| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 15, Number 2, April 2022, pages 67-74
The Incidence of Venous Thromboembolism and Practice of Deep Venous Thrombosis Prophylaxis Among Hospitalized Cirrhotic Patients
Mira Alsheikha, e, Khalil Kamarb, Malek Kreidiehc, Rola Sassoc, Samragnyi Madalac, Rubal Sharmad, Hassan Al Moussawia, Liliane Deeba
aDepartment of Gastroenterology and Hepatology, Northwell Health Staten Island University Hospital, Staten Island, NY 10305, USA
bDepartment of Hematology and Oncology at SUNY Downstate Medical Center, Brooklyn, NY, USA
cDepartment of Internal Medicine, Northwell Health Staten Island University Hospital, Staten Island, NY 10305, USA
dDepartment of Hematology and Oncology, Northwell Health Staten Island University Hospital, Staten Island, NY 10305, USA
eCorresponding Author: Mira Alsheikh, Department of Gastroenterology and Hepatology, Northwell Health Staten Island University Hospital, Staten Island, NY 10305, USA
Manuscript submitted January 30, 2022, accepted March 22, 2022, published online April 12, 2022
Short title: VTE Prophylaxis in Patients With Liver Cirrhosis
doi: https://doi.org/10.14740/gr1493
| Abstract | ▴Top |
Background: Patients with liver cirrhosis have altered hepatic synthetic functions which theoretically result in reduced levels of pro-and anti-coagulant factors as well as thrombocytopenia. Initially, cirrhotic patients were thought to be at an increased risk of bleeding and a reduced risk of thrombosis. Several studies have recently reported an increased occurrence of venous thromboembolism (VTE) in cirrhotic patients. In this study, we aimed to assess the current practice of deep venous thrombosis (DVT) prophylaxis, the incidence and predictors of VTE, and the associated bleeding sequelae in patients with liver cirrhosis.
Methods: A retrospective cohort study was performed. We included all adult patients with a diagnosis of liver cirrhosis from January 2010 to June 2019 admitted to the hospital. Our cohort patients were divided into two groups, cirrhotic patients with pharmacological VTE prophylaxis and those with mechanical or no VTE prophylaxis.
Results: We included 601 cirrhotic patients in our study. The incidence of VTE occurring within the first 6 months of their admission was 1.5%. Seven patients (1.49%) developed VTE with the majority being DVTs while not on pharmacologic prophylaxis, and two patients developed VTE despite being on pharmacologic prophylaxis; however, there was no statistical difference. Alcohol use was the most common underlying cause of liver cirrhosis (40.4%), followed by chronic hepatitis C (21.1%), and nonalcoholic steatohepatitis (11.3%). Out of the 601 patients included, 69 patients received neither pharmacologic nor mechanical VTE prophylactic agent (11.48%), while the remaining majority received either pharmacological or mechanical prophylaxis (88.52%).
Conclusions: Our study did not show a statistically significant association between the use of pharmacological VTE prophylactic agents and a reduction in the risk of VTE in cirrhotic patients. The rates of usage of DVT prophylactic agents among our Northwell hospitals during the study period appeared to be no longer suboptimal when compared to prior studies. Low albumin appears to be a predictor factor to develop VTE. There was a statistically significant increase in bleeding risk and transfusion requirement in cirrhotic patients receiving no pharmacological VTE prophylactic agents. Further prospective trials are needed to shed more light on this subject and identify the group of cirrhotic patients who could safely benefit from pharmacologic VTE prophylaxis.
Keywords: Cirrhosis; Deep vein thrombosis; Bleeding; Deep vein thrombosis prophylaxis
| Introduction | ▴Top |
Hemostasis is a complex physiological process to control bleeding at the site of endothelial injury. The coagulation cascade integrates both cellular and humoral reactions that drive in opposite directions, with one resulting in blood clot formation and another ending in the activation of the anticoagulation system [1]. The liver plays a crucial role in hemostasis as it influences the coagulation cascade and fibrinolysis by producing and regulating most pro- and anti-coagulant factors except calcium and von Willebrand factor, which is produced by endothelial cells.
