| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Case Report
Volume 15, Number 3, June 2022, pages 148-154
Budd-Chiari Syndrome as an Initial Presentation of Non-Promyelocytic Acute Myelogenous Leukemia
Jiten P. Kothadiaa, b, g, Vanisha Patelc, Rajiv Hedac, Wesley A. Angeld, Vishwas Vanare, Benedict J. Maliakkalf, Rajanshu Vermac
aJames D. Eason Transplant Institute, University of Tennessee Health Science Center, Methodist University Hospital, Memphis, TN, USA
bDivision of Transplant Surgery, Department of Surgery, University of Tennessee Health Science Center, Memphis, TN, USA
cDivision of Gastroenterology, Department of Medicine, University of Tennessee Health Science Center, Memphis, TN, USA
dMemphis Vascular Center, Germantown, TN, USA
eAdvent Health Medical Group, Department of Gastroenterology/Hepatology, Advent Health Hospital Altamonte Springs, Altamonte Springs, FL, USA
fDepartment of Gastroenterology, Nashville General Hospital, Nashville, TN, USA
gCorresponding Author: Jiten P. Kothadia, James D. Eason Transplant Institute, University of Tennessee Health Science Center, Methodist University Hospital, Memphis, TN 38104, USA
Manuscript submitted April 13, 2022, accepted May 12, 2022, published online June 2, 2022
Short title: Acute BCS as an Initial Presentation of AML
doi: https://doi.org/10.14740/gr1530
| Abstract | ▴Top |
Budd-Chiari syndrome (BCS) is a rare disease characterized by hepatic venous outflow tract obstruction, frequently due to an underlying thrombophilic disorder. Acute myeloid leukemia rarely presents as acute BCS due to hyperfibrinolysis, hyperleukocytosis, nonspecific proteolytic activity, and disseminated intravascular coagulation causing acute hepatic vein thrombosis. In patients presenting with acute BCS with acute liver failure (ALF), a high index of suspicion and exclusion of underlying malignancy is a must, as it is a contraindication for liver transplantation. We report a case of a 19-year-old Caucasian male who presented with acute BCS causing ALF as an initial presentation of acute myelogenous leukemia.
Keywords: Budd-Chiari syndrome; Acute promyelocytic leukemia; Acute myeloid leukemia; Thrombophilia; Leukemia; Acute liver failure
| Introduction | ▴Top |
Budd-Chiari syndrome (BCS) is a rare disease characterized by hepatic venous outflow obstruction (HVOO) independent of the level or mechanism of obstruction [1, 2]. The prevalence of BCS is estimated to be 1 in 100,000 in the general population [3, 4]. The clinical presentation of BCS is highly variable, from asymptomatic to acute liver failure (ALF) [2]. Multiple risk factors have been identified for BCS, such as primary myeloproliferative disorders (MPDs) (most common), inherited thrombophilia, oral contraceptives, pregnancy, antiphospholipid syndrome, sarcoidosis, hyperhomocysteinemia, paroxysmal nocturnal hemoglobinuria (PNH), Behcet disease, and systemic inflammatory diseases [1, 2, 4].
Although bleeding complications were thought to be the most common complications of hematological malignancies, now it is described that these patients are also at risk of venous thromboembolism (VTE), similar to patients with solid tumors [5]. The patients may present with VTE as a presenting symptom of hematological malignancy [6]. There are only seven reported cases of acute myeloid leukemia (AML) presenting with acute BCS as the initial presentation in the literature [5, 7-14]. In patients presenting with acute BCS with ALF, a high index of suspicion and exclusion of underlying malignancy is a must, as it is a contraindication for liver transplantation (LT). We report a case of a 19-year-old Caucasian male who presented with acute BCS causing ALF as an initial presentation of acute myelogenous leukemia.
| Case Report | ▴Top |
A 19-year-old Caucasian male with a past medical history significant for asthma, left second rib Ewing sarcoma diagnosed in 2011 and treated with chemotherapy (in remission) presented to the hospital with 1-week history of jaundice, fatigue, and right upper quadrant abdominal pain. Past surgical history was significant for tonsillectomy. No record of alcohol, smoking, recreational drug, or hepatotoxic drug intake was reported. His family history was non-contributory.
