| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website https://www.gastrores.org |
Original Article
Volume 14, Number 4, August 2021, pages 220-226
Usefulness of the Eosinophilic Esophagitis Histologic Scoring System in Distinguishing Active Eosinophilic Esophagitis From Remission and Gastroesophageal Reflux Disease
Bo Lina, b, Simon Rabinowitzc, M.A. Haseeba, b, d, Raavi Guptaa, e
aDepartment of Pathology, University of New York, Downstate Health Sciences University, Brooklyn, NY 11203, USA
bDepartment of Pathology, Kings County Hospital Center, Brooklyn, NY 11203, USA
cDepartment of Pediatrics, University of New York, Downstate Health Sciences University, Brooklyn, NY 11203, USA
dDepartment of Medicine, State University of New York, Downstate Health Sciences University, Brooklyn, NY 11203, USA
eCorresponding Author: Raavi Gupta, Department of Pathology, SUNY Downstate Health Sciences University, Brooklyn, NY 11203, USA
Manuscript submitted May 28, 2021, accepted June 26, 2021, published online July 28, 2021
Short title: Utility of Histologic Score in EoE
doi: https://doi.org/10.14740/gr1423
| Abstract | ▴Top |
Background: Eosinophilic esophagitis (EoE) is defined as esophageal dysfunction in the presence of > 15 intraepithelial eosinophils per high-power field (eos/hpf) in either the mid or distal esophagus. The current focus of EoE pathologic evaluation is the peak eosinophil count (PEC), although histologic features other than eosinophilic inflammation are also commonly observed. In addition, histologic variance between the mid and distal esophagus in EoE has not been rigorously studied. The aim of our study was to utilize a recently developed EoE histologic scoring system (EoEHSS) to compare the mid and the distal esophageal histology in patients with active EoE (EoE-A), EoE in remission (EoE-R), and gastroesophageal reflux disease (GERD).
Methods: EoEHSS was used to prospectively evaluate the severity and extent of changes in multiple histopathologic features (PEC; basal zone hyperplasia (BZH); eosinophilic abscesses (EA); eosinophil surface layering (ESL); dilated intercellular spaces (DIS); surface epithelial alteration (SEA); dyskeratotic epithelial cells (DEC); lamina propria fibrosis (LPF)) in the mid and distal esophageal biopsies in 85 pediatric patients at a tertiary medical center. These patients were divided into three cohorts: EoE-A (n = 36), EoE-R (n = 12) and GERD (n = 37).
Results: Total grade (severity) and stage (extent) scores were significantly higher in EoE-A compared to EoE-R and GERD patients in both the mid and the distal esophagus. The mean total grade scores in the mid esophagus, but not the distal esophagus remained higher in EoE-R as compared to GERD patients. Specific histopathologic features independent of PEC were different in distal and mid esophagus in EoE-A. About one-half of children with active EoE had different EoEHSS scores in their mid and distal esophageal biopsies.
Conclusions: EoEHSS yields histologic insights beyond those derived from PEC and helps in more objective, reproducible and accurate diagnosis of EoE and GERD. It also provides a more comprehensive understanding into the pathophysiology of EoE.
Keywords: Eosinophilic esophagitis; EoEHSS; Peak eosinophil count
| Introduction | ▴Top |
Eosinophilic esophagitis (EoE) is a clinicopathological condition defined clinically by non-specific manifestations of esophageal dysfunction in young children, and dysphagia and food impaction in older adolescents, in the presence of > 15 intraepithelial eosinophils per high-power field (eos/hpf) [1-3]. Esophageal eosinophilia itself is a relatively non-specific finding, and the diagnosis of EoE requires a close correlation between clinical findings and histopathological features [2, 4]. Although esophageal eosinophilia is seen in a variety of diseases, the principal mimic of EoE is gastroesophageal reflux disease (GERD) [5, 6]. Histologically, GERD is characterized by mild to moderate eosinophilia generally localized to the distal esophagus. EoE often also includes mid esophageal eosinophilia, superficial eosinophilic abscesses (EA), degranulation of eosinophils, submucosal fibrosis, and deeper involvement of the esophageal wall [5, 6]. Clinical symptoms such as dysphagia and heartburn can be seen in both conditions; therefore, it can sometimes be a clinical challenge to differentiate EoE and GERD [7].