In case of hepatic dysfunction from progressive fibrosis, as seen in chronic liver disease and liver cirrhosis, this synthetic and regulatory function is impaired. This results in prolongation of the prothrombin time which reflects the decreased activity of factors I, II, V, VII, and X. The reduction in the hepatic synthetic function increases bleeding risk secondary to a reduction in coagulation factors, as well as a reduction in thrombopoietin secretion which regulates the proliferation and differentiation of megakaryocytes and platelet formation. In addition, portal hypertension and splenomegaly resulting from liver disease contribute to platelet destruction and sequestration in the enlarged spleen leading to thrombocytopenia. Portal hypertension also results in the formation of esophageal, gastric, and rectal varices which places cirrhotic patients at higher risks of gastrointestinal bleeding. In light of the above-mentioned, patients with advanced liver disease and cirrhosis have been initially thought to be at increased risk of bleeding and reduced risk of thrombosis. As a result, the in-patient use of pharmacologic venous thromboembolism (VTE) prophylaxis in these patients has been limited.
Several studies have recently reported an increased occurrence of VTE in cirrhotic patients. For instance, the incidence of non-portal VTE in patients with chronic liver disease has been shown to range between 0.5% and 6.3% in multiple reports [2-4]. This has been thought to be related to both, a reduction in the anticoagulant factors, antithrombin III and proteins S or C, and an increase in procoagulant factors, including factor VIII and von Willebrand factor, in cirrhosis. Also, cirrhotic patients are predisposed to VTE due to frequent hospitalizations with decompensated disease, immobility secondary to lower extremities edema and ascites, and raised levels of estrogen and inflammation [5]. As such, the novel idea of “rebalanced hemostasis” emerged, whereby cirrhotic patients can have an inclination toward bleeding or thrombotic events depending on the balance between their pro- and anti-coagulant factors as determined by their corresponding clinical settings.
The current guidelines recommend using low-molecular-weight heparin, low-dose unfractionated heparin, or fondaparinux as VTE prophylaxis agents for all hospitalized patients with acute medical illnesses who are at increased risk of thrombosis [6]. Those guidelines excluded cirrhotic patients from the studies evaluating the benefit of VTE prophylaxis. This lack of clear recommendations in cirrhotic patients, in addition to the bleeding concerns, limits the use of VTE prophylaxis in this population.
Thus, this study was conducted to assess the practice of VTE prophylaxis and the incidence and predictors of VTE and bleeding sequelae in patients with liver cirrhosis.
| Materials and Methods | ▴Top |
Study population
A retrospective cohort study with chart review was conducted on cirrhotic patients who were admitted to any of the following Northwell hospitals: Glen Cove Hospital, Huntington Hospital, Lenox Health Greenwich Village, Lenox Hill Hospital, LIJ (Long Island Jewish) Forest Hills, LIJ Valley Stream, Long Island Jewish, NSUH (Northshore University hospital), Plainview Hospital, SIUH (Staten Island University Hospital) North and South, Southside Hospital and Syosset Hospital. Electronic medical records were reviewed between January 2010 and June 2019. Included patients were older than 18 years who were admitted for at least 2 days with the International Classification of Diseases (ICD Ninth and 10th editions (ICD-9 and ICD-10)) diagnosis codes of 571.5 and K74.60. Patients on anticoagulation, oral contraceptives, or other hormonal replacement therapies at the time of admission were excluded from the study. Pregnant patients and patients with a history of VTE, active malignancy, or thrombophilia were also excluded. The study was approved by the Northwell Institutional Review Board. The approval allowed for retrospective chart review and anonymous results reporting without informed consent. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data collection
For each patient, the following information was collected: age, gender, race, body mass index (BMI), admission platelet count, hemoglobin, serum albumin, total bilirubin, and international normalized ratio (INR). History of alcohol or tobacco was also recorded. Known risk factors for VTE, including surgery within the preceding 30 days, infection, and intensive care stay were also collected. The presence of ascites, esophageal varices, and hepatic encephalopathy was documented. Child-Pugh score and MELD-Na score were determined accordingly.