On physical exam, he had scleral icterus, mild abdominal distension, and generalized discomfort on palpation without guarding, rebound, or rigidity. He had palpable hepatomegaly with the lower edge of the liver 5 cm below the right costal margin. There was no shifting dullness or ascites. The skin was notable for purple-blue discoloration of toes and soles bilaterally (Fig. 1). His vital signs showed a blood pressure of 77/45 mm Hg, a temperature of 36.5 °C, heart rate of 118 beats/min, a respiratory rate of 24 breaths/min, and oxygen saturation of 96% on room air. Labs including complete blood count, liver function tests, coagulation studies, and renal function tests are displayed in Table 1. The patient was admitted to the intensive care unit (ICU) for further management. He received two units of platelets, two units of fresh frozen plasma (FFP), eight units of cryoprecipitate, and two units of packed red blood cells (PRBCs). He continued to have persistent hypotension requiring norepinephrine infusion, and continuous renal replacement therapy was initiated due to persistent metabolic acidosis. The patient was treated empirically with broad-spectrum antibiotics. The blue-purple discoloration of his lower extremities was consistent with blue toe syndrome (BTS) that was attributed to arterial microthrombi from disseminated intravascular coagulation (DIC).
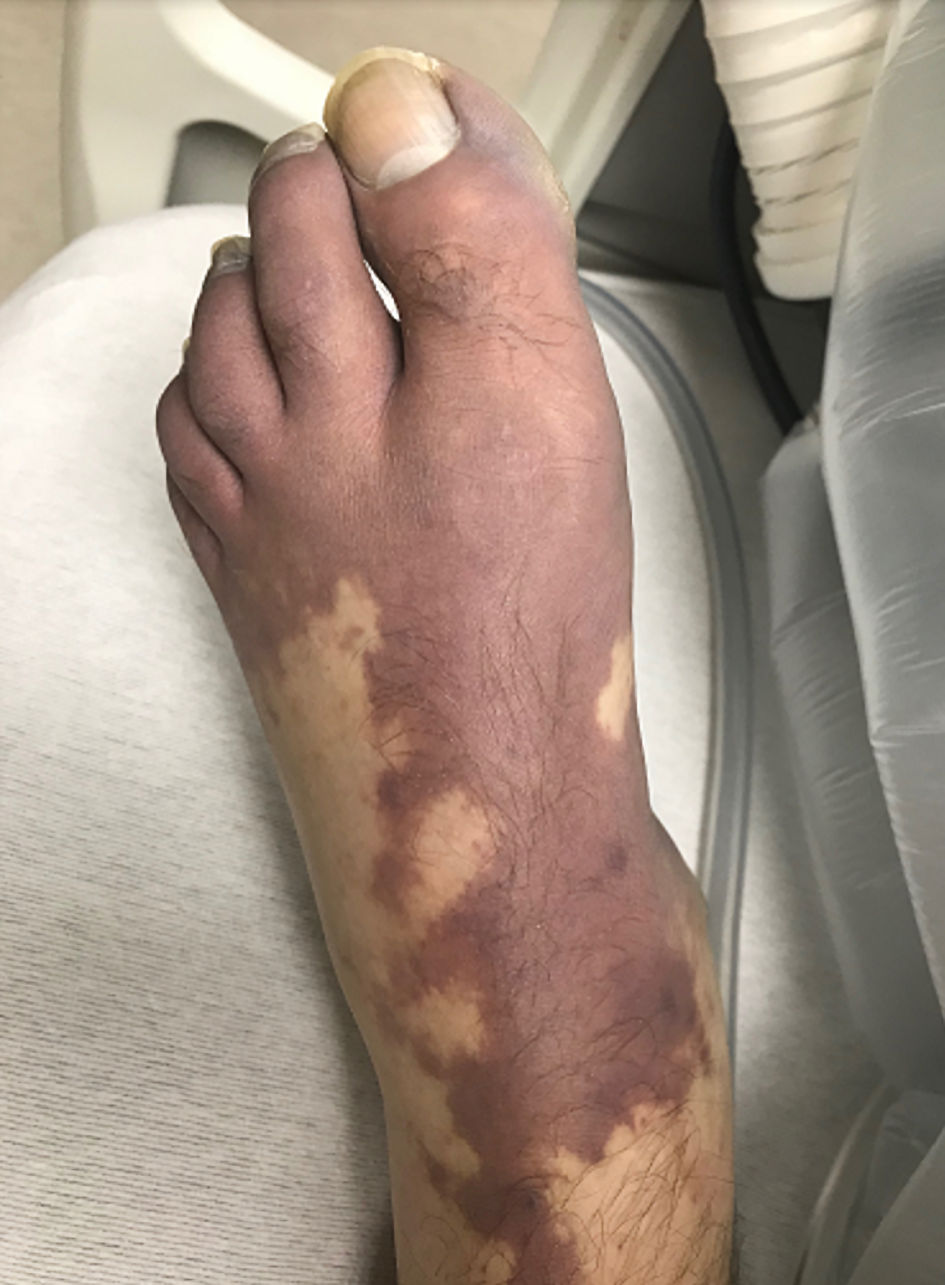 Click for large image | Figure 1. Image of skin changes associated with blue toe syndrome in this patient. |
 Click to view | Table 1. Laboratory Studies Done During Hospitalization |
Peripheral blood smear revealed leukocytosis with 65% circulating blasts, normocytic anemia, and thrombocytopenia (Fig. 2a). Flow cytometry showed 76% blasts that were positive for CD117, CD33, CD13, and myeloperoxidase (MPO) with human leukocyte antigen-DR isotype (HLA-DR). A bone marrow biopsy was obtained and showed bone marrow replaced by sheets of blasts consistent with AML (Fig. 2b). Cytogenic analysis showed a t(11;19)(q23;p13.3) and negative fluorescence in situ hybridization (FISH) studies for BCR/ABL1 rearrangement and t(15;17), which confirmed the diagnosis of non-promyelocytic acute myelogenous leukemia.