The current focus of evaluating esophageal biopsies in patients with suspected EoE is the severity of eosinophilic infiltration, i.e., peak eosinophil count (PEC). Reduced PEC constitutes an endpoint in clinical trials of therapies for EoE and is a universal goal in clinical management [4]. Several previous investigators have attempted to identify additional histologic features that would further characterize EoE [8-10]. Microabscesses, superficial layering, surface sloughing, desquamation, and degranulation have been observed more commonly in patients with EoE than those with GERD [8]. Other more comprehensive scoring systems to evaluate EoE have been proposed. These have uniformly included several “core features”: eosinophil microabscesses, basal zone hyperplasia (BZH), dilated intercellular spaces (DIS), and lamina propria fibrosis (LPF) [8-10]. Subsequently, additional features were introduced to compare two clinical subgroups of EoE: EoE-dysphagia (EoE-D) and EoE-abdominal pain (EoE-AP). Microabscesses, superficial layering of eosinophils, desquamation, and eosinophilic distribution around rete pegs were found to be significantly more common in EoE-D than EoE-AP [9]. A slightly different scoring system was created to account for eosinophils in the epithelium and lamina propria, basal zone hyperplasia, dilated intercellular spaces, desquamation and lamina propria fibrosis to evaluate EoE [10].
A recently published EoE histologic scoring system (EoEHSS) employs eight histologic features including: PEC; BZH; EA; eosinophil surface layering (ESL); DIS; surface epithelial alteration (SEA); dyskeratotic epithelial cells (DEC); and lamina propria fibrosis (LPF). Each feature is assigned two scores: grade (severity) and stage (extent) using a four-point Linkert scale [11]. This scoring system was able to distinctly identify untreated and treated EoE patients based on grade and stage scores. Subsequently, other investigators have independently established the value of EoEHSS [12]. It is now recognized as the most valid, reliable index currently available for measurement of histological disease activity in EoE [13]. The present study employs a novel application of the EoEHSS in which the total grade and stage scores and the individual histologic features were compared between the mid and distal esophagus in children with active EoE (EoE-A), EoE in remission (EoE-R), and GERD.
| Materials and Methods | ▴Top |
Patient groups
Eighty-five patients with suspected, diagnosed, and treated EoE were enrolled in a prospective study at a tertiary care center. Parents of children under 18 years and patients who were 18 years old consented and children above 7 years assented prior to participation. Patients were divided into three cohorts: 1) EoE-A diagnosed by consensus guidelines [14] (n = 36; 76 esophageal biopsies, 39 distal, 37 mid), including proton pump inhibitor (PPI) non-responders; 2) EoE patients who received treatment with PPI, dietary exclusion, and topical steroids that yielded clinical and histopathologic remission defined as < 15 eosinophils/hpf in at least six biopsies from the mid and distal esophagus (EoE-R) (n = 12; 24 esophageal biopsies, 12 distal, 12 mid); and 3) control patients who underwent endoscopy for evaluation of esophageal symptoms, but had neither endoscopic or histologic features of EoE, and GERD accounted for their esophageal dysfunction (n = 37; 74 esophageal biopsies, 37 distal, 37 mid). Majority of the patients with EoE had a history of asthma or atopic conditions as part of the disease spectrum, and none of the participants reported smoking.
This study was conducted in compliance with the ethical standards of the institution on human tissue, and it was approved by the Institutional Review Board and Privacy Board (IRB) of the State University of New York Downstate Medical Center (IRB: 703018-5).