The diagnosis of cirrhosis was defined based on clinical and pathologic criteria. Only patients with documented cirrhosis in the medical records were included. Histologic diagnosis was not required. Etiologies of cirrhosis were identified as alcohol-related, chronic viral hepatitis C, chronic hepatitis B, nonalcoholic fatty liver disease, and others which included autoimmune disease, drug-related, hemochromatosis, primary biliary cirrhosis, cystic fibrosis, primary sclerosing cholangitis, Wilson disease, etc.
Furthermore, any type of mechanical or pharmacologic VTE prophylaxis administered during hospitalization was recorded, such as sequential compression device (SCD), low-molecular-weight heparin, unfractionated heparin, or fondaparinux.
Patients were followed until 6 months post-discharge.
Outcome measures
The primary outcome was defined as the development of VTE including deep venous thrombosis (DVT), splanchnic vein thrombosis, or pulmonary embolism, as confirmed by an extremity Doppler ultrasound, computed tomography scan of the chest or abdomen, or ventilation-perfusion scan.
The secondary outcomes included bleeding events, transfusion requirements, a 6-month readmission rate, and hospital length of stay.
Statistical analysis
Patients were divided into two study groups: patients who were started on pharmacological VTE prophylaxis and those who were placed on mechanical or no prophylaxis. Baseline characteristics were compared among the two groups.
Variables were expressed as means or medians with standard deviation (SD) or interquartile range (IQR) according to normality testing using Kolmogorov-Smirnov tests. Categorical values were expressed as units and percentages. Comparisons were performed using analysis of variance, Kruskal-Wallis test for continuous variables, and χ2 or Fisher exact tests for categorical variables. Significance was defined as a P value ≤ 0.05, and all tests were two-sided.
| Results | ▴Top |
Study identification algorithm