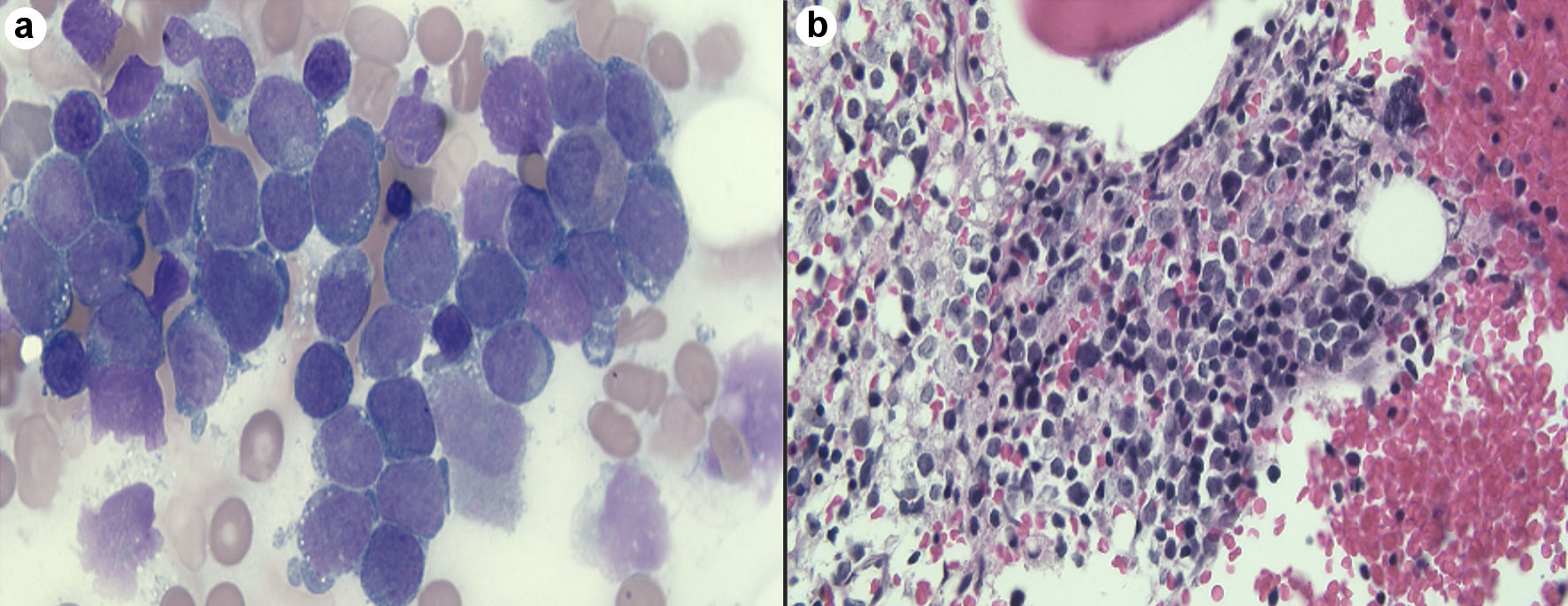 Click for large image | Figure 2. (a) Peripheral smear showed large number of blast cells. (b) bone marrow shows bone marrow replaced by sheets of blasts consistent with acute myeloid leukemia. |
He was planned for treatment with all-trans retinoic acid (ATRA), etoposide, and rasburicase. Hepatology was consulted for evaluation of elevated liver enzymes and jaundice. Ultrasound vascular Doppler showed no hepatic vein outflow and sluggish but hepatopedal portal flow (Fig. 3). A computed tomography (CT) scan of the abdomen with and without contrast revealed the presence of small ascites, hepatic vein thrombosis, mottled inhomogeneous enhancement of the liver with caudate lobe hypertrophy, and hyperenhancement (Figs. 4, 5). These imaging findings suggest acute BCS as the underlying etiology.