EoEHSS scoring
Eight histologic features as described in EoEHSS were identified and scored at mid and distal esophagus separately [11]. These features include: PEC; BZH; EA; ESL; DIS; SEA; DEC; and LPF. Each feature was quantitatively assigned grade (severity) and stage (extent) scores using a four-point scale (0 normal; 3 maximum change). Total grade and stage scores were calculated and compared among the cohorts for both mid and distal esophagus.
Severity of PEC was categorized into three groups: low < 15 eos/hpf, moderate 15 - 59 eos/hpf and high > 60 eos/hpf as has been previously described [11]. The extent of eosinophils was categorized as low (involving < 1/3 of epithelium), moderate (involving 1/3 to 2/3 of epithelium) and high (involving > 2/3 of epithelium).
Two of authors (BL and RG) independently evaluated the biopsies and for the few cases with different scores, consensus was reached through mutual agreement.
Statistical analysis
Data were analyzed using SPSS (v. 18.0). Mann-Whitney U test was used to compare the scores between cohorts and P < 0.05 was considered statistically significant. Pearson correlation coefficient test was used to compare the severity and extent of PEC and the total grade and stage scores, respectively.
| Results | ▴Top |
Demographics
There were 60 males and 25 females, and their median age was 10.2 years (range: 1 - 21 years) at the time of endoscopy. Thirty-seven patients diagnosed with GERD who served as controls had a median age of 11 years (range: 1 - 21 years) and a male/female ratio of 1.3:1. Thirty-six patients had EoE-A with a median age of 9.5 years (range: 1 - 20 years) and a male/female ratio of 4:1. Twelve patients with EoE-R had a median age of 10.5 years (range: 2 - 21 years) and a male/female ratio of 5:1 (Table 1).
 Click to view | Table 1. Characteristics of Patients With GERD, EoE-A and EoE-R |
EoEHSS grade and stage scores are different between EoE-A, EoE-R and GERD