Using the above-mentioned ICD-9 and ICD-10 codes for cirrhosis, 16,004 charts were identified and reviewed between January 2010 and June 2019 across the listed Northwell hospitals. A total of 10,637 charts corresponding to patients with cirrhosis who were not hospitalized were consequently excluded. Similarly, 700 other charts that met the exclusion criteria were excluded thereafter. Out of the remaining pool of 4,677 charts corresponding to hospitalized patients with cirrhosis, 1,191 ones were randomly selected. A total of 601 patients met the inclusion criteria and were included in the final analysis (Fig. 1).
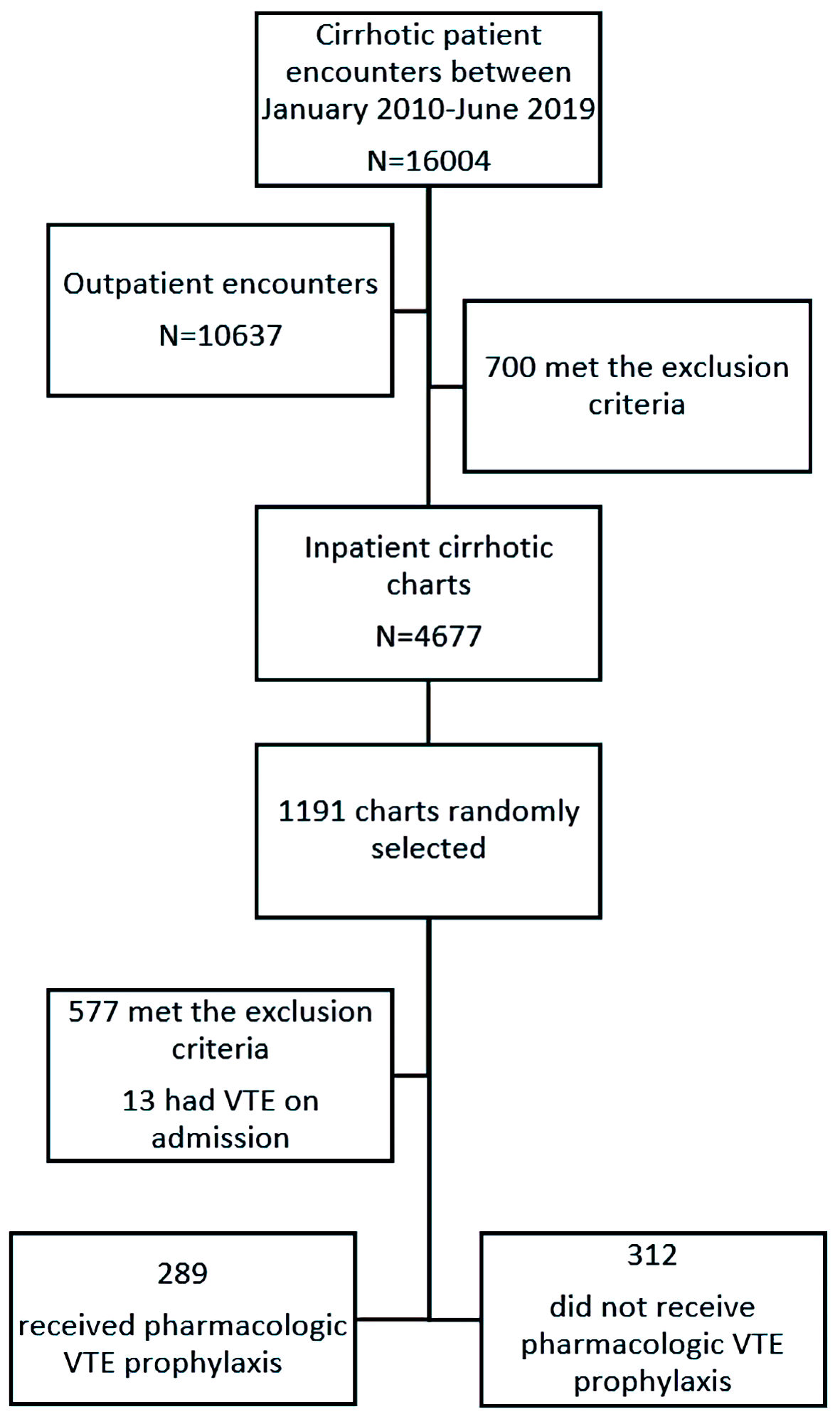 Click for large image | Figure 1. Study identification algorithm. VTE: venous thromboembolism. |
The 601 study subjects were divided into two groups depending on whether a pharmacologic VTE prophylactic agent was administered (first group) or if mechanical VTE prophylaxis or no prophylaxis was initiated (second group). The baseline characteristics are presented in Table 1.
 Click to view | Table 1. Baseline Characteristics of Included Patients With Liver Cirrhosis |
General characteristics and practices of VTE prophylaxis
Among the included 601 patients, 69 patients (11.48%) received neither pharmacologic nor mechanical thromboembolism prophylactic agent, while the remaining majority received either pharmacological or mechanical prophylaxis. Cirrhotic patients receiving either mechanical or no VTE prophylaxis had a significantly lower median age of 61.42 compared to 65.62 in those receiving pharmacologic VTE prophylactic agents with a significant P value of 0.001 (Table 1).
In terms of comorbid conditions, cirrhotic patients receiving pharmacologic VTE prophylactic agents were significantly more likely to have a history of coronary artery disease (21.1%), heart failure (22.1%), hypertension (61.6%), end-stage renal disease on hemodialysis(7.3%), diabetes mellitus (37.7%) and active infection (34.3%) than those receiving no pharmacologic VTE prophylaxis (11.3%, 11.2%, 44.9%, 3.5%, 29.8%, and 24.4%, respectively) as reflected by the significant P value of < 0.05 (Table 1).