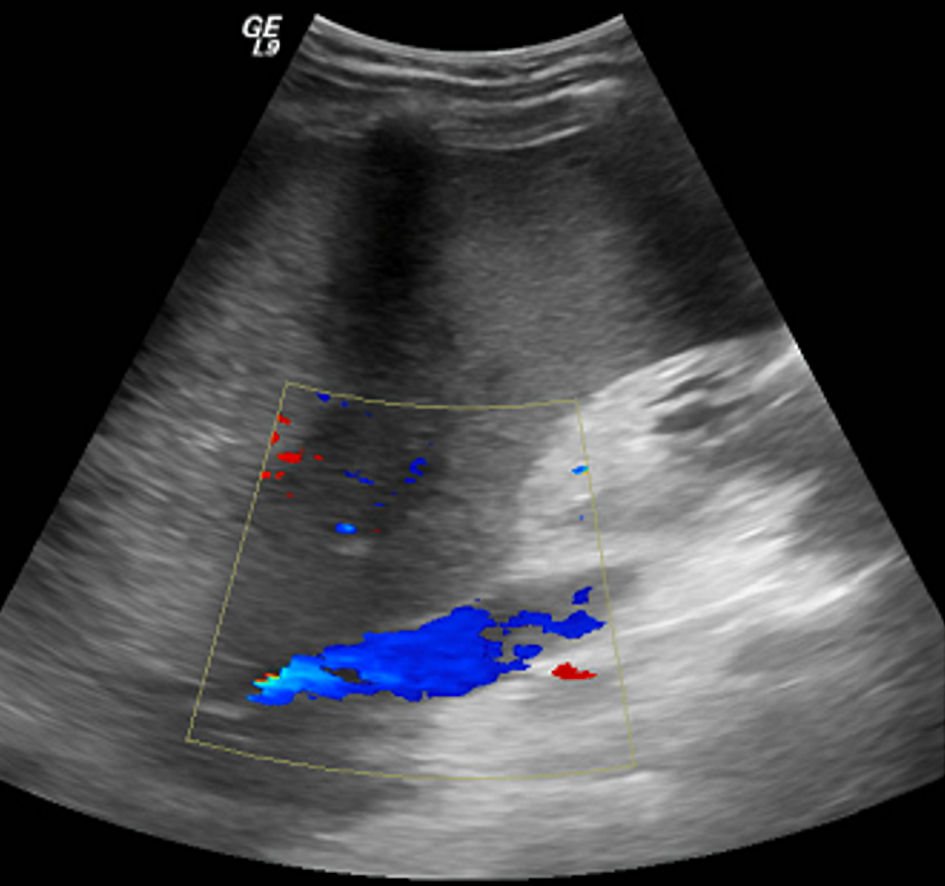 Click for large image | Figure 3. Ultrasound vascular Doppler image shows no hepatic vein outflow and sluggish but hepatopedal portal flow. |
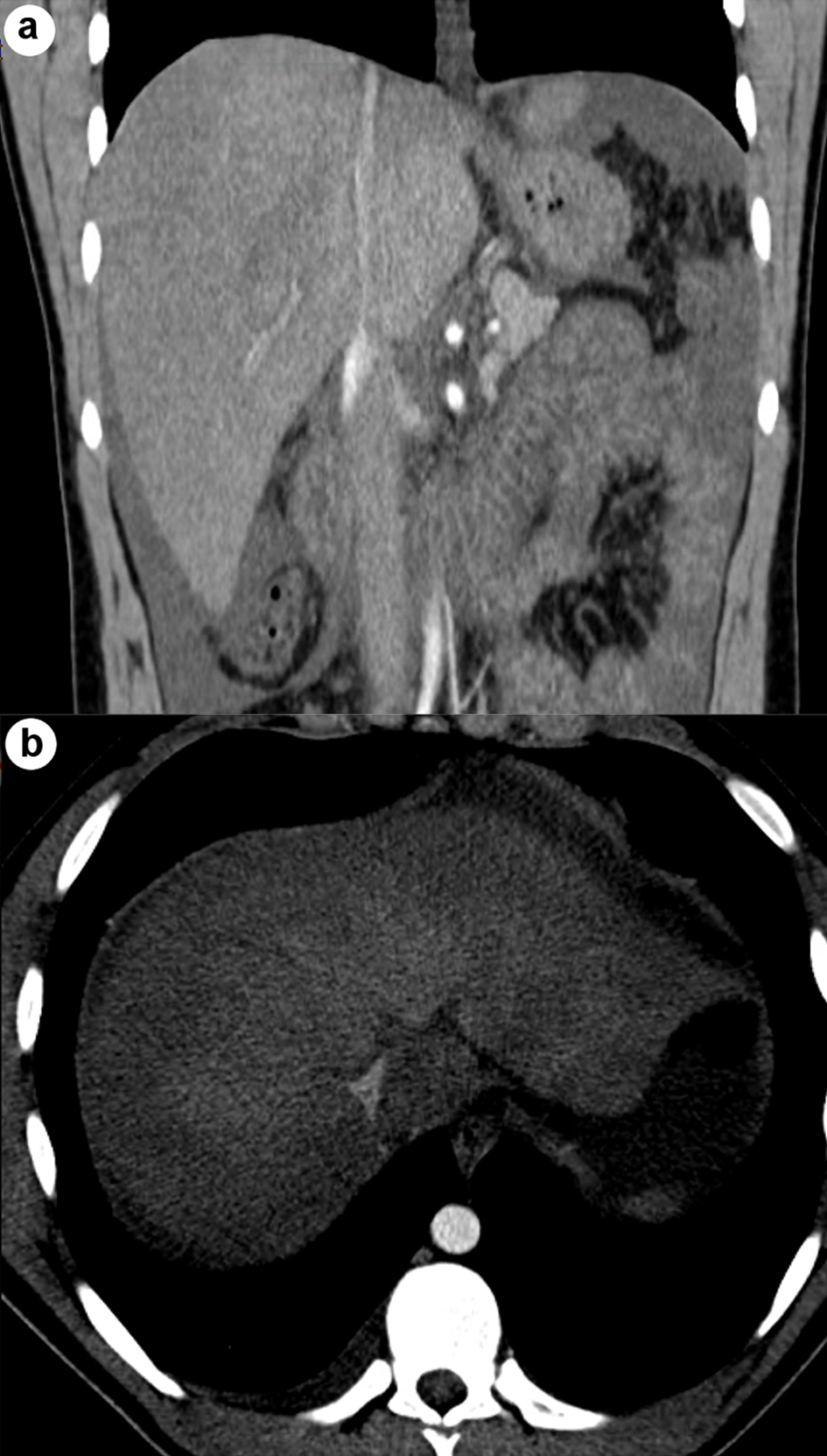 Click for large image | Figure 4. Coronal (a) and axial (b) CT abdomen images show no hepatic vein outflow with mottled inhomogeneous enhancement of the liver. CT: computed tomography. |
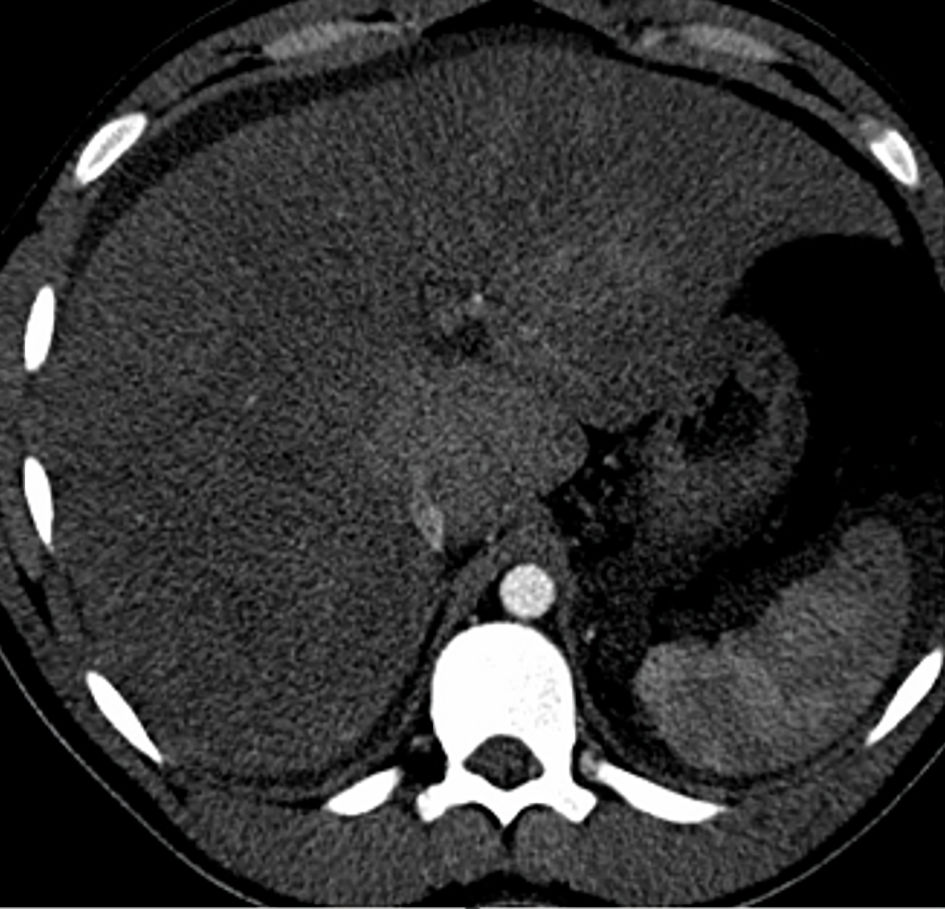 Click for large image | Figure 5. Axial CT abdomen image shows caudate hypertrophy with hyperenhancement. CT: computed tomography. |
Given the patient’s hypercoagulability, anticoagulation was not initiated, and he was not deemed suitable for a transjugular intrahepatic portosystemic shunt (TIPS) procedure due to clinical instability. However, the patient had persistent hemodynamic instability despite aggressive ICU management. Unfortunately, he succumbed to multiorgan system failure.
| Discussion | ▴Top |
BCS is a rare disease characterized by HVOO independent of the level or mechanism of obstruction [1, 2]. This excludes venous obstruction due to sinusoidal obstruction syndrome, pericardial disease, and right-sided heart disease. Venous pooling and increased pressure in the hepatic sinusoids, and the portal system cause hypoxic damage to the hepatocytes. If this occurs acutely, serum transaminases may rise to five times the upper limit of normal, with