The mean total grade scores from mid and distal esophagus were significantly higher in the EoE-A compared to the EoE-R and GERD (P < 0.001, for both sites and both groups) (Fig. 1a). The mean total grade scores were similar between the mid and distal biopsies in EoE-A patients (P > 0.05). In EoE-R and GERD patients, the mean total grade score at distal esophagus is similar (P > 0.05); however, it is significantly higher at mid esophagus in EoE-R patients (P < 0.05) (Fig. 1a). The grade scores of individual features assessed were significantly higher in the EoE-A patients as compared to those with EoE-R or GERD (Table 2).
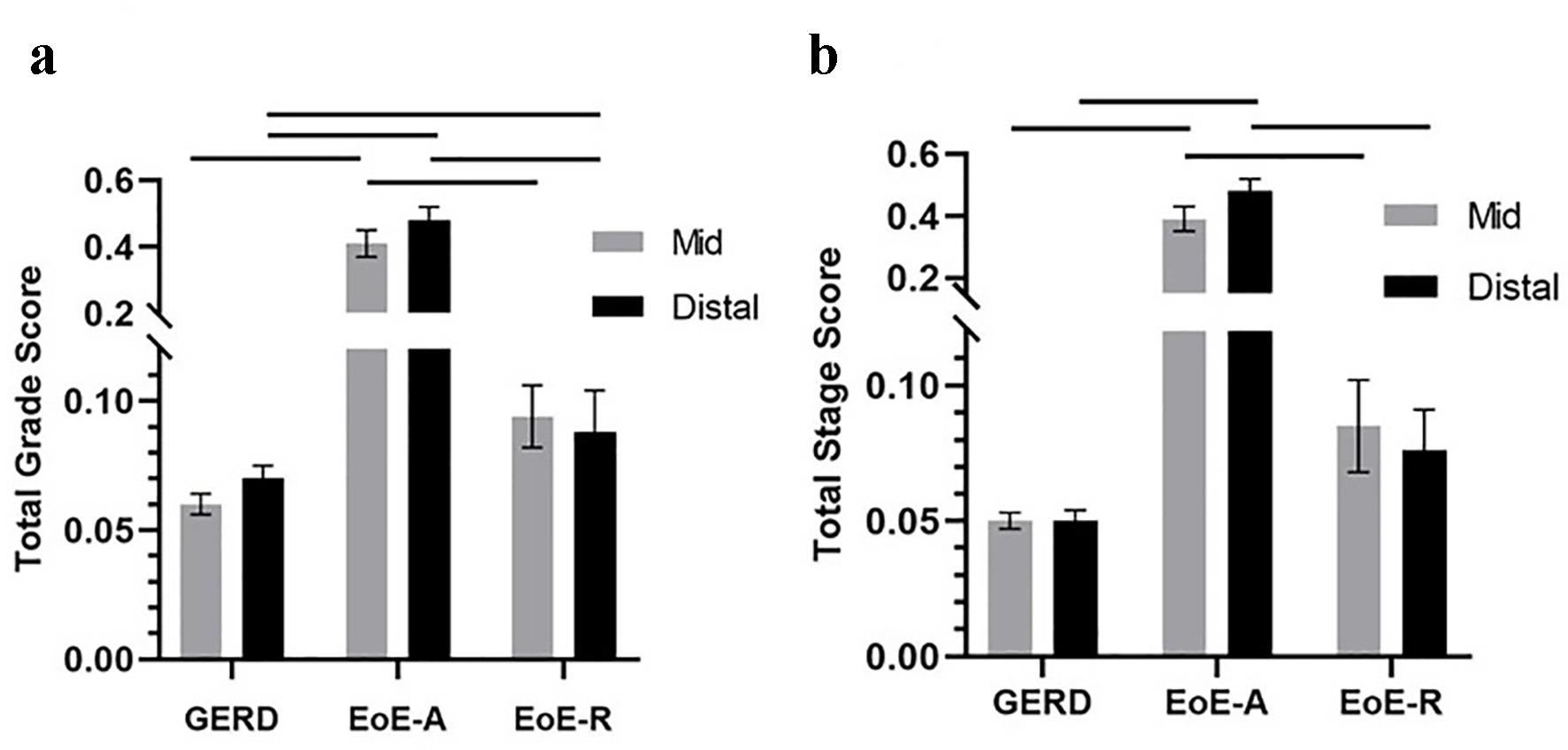 Click for large image | Figure 1. Total grade scores (a) and total stage scores (b) in patients with gastroesophageal reflux disease (GERD), active eosinophilic esophagitis (EoE-A) and eosinophilic esophagitis in remission (EoE-R) in distal and mid esophagus. Horizontal lines above the bars show the groups that were compared and had a significant difference (P < 0.05). |
 Click to view | Table 2. Mean Grade and Stage Scores of Histologic Features of Patients With GERD, EoE-A and EoE-R |
Mean total stage scores were significantly higher in patients with EoE-A compared to patients with EoE-R or GERD at both mid and distal esophageal sites (P < 0.001, for both sites and both groups). The stage scores were similar between the mid and distal esophagus in the EoE-A patients (P > 0.05) (Fig. 1b). The mean total stage scores were similar between EoE-R and GERD patients in both the mid and distal esophagus (Fig. 1b).
EoEHSS grade and stage scores correlate with the severity and extent of PEC
PEC severity (r = 0.781) and extent (r = 0.68) correlated with the mean total grade and stage scores respectively in EoE-A (Fig. 2a, b). EoE-A patients with low PEC severity and extent still had significantly higher total grade and stage scores compared to EoE-R and GERD patients. There was no difference in PEC between mid and distal esophagus in EoE-A patients (P > 0.05). There was no difference in PEC at mid and distal esophagus, between the EoE-R and GERD patients (P > 0.05); both were low.