When it comes to social history, cirrhotic patients who drink alcohol were significantly more likely (47.1%) to receive either mechanical or no VTE prophylaxis than to receive pharmacologic VTE prophylactic agents (37.6%) as reflected by the significant P value of 0.02.
Among our study population, the most common causes of liver cirrhosis were alcohol (243/601, 40.4%), followed by hepatitis C (127/601, 21.1%), and nonalcoholic steatohepatitis (68/601, 11.3%). Cirrhotic patients receiving pharmacologic VTE prophylaxis were significantly less likely to have a history of hepatic encephalopathy, esophageal varices, or ascites upon admission (24.4%, 31%, and 46.9%, respectively) compared to those receiving either mechanical or no VTE prophylaxis (16.3%, 9.8%, and 34.9%, respectively) (Table 1).
Labs on admission including platelet counts, hemoglobin, INR, albumin, creatinine; and bilirubin were significantly different between the two groups. No statistically significant difference in the MELD-Na score existed between both groups (P value > 0.05). Of note, included cirrhotic patients receiving either mechanical or no VTE prophylaxis were significantly more likely to have a lower platelet count (mean of 119), higher INR (mean of 1.53), and lower hemoglobin (mean of 10.71) than those receiving pharmacologic VTE prophylactic agents (means of 172.03, 1.33, and 11.88, respectively) as reflected by significant P values of 0.001 or less (Table 1).
Incidence and risk factors for VTE
The incidence of VTE occurring within the first 6 months of admission was 1.5% (9/601) (Tables 2 and 3). A total of nine patients developed VTE within 6 months of admission, seven in the group receiving either mechanical or no pharmacologic VTE prophylaxis compared to two patients in the group receiving pharmacologic prophylaxis with a nonsignificant P value of 0.118 (Table 2). Among the seven cirrhotic patients who developed VTE, four (4/7, 57.1%) were on sequential compression devices and three (3/7, 42.8%) were not on any prophylaxis. Of note, the two patients who developed VTE while on pharmacologic VTE prophylaxis were receiving subcutaneous unfractionated heparin. Although the likelihood of VTE in the pharmacologic thromboembolism prophylaxis group was lower (2/289, 0.69%) compared to the non-pharmacologic thromboembolism prophylaxis group (7/312, 2.2%), these results were statistically insignificant (P value > 0.05).
 Click to view | Table 2. The Impact of VTE Prophylaxis on the Incidence of VTE and Outcomes Among Subgroups of Hospitalized Cirrhotic Patients |
 Click to view | Table 3. List of Hospitalized Cirrhotic Patients With Newly Diagnosed VTE: Risk Factors, Diagnostic Modality, Location, and Days to Diagnosis |
The majority of VTE cases (6/9, 66.7%) were lower extremities deep vein thrombosis involving, common femoral (3/6, 50%), the iliac, and popliteal veins. One out of the six cases involved the popliteal vein and was associated with left pulmonary embolism, ultimately requiring Coumadin initiation. Interestingly, this patient was the one with the highest INR (3.42) among the nine cases and the second-highest INR among the 601 patients. The remaining three venous thrombosis cases involved either the subclavian, brachial, or portal veins.
Four of the nine VTE cases occurred while the patients were still hospitalized, with a mean duration of 12.8 days to developing VTE (Table 3). Of note, the mean duration to developing VTE was longer (25.5 days) in the group receiving pharmacologic VTE prophylaxis compared to the second group receiving either mechanical or no prophylaxis (9.3 days).
The nine cirrhotic patients who developed VTE were older (mean age 67.8 years) than the remaining 592 cirrhotic patients who did not (mean age 63.56 years). In addition, these patients had higher INR on presentation with a mean INR of 1.56 compared to 1.44, lower serum albumin levels with a mean of 2.54 compared to 3.01 among the patients without VTE. The platelet counts were similar among both groups (mean 145.2 compared to 144.4).