elevated total bilirubin and a concomitant decrease in albumin [15]. Our patient presented with profoundly elevated serum transaminases. Ascitic fluid analysis in patients with BCS reveals serum-ascitic albumin gradient (SAAG) > 1.1 with total protein greater than 2.5 g/dL [15]. Our patient had small ascites revealed in imaging, so no paracentesis was done.
The clinical presentation of BCS is heterogeneous, from asymptomatic to ALF [1, 2]. The classic triad of ascites, abdominal pain, and hepatomegaly are common in patients with BCS. Four main clinical presentations of BCS have been described depending on the extent and rapidity of HVOO and the presence of collaterals: fulminant liver disease, acute liver disease, subacute liver disease, or chronic [1, 2, 15]. BCS can be further classified depending on etiology as being primary or secondary depending on the exact nature of the HVOO [1, 2, 15]. Multiple risk factors have been identified for BCS, such as primary MPD, inherited thrombophilia (factor V Leiden, G20210A prothrombin, protein C deficiency, protein S deficiency, antithrombin deficiency), oral contraceptives, pregnancy, antiphospholipid syndrome, sarcoidosis, hyperhomocysteinemia, PNH, Behcet disease, and systemic inflammatory diseases [1, 2, 4]. Among these, MPD are commonly associated with BCS in 30-50% of cases [1].