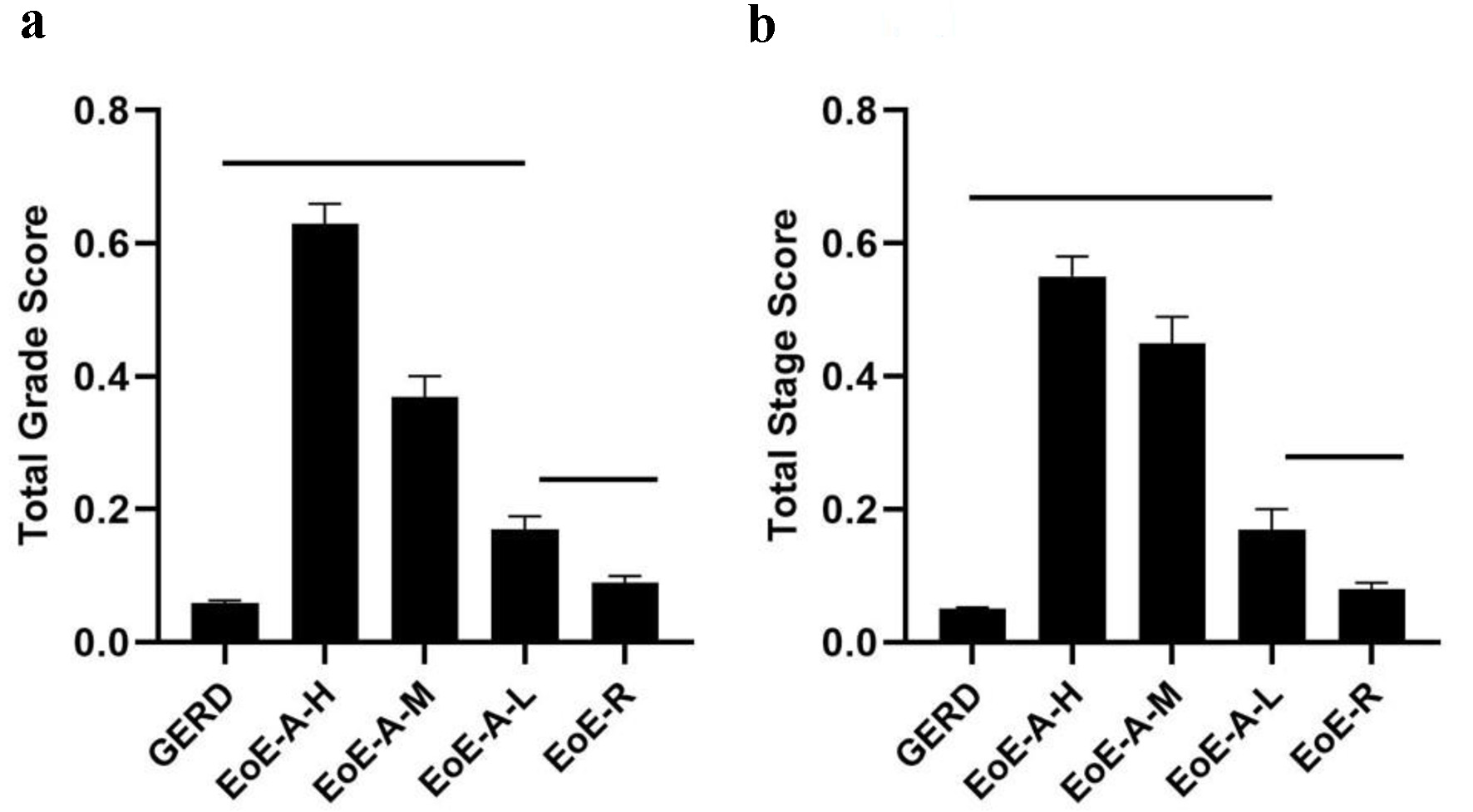 Click for large image | Figure 2. Total grade scores (a) and total stage scores (b) in patients with gastroesophageal reflux disease (GERD), active eosinophilic esophagitis (EoE-A) relative to PEC (high (EoE-A-H), moderate (EoE-A-M), low (EoE-A-L)) and eosinophilic esophagitis in remission (EoE-R). Horizontal lines above the bars show the groups that were compared and had a significant difference (P < 0.05). |
Histopathologic differences are present between the mid and distal esophagus in EoE-A
The mean grade score for each of the eight individual features of the EoEHSS was higher in EoE-A compared to EoE-R and GERD (all P < 0.05). Grade scores for BZH and DIS were independent of PEC and were significantly higher in the distal compared to mid esophagus in EoE-A (both P < 0.05) (Fig. 3a). Mean PEC did not differ between distal and mid esophagus in these cases (P > 0.05). LPF was more severe in mid than distal esophagus (few biopsies with adequate submucosal tissue were available for analysis) (Fig. 3a). The mean stage scores of BZH, DIS, LPF and PEC in EoE-A patients were not significantly different between mid and distal esophagus (Fig. 3b).
