pubmed - Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705-1713.
doi pubmed - Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36(3):437-441.
doi pubmed - Afdhal NH. Diagnosing fibrosis in hepatitis C: is the pendulum swinging from biopsy to blood tests? Hepatology. 2003;37(5):972-974.
doi pubmed - Chang PE, Goh GB, Ngu JH, Tan HK, Tan CK. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World J Gastrointest Pharmacol Ther. 2016;7(1):91-106.
doi pubmed - Srinivasa Babu A, Wells ML, Teytelboym OM, Mackey JE, Miller FH, Yeh BM, Ehman RL, et al. Elastography in chronic liver disease: modalities, techniques, limitations, and future directions. Radiographics. 2016;36(7):1987-2006.
doi pubmed - Lesmana CR, Salim S, Hasan I, Sulaiman AS, Gani RA, Pakasi LS, Lesmana LA, et al. Diagnostic accuracy of transient elastography (FibroScan) versus the aspartate transaminase to platelet ratio index in assessing liver fibrosis in chronic hepatitis B: the role in primary care setting. J Clin Pathol. 2011;64(10):916-920.
doi pubmed - Salameh N, Larrat B, Abarca-Quinones J, Pallu S, Dorvillius M, Leclercq I, Fink M, et al. Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology. 2009;253(1):90-97.
doi pubmed - Legros L, Bardou-Jacquet E, Latournerie M, Guillygomarc'h A, Turlin B, Le Lan C, Desille Y, et al. Non-invasive assessment of liver fibrosis in C282Y homozygous HFE hemochromatosis. Liver Int. 2015;35(6):1731-1738.
doi pubmed - Ronot M, Di Renzo S, Gregoli B, Duran R, Castera L, Van Beers BE, Vilgrain V. Characterization of fortuitously discovered focal liver lesions: additional information provided by shearwave elastography. Eur Radiol. 2015;25(2):346-358.
doi pubmed - Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190(6):1534-1540.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