Doppler ultrasonography is readily available and noninvasive in diagnosing BCS with high sensitivity and specificity [1, 2]. Contrast-enhanced CT scan or magnetic resonance imaging (MRI) can also assist in confirming the diagnosis, evaluating ischemia-related changes involving hepatic parenchyma and therapeutic planning for a procedure such as TIPS placement [1, 2, 16]. Venography can be utilized if other noninvasive modalities are not inconclusive, but the suspicion is extremely high [4]. Our patient’s abdominal ultrasound vascular Doppler showed no hepatic vein outflow, but the diagnosis of BCS was confirmed with characteristic CT abdomen findings (Figs. 4, 5).
The risk of venous thrombosis associated with AML is substantial. Acute promyelocytic leukemia (APL) (French-American-British (FAB)-M3) is most frequently associated with DIC and thrombi formation [9, 14]. The factors involved in the pathogenesis of thrombosis include hyperleukocytosis, hyperfibrinolysis, nonspecific proteolytic activity, DIC, presence of short PML-RARa isoform (bcr3), FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication, as well as CD2 and CD15 expression [17, 18]. Thrombosis in acute leukemia can involve any circulations, both venous and arterial. The most common is deep vein thrombosis (DVT, upper extremity) and other sites such as intracranial, pulmonary, and coronary circulation [11, 17]. Hepatic venous thrombosis resulting in acute BCS is very uncommon in patients with acute leukemia.