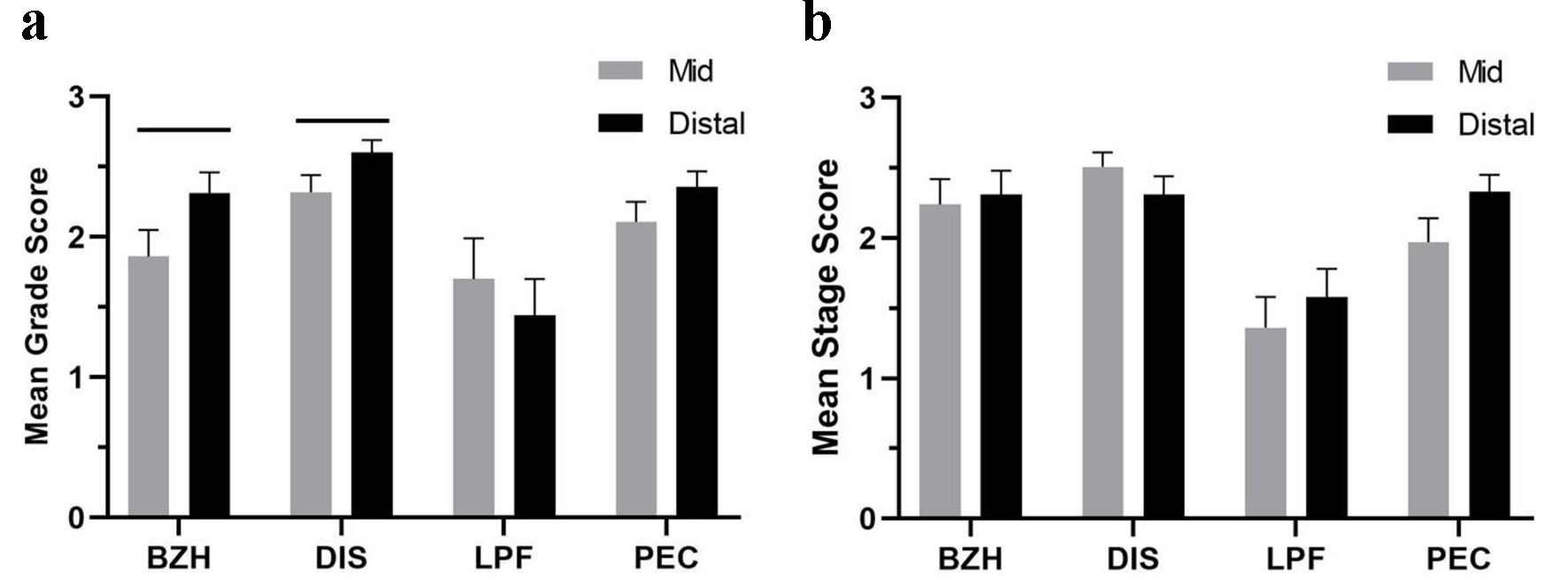 Click for large image | Figure 3. Mean grade scores (a) and mean stage scores (b) of basal zone hyperplasia (BZH), dilated intercellular space (DIS), lamina propria fibrosis (LPF) and peak eosinophil count (PEC) in mid and distal esophagus of patients with EoE-A. Horizontal lines above the bars show the groups that were compared and had a significant difference (P < 0.05). |
Discordance is common between mid and distal esophageal EoEHSS scores in EoE-A
In patients with EoE-A, 18/36 (50%) had four-point difference in EoE grade scores and 20/36 (56%) had four-point difference in stage score between the distal and mid esophagus. Of these patients with discordant scores, 15 patients showed disparity in both the grade and stage scores. Remaining five patients had disparity in only the stage score and three patients had disparity in only the grade score. Of the 18 patients with grade score disparities between mid and distal esophagus, six had higher scores in the mid esophagus and 12 had higher scores in the distal esophagus. Of the 20 patients with stage score disparities between mid and distal esophagus, eight had higher stage scores in the mid esophagus while 12 had higher scores from their distal biopsies (Table 3).