Bleeding risk associated with VTE prophylaxis in cirrhotic patients
It was noted that patients receiving either mechanical or no VTE prophylaxis were significantly more likely to bleed (20.2%) or require blood transfusions (31.1%) compared to those receiving pharmacologic VTE prophylaxis (2.8% and 12.5%, respectively) (P values < 0.001) (Table 2).
Length of stay in the hospital and readmission rates associated with VTE prophylaxis in cirrhotic patients
Concerning secondary outcomes of 6-months readmission rates and lengths of stay, no statistically significant difference was noted among cirrhotic patients receiving pharmacologic VTE prophylaxis and those receiving mechanical or no prophylaxis (P values of 0.42 and 0.118, respectively) (Table 2).
| Discussion | ▴Top |
Although our study results show that most cirrhotic patients who developed VTE (7/9, 77.7%) belonged to the group receiving either mechanical or no pharmacologic VTE prophylaxis, the difference was not statistically significant (P value = 0.118).
The incidence of VTE occurring within the first 6 months of our cirrhotic patients’ admission was 1.5% (9/601). This aligns well with the rate of 0.5-6.3%, reported in the literature, of VTE occurring in hospitalized patients with chronic liver disease [2-4, 7-10]. More so, the risk of VTE in cirrhotic patients seems to be higher than the general population even outside the hospital, as many reports have shown that around 1.8% of cirrhotic patients’ admission diagnoses are related to VTE [4, 7, 11]. This predisposition to VTE in cirrhotic patients has been thought to be related to both, a reduction in the anticoagulant factors and an increase in procoagulant factors. It has also been associated with increased hospitalizations from decompensated disease, immobility secondary to lower extremities edema and ascites, and increased levels of estrogen and inflammation.
The nine cirrhotic patients who developed VTE in our study were older in contrast to a previous study by Wu et al, which showed an increased incidence of VTE in patients with cirrhosis (compensated or decompensated) younger than 45 years. This difference in results could be related to the fact that younger patients are less likely to receive VTE prophylaxis, nonetheless, cirrhotic patients are at increased risk of VTE regardless of age [5].
Elevated INR and low albumin were identified as risk factors for VTE in our study, but platelets count seems to have no influence. According to results from two retrospective cohort studies designed by Northup et al [2] and Garcia Fuster et al [3], the risk of developing VTE increases in patients with low serum albumin, but seems to be independent of elevated INR or low platelet count. The association between low serum albumin and risk of VTE could be related to the fact that serum albumin is a marker of liver synthetic function, and it indirectly reflects an imbalance of anticoagulant factors, including antithrombin III, protein C, and protein S. In another retrospective study by Dabbagh et al [4], it was confirmed that elevated INR values exceeding 2.2 do not necessarily protect patients with the chronic liver disease against the risk of VTE.
The belief that patients with cirrhosis are at a higher risk of bleeding rather than thrombosis has been reflected by several studies in the past demonstrating limited administration of VTE prophylaxis to cirrhotic patients. In a retrospective cohort study on DVT prophylaxis in hospitalized cirrhotic patients, by Aldawood et al in 2009, around 76% of cirrhotic patients received neither pharmacological nor mechanical DVT prophylaxis [10]. In our study, only 11.48% of the 601 included cirrhotic patients received neither pharmacologic nor mechanical thromboembolism prophylaxis, reflecting an increased awareness about the risk of VTE in cirrhotic patients. In addition, our study results demonstrated that patients receiving no pharmacologic VTE prophylaxis were more likely to bleed or require blood transfusions. This might reflect the health care providers’ fear to provide pharmacologic VTE prophylaxis to certain groups with higher bleeding risk. More studies are needed to identify this subgroup of patients.