In our case, BCS was likely secondary to undiagnosed AML on admission. On review of the literature, there are 10 reported cases of BCS in association with AML, of which seven cases were presented with BCS as an initial presentation of AML (Table 2) [5, 7-14, 19]. Of the reported cases, seven cases occurred in APL, including one microgranular variant. Four of these seven cases of APL had fatal outcomes [8, 9, 11, 14], two patients were treated with ATRA with anticoagulation, resulting in vein recanalization with a favorable outcome [13, 19], and the disease course of one case is not available [10]. In another case of fulminant BCS, a patient with non-promyelocytic acute myelogenous leukemia presented with persistent DVT while on anticoagulation and was found to have hepatic venous occlusion [7]. Our patient had a t(11;19)(q23;p13.3) translocation, which has been noted as a variant of both myeloid and lymphoid leukemias and is associated with secondary leukemia. Our patient has a history of Ewing sarcoma that was treated with chemotherapy, which might be the cause of AML in our patient.
 Click to view | Table 2. Summary of Patients With Budd-Chiari Syndrome in Association With AML |
DIC at the time of AML diagnosis is noted to be a predictor of subsequent thrombosis [20]. Diagnosis of DIC requires both a clinical (i.e., bleeding and/or thrombosis) and laboratory component, with findings of prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), decreased fibrinogen, and increased D-dimer levels. Of the laboratory values, D-dimer levels > 4 mg/L were shown to be most predictive of thrombosis [20]. Although we did not initially do so, we recommend considering checking D-dimer levels in the evaluation of newly diagnosed AML patients. DIC commonly occurs in the setting of BCS due to APL with prevalence ranging from 17% to 100% [7, 11, 21]. DIC is mainly associated with bleeding in APL, while thrombosis is common in acute lymphocytic leukemia (ALL) and non-APL AML [21]. In the study reported by Amitrano et al [7] of BCS due to non-promyelocytic AML, no DIC was reported; however, in our case, DIC was suspected due to the patient having both clinical (hepatic vein thrombosis) and laboratory findings consistent with DIC.
Another unique finding in this case, was the patient’s foot pain and ischemic skin changes noted on the patient’s lower extremities (Fig. 1). BTS is caused by conditions that compromise arterial blood circulation and/or venous outflow, causing the blue or violaceous discoloration that occurs in the toes [22-24]. BTS is often attributed to atheroembolism [22, 23]. BTS as the initial presentation of DIC has been reported in an isolated case [22].
In summary, our case highlights uncommon arterio-venous thromboses due to non-promyelocytic AML. To the best of our knowledge, this is first reported case of non-promyelocytic AML who presented with BCS and DIC as presenting symptoms in the USA. In patients presenting with acute BCS with ALF, a high index of suspicion and exclusion of underlying malignancy is a must, as it is a contraindication for LT. Our patient also emphasizes that a hypercoagulable state can present with multiple thromboses as evidenced by concurrent portal vein thrombosis and BTS. Interestingly, BTS is novel in our case, as it was not reported in prior cases of BCS secondary to acute AML. Prompt diagnosis and treatment is critical given the high mortality of both AML and BCS.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors indicated no potential conflict of interest.
Informed Consent
Informed consent was obtained from the patient’s family for publication of this manuscript.
Author Contributions
Jiten P. Kothadia, MD was involved in drafting the case report and the acquisition of available literature and revision of the manuscript. Vanisha Patel, MD and Rajiv Heda, MD were involved in drafting the case report and reviewing literature. Wesley A. Angel provided radiology images and reviewed manuscript. Vishwas Vanar, MD, Benedict J. Maliakkal, MD, and Rajanshu Verma, MD were involved in critical revision of the manuscript for important intellectual content.