 Click to view | Table 3. Grade and Stage Scores of Histologic Features for Mid and Distal Esophageal Regions in EoE-A Patients |
As the EoEHSS scores for EoE-R and GERD were substantially lower in both the mid and distal esophagus, no significant deviations in histopathologic features were noted in these two cohorts.
| Discussion | ▴Top |
The present study corroborates that EoEHSS is a reproducible diagnostic score to evaluate histologic features of esophageal biopsies of EoE patients. EoEHSS in our patient cohort yielded significantly higher grade and stage scores in EoE-A compared to patients with EoE-R or GERD. Our study confirms a positive correlation between PEC and the total grade and stage scores in EoE-A patients. The clinical value of introducing EoEHSS in routine clinical practice is supported by the observation that PEC, the histologic hallmark presently employed to characterize EoE, does not always correspond to the extent of tissue injury, as the eosinophils may resolve in EoE-R; however, tissue injury is still present in the mid esophagus.
The present study employs a novel application of EoEHSS, to compare mid and distal esophageal scores in patients with EoE-A, EoE-R, and GERD. In our cohort, the mean total grade (severity) scores in the mid esophagus, but not in the distal esophagus, of EoE-R patients remained above the baseline noted in GERD. Thus, the EoEHSS (unlike the PEC) highlights for the first time a divergence in the histologic recovery between mid and distal esophagus in EoE patients that have resolved their clinical symptoms and tissue eosinophilia. If established in larger cohorts, this would suggest that full resolution of esophageal remodeling in EoE may require a longer period of recovery after eosinophilia has resolved.
EoEHSS also establishes a more comprehensive understanding of EoE histology by analyzing individual features associated with EoE that are independent of PEC, such as BZH, DIS and LPF. BZH and DIS were more prominent in the distal esophagus compared to the mid esophagus in our EoE-A cohort, whereas LPF was more severe in the mid than distal esophagus in this group. This difference is not reflected when comparing mid and distal PEC, extending the utility of using EoEHSS to provide insights beyond those derived from simply counting eosinophils. A similar conclusion was reached in another study that found that BZH has a higher correlation with the transcriptome expression in active EoE than PEC [15].
Total and individual EoEHSS scores frequently varied between the mid and distal esophagus in a single patient with EoE-A, not always higher in distal esophagus. This variation in severity between mid and distal esophagus could present as different clinical symptoms, correspond to distinct EoE subtypes, represent variations in responses to different antigens, or to different treatment approaches.
Our study has several limitations. The patient cohorts are relatively small in number and all patients are from a single institution which serves patients from a limited geographic area. Larger diverse cohorts may be required to confirm the observations made in our study. The definition of EoE-R of < 15 eos/hpf is widely utilized but some investigators have recently proposed a cutoff of < 5 eos/hpf. As the guidelines are continuously being revised, further studies with updated criteria may be helpful in better understanding of the disease.