Study strengths
Since our study evaluates the incidence of a rare outcome, which is VTE in cirrhotic patients, we included a large sample size (601) collected over 10 years from January 2010 to June 2019 to minimize and avoid type II (beta) error. To ensure that our included pool of patients is heterogeneous, from different cultural, social, and ethnical backgrounds, we aimed at reviewing charts corresponding to patients admitted to different Northwell hospitals spread across many geographical areas, namely the Glen Cove Hospital, Huntington Hospital, Lenox Health Greenwich Village, Lenox Hill Hospital, LIJ Forest Hills, LIJ Valley Stream, Long Island Jewish, NSUH, Plainview Hospital, SIUH North and South, Southside Hospital and Syosset Hospital.
Study limitations
The major limitation of our study is its retrospective design which relies on the data entered into the electronic medical record system. In our study, however, we followed a certain methodological approach to overcome the limitations and errors associated with retrospective chart reviews.
The diagnosis of cirrhosis was initially extracted from the ICD-9 and ICD-10 codes; however it was subsequently confirmed with a comprehensive review of blood tests, imaging studies, and gastroenterologists’ documentation. Moreover, the VTE diagnosis was based on official reports of ultrasonographic or computed tomographic imaging rather than just ICD-9 or ICD-10 codes. The documentation of bleeding on chart notes drops in hemoglobin, or blood transfusions administered. Another study limitation is that patients were only followed up for 6 months post-discharge. In addition, the two study groups had variable baseline characteristics. Moreover, although we excluded pregnant patients and patients with a history of VTE, active malignancy, or thrombophilia, patients with recent orthopedic surgeries were included which might have falsely increased the rates of VTE development in cirrhotic patients.
Conclusions
In conclusion, the incidence of VTE in cirrhosis occurring within the 6 months of admission was 1.5%. The trends of usage of DVT prophylactic agents seemed to be no longer suboptimal compared to previously reported practices. However, our study results did not show a statistically significant association between the use of pharmacological VTE prophylactic agents and a reduction in the risk of VTE in cirrhotic patients.
Cirrhotic patients tend to have a complex coagulation profile. This makes it very challenging for physicians to determine whether and when to institute prophylactic anticoagulation in patients with advanced liver disease. Large prospective interventional randomized controlled trials are needed to better identify the clinical and demographic predictors of VTE, as well as mitigate current safety concerns perceived with the use of anticoagulation in cirrhotic patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The study was approved by the Northwell Institutional Review Board. The approval allowed for retrospective chart review and anonymous results reporting without informed consent.
Author Contributions
Mira Alsheikh and Khalil Kamar carried out the study design, data entry, and critical revision of the manuscript. Malek Kreidieh carried out the drafting of the manuscript and its critical revision. Rola Sasso carried out data entry and statistical analysis. Samragnyi Madala and Rubal Sharma carried out data entry. Liliane Deeb and Hassan Al Moussawi carried out the critical revision of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, de Franchis R, Colombo M, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137(6):2105-2111.
doi pubmed - Northup PG, McMahon MM, Ruhl AP, Altschuler SE, Volk-Bednarz A, Caldwell SH, Berg CL. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101(7):1524-1528.
doi pubmed - Garcia-Fuster MJ, et al. VTE and liver cirrhosis. Rev Esp Enferm Dig. 2008;100(5):259-262.
doi pubmed - Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145-1149.
doi pubmed - Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8(9):800-805.
doi pubmed - Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S-e226S.
doi pubmed - Ali M, Ananthakrishnan AN, McGinley EL, Saeian K. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig Dis Sci. 2011;56(7):2152-2159.
doi pubmed - Ambrosino P, et al. The risk of VTE in patients with cirrhosis. A systematic review and meta-analysis. Thromb Haemost. 2017;117(1):139-148.
doi pubmed - Gulley D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53(11):3012-3017.
doi pubmed - Aldawood A, Arabi Y, Aljumah A, Alsaadi A, Rishu A, Aldorzi H, Alqahtani S, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1.
doi pubmed - Sogaard KK, Horvath-Puho E, Gronbaek H, Jepsen P, Vilstrup H, Sorensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104(1):96-101.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