Data Availability
The authors declare that the data supporting the findings of this study are presented within the content of this article.
| References | ▴Top |
- Martens P, Nevens F. Budd-Chiari syndrome. United European Gastroenterol J. 2015;3(6):489-500.
doi pubmed - DeLeve LD, Valla DC, Garcia-Tsao G, American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49(5):1729-1764.
doi pubmed - Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38(4):793-803.
doi pubmed - Aydinli M, Bayraktar Y. Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol. 2007;13(19):2693-2696.
doi pubmed - Costa ARG, Freitas I, Raposo J, Barbosa G, Miranda HP, Nery F. Budd-Chiari syndrome and acute liver failure: an uncommon presentation of acute myeloid leukaemia. GE Port J Gastroenterol. 2020;28(1):62-66.
doi pubmed - Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848-4857.
doi pubmed - Amitrano L, Guardascione MA, Schiavone EM, Brancaccio V, Antinolfi I, Iannaccone L, Ferrara F, et al. Hepatic vein thrombosis leading to fulminant hepatic failure in a case of acute non-promyelocytic myelogenous leukemia. Blood Coagul Fibrinolysis. 2006;17(1):59-61.
doi pubmed - Bandyopadhyay S, Bandyopadhyay D. Acute Budd-Chiari syndrome as an initial presentation of acute promyelocytic leukemia. J Cancer Res Ther. 2010;6(4):567-569.
doi pubmed - Chillar RK, Paladugu RR. Case report: hepatic vein thrombosis (acute Budd-Chiari syndrome) in acute leukemia. Am J Med Sci. 1981;282(3):153-156.
doi pubmed - Havelange V, Annet L, Poire X, Lambert C, Vekemans MC. Budd-Chiari syndrome in a patient with acute promyelocytic leukaemia. Br J Haematol. 2014;166(1):1.
doi pubmed - Kayal S, Singhal B, Thulkar S, Mishra J, Kumar R, Bakhshi S. Acute Budd-Chiari syndrome in pediatric acute promyelocytic leukemia. Leuk Lymphoma. 2011;52(8):1611-1614.
doi pubmed - Kurt M, Shorbagi A, Altundag K, Elkiran T, Gullu I, Kansu E. Possible association between Budd-Chiari Syndrome and gemtuzumab ozogamicin treatment in a patient with refractory acute myelogenous leukemia. Am J Hematol. 2005;80(3):213-215.
doi pubmed - Natasa C, Nada S, Ana V, Irena D, Dragica T. Favorable outcome of Budd-Chiari syndrome in acute promyelocytic leukemia. International Journal of Medical and Pharmaceutical Case Reports. 2014;2(5):126-130.
doi - Riccio JA, Colley AT, Cera PJ. Hepatic vein thrombosis (Budd-Chiari syndrome) in the microgranular variant of acute promyelocytic leukemia. Am J Clin Pathol. 1989;92(3):366-371.
doi pubmed - Menon KV, Shah V, Kamath PS. The Budd-Chiari syndrome. N Engl J Med. 2004;350(6):578-585.
doi pubmed - Noone TC, Semelka RC, Siegelman ES, Balci NC, Hussain SM, Kim PN, Mitchell DG. Budd-Chiari syndrome: spectrum of appearances of acute, subacute, and chronic disease with magnetic resonance imaging. J Magn Reson Imaging. 2000;11(1):44-50.
doi - Breccia M, Avvisati G, Latagliata R, Carmosino I, Guarini A, De Propris MS, Gentilini F, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007;21(1):79-83.
doi pubmed - Kwaan HC, Vicuna B. Incidence and pathogenesis of thrombosis in hematologic malignancies. Semin Thromb Hemost. 2007;33(4):303-312.
doi pubmed - Assouline D, Negrier C, Coguard I, Archimbaud E, Fiere D, Bret M, Bodnar D. Successful systemic thrombolysis of hepatic vein thrombosis in a patient with promyelocytic leukemia treated with all-trans retinoic acid. Am J Hematol. 1995;48(4):291-292.
doi pubmed - Libourel EJ, Klerk CPW, van Norden Y, de Maat MPM, Kruip MJ, Sonneveld P, Lowenberg B, et al. Disseminated intravascular coagulation at diagnosis is a strong predictor for thrombosis in acute myeloid leukemia. Blood. 2016;128(14):1854-1861.
doi pubmed - Ten Cate H, Leader A. Management of disseminated intravascular coagulation in acute leukemias. Hamostaseologie. 2021;41(2):120-126.
doi pubmed - Choi KH, Yoo J, Huh JW, Jeong YI, Kim MS, Jue MS, Park HJ. Blue toe syndrome as an early sign of disseminated intravascular coagulation. Ann Dermatol. 2016;28(3):400-401.
doi pubmed - Shah N, Nagalli S. Cholesterol Emboli. In: StatPearls. Treasure Island (FL), 2022.
- Hirschmann JV, Raugi GJ. Blue (or purple) toe syndrome. J Am Acad Dermatol. 2009;60(1):1-20; quiz 21-22.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