In summary, EoEHSS was successfully applied to evaluate the difference in mid and distal esophagus in patients with EoE-A, EoE-R, and GERD. EoEHSS scores in mid and distal esophagus were significantly higher in children with EoE-A compared to EoE-R and GERD. As EoEHSS identified discordance in mid and distal esophagus pathology in both EoE-A and EoE-R, it emphasizes the importance of obtaining tissue from both sites, as has been recommended in the clinical guidelines [14]. Our study concluded that features of active EoE, independent of PEC, often vary between mid and distal esophagus and that healing may not be uniform in the entire esophagus. EoEHSS helped quantitate disease activity beyond those derived from PEC, and thus provided a more comprehensive understanding into the pathophysiology of EoE. We propose using EoEHSS in daily clinical practice as it will help make the diagnosis of EoE more objective, reproducible and accurate and better understand the pathophysiology of EoE.
Acknowledgments
We would like to acknowledge expert assistance of the histology laboratory staff at Downstate.
Financial Disclosure
This study was supported in part by Madu Rao Award to BL and SR.
Conflict of Interest
The authors declare that there is no conflict of interest.
Informed Consent
All subjects provided written informed consent.
Author Contributions
BL, SR, RG and MAH designed the study. BL, SR and RG conducted the study. BL, SR and MAH wrote the manuscript. All authors reviewed and edited the manuscript.
Data Availability
The data supporting the findings of this study are available within the article.
Abbreviations
BZH: basal zone hyperplasia; DIS: dilated intercellular spaces; DEC: dyskeratotic epithelial cells; EA: eosinophil abscesses; EoE: eosinophilic esophagitis; EoEHSS: eosinophilic esophagitis histologic scoring system; ESL: eosinophil surface layering; GERD: gastroesophageal reflux disease; LPF: lamina propria fibrosis; PEC: peak eosinophil count; SEA: surface epthelial alteration
| References | ▴Top |
- Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201-218.
doi pubmed - Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, American College of G. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679-692; quiz 693.
doi pubmed - Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):21-22.
doi - Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342-1363.
doi pubmed - Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19(1):90-96.
doi pubmed - Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43(2):257-268.
doi pubmed - Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, Shaheen NJ. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7(12):1305-1313; quiz 1261.
doi pubmed - Odze RD. Pathology of eosinophilic esophagitis: what the clinician needs to know. Am J Gastroenterol. 2009;104(2):485-490.
doi pubmed - Gunasekaran TS, Chu C, Ronquillo N, Jr., Chennuri R, Adley B, Borgen K, Schwartz A, et al. Detailed histologic evaluation of eosinophilic esophagitis in pediatric patients presenting with dysphagia or abdominal pain and comparison of the histology between the two groups. Can J Gastroenterol Hepatol. 2017;2017:3709254.
doi pubmed - Rajan J, Newbury RO, Anilkumar A, Dohil R, Broide DH, Aceves SS. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol. 2016;137(1):147-156 e148.
doi pubmed - Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, Pentiuk S, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2017;30(3):1-8.
doi pubmed - Warners MJ, Ambarus CA, Bredenoord AJ, Verheij J, Lauwers GY, Walsh JC, Katzka DA, et al. Reliability of histologic assessment in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2018;47(7):940-950.
doi pubmed - Pai RK, Bredenoord AJ, Feagan BG, Jairath V. Editorial: validating reliability of the eosinophilic oesophagitis histological scoring system (EOE-HSS)-an important first step. Authors' reply. Aliment Pharmacol Ther. 2018;47(12):1714-1715.
doi pubmed - Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, Spechler SJ, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155(4):1022-1033 e1010.
- Shoda T, Wen T, Caldwell JM, Kuhl J, Aceves SS, Bonis P, Capocelli KE, et al. Correlation of the eosinophilic histopathological scoring system with esophageal gene expression in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2018;141(2 Supplement):AB138.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


