| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Short Communication
Volume 12, Number 6, December 2019, pages 312-314
Re-Educating Residents About Non-Invasive Colorectal Cancer Screening: An Approach to Improving Colon Cancer Screening Compliance
Gabriel Melkia, c, Moutaz Ghrewatia, Hadir Mohameda, Shaker Barhamb, Ashima Kapoora, Farhan Ayouba, Abdalla Mohameda, Alexander Wua, Sugabramya Kurua, Alisa Farokhiana, Rana Garrisa, Chandra Chandrana, Matthew Grossmanb, Walid Baddourab
aDepartment of Internal Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
bDepartment of Gastroenterology, St. Joseph’s University Medical Center, Paterson, NJ, USA
cCorresponding Author: Gabriel Melki, Department of Internal Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
Manuscript submitted July 31, 2019, accepted September 6, 2019
Short title: Re-Educating Residents About CRC Screening
doi: https://doi.org/10.14740/gr1205
| Abstract | ▴Top |
Background: Colorectal cancer is the third leading cause of cancer death; therefore early detection by screening is beneficial. Residents at a clinic in NJ, USA were not offering other forms of colon cancer screening when patients refused colonoscopy, which lead to the creation of the quality improvement project.
Methods: Residents practicing at the clinic were given an anonymous survey determining which method of colon cancer screening they used and which alternative method they offered when patients refused the original method. The residents were educated about all methods of colon cancer screening and the residents were resurveyed.
Results: A total of 64% of residents offered less invasive testing when colonoscopy was refused. Six months after education, 95% of residents offered less invasive testing when colonoscopy was refused.
Conclusions: Early detection and removal of polyps by colonoscopy reduce the risk of cancer development. Colonoscopy is the gold standard for colon cancer screening; however other less invasive modalities are approved. This quality improvement project lead to offering the fecal immunochemical test or fecal occult blood test once patients refused colonoscopy at the clinic, increasing the number of patients receiving colorectal cancer screening, and thus providing better medical care.
Keywords: Colon cancer; Colon cancer screening; Fecal occult blood test; Quality improvement
| Introduction | ▴Top |
Colorectal cancer (CRC) is the third leading cause of cancer death [1], and therefore early detection by screening is beneficial [2]. Residents in primary care clinics offer screening modalities for CRC screening starting at the age of 50. At a clinic in NJ, USA, the primary method of CRC screening used was colonoscopy every 10 years starting at the age of 50; however when patients refused colonoscopies, other less invasive testing were not being offered. This prompted the investigators to intervene and assess the percentage of residents not offering less invasive testing. Residents were re-educated about alternate screening modalities for CRC and residents were reassessed.
| Materials and Methods | ▴Top |
Sixty-three residents were given survey (Supplementary Material 1, www.gastrores.org) based on multiple choice questionnaires, which asked first test used for CRC screening and what they would do if their first screening test was refused (if invasive). The questionnaire was anonymous. Results were obtained to assess the percentage of residents offering less invasive testing. Residents were re-educated about all methods of CRC screening that are approved as of 2018 [3]. At 6 months, the residents were given the questionnaire and results were used to compare the percentage of residents offering less invasive testing. Statistical methods used to analyze the study were the percent change and the percent difference. All analyses were performed using Microsoft Office Excel 2007 (Microsoft Corp., Redmond, WA, USA). The Institutional Review Board approved this study, which was conducted in compliance with the ethical standards of the responsible institution on human subjects.
| Results | ▴Top |
At this institution, the first test used for CRC screening was colonoscopy at 73%, followed by fecal occult blood test (FOBT) at 19% (Fig. 1). A total of 64% of residents were offered less invasive testing when colonoscopy was refused, usually fecal immunochemical test (FIT) (Fig. 2). After 6 months of resident re-education, colonoscopy was still the most used at 73%. A total of 95% residents offered less invasive testing when colonoscopy was refused (Fig. 3). The percent change revealed an increase of 48.4375% in terms of residents offering less invasive testing when patients refused colonoscopy. The percent difference indicated a difference of action from the residents of 38.9937%.
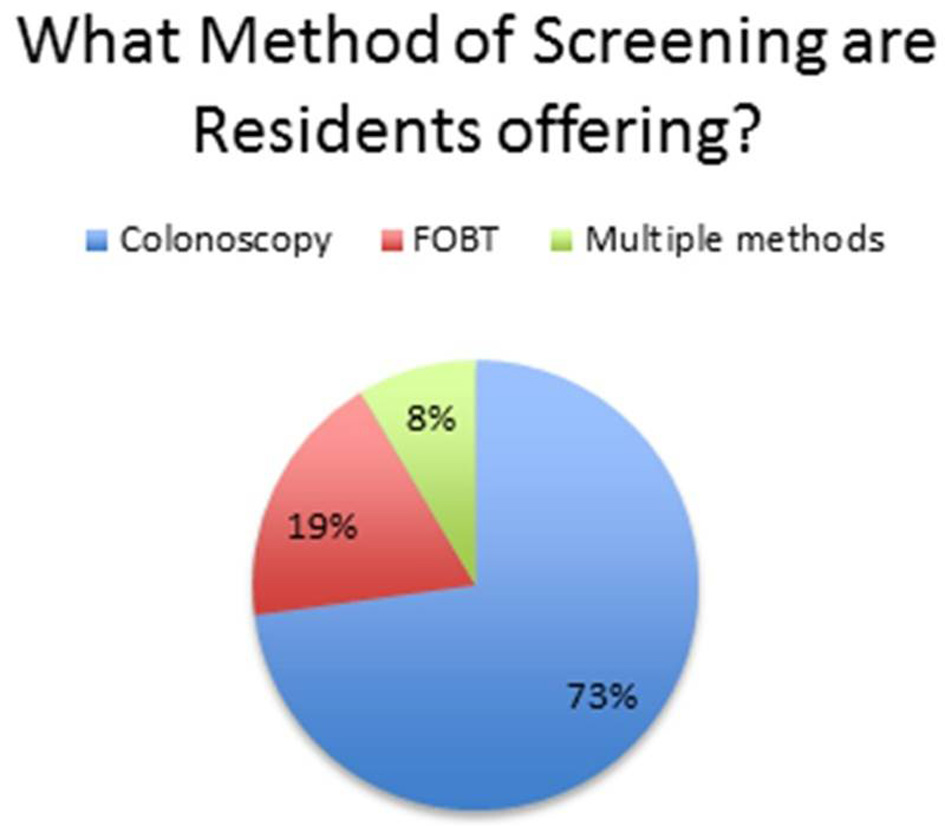 Click for large image | Figure 1. Pie chart revealing what residents offer as a primary mode of screening. |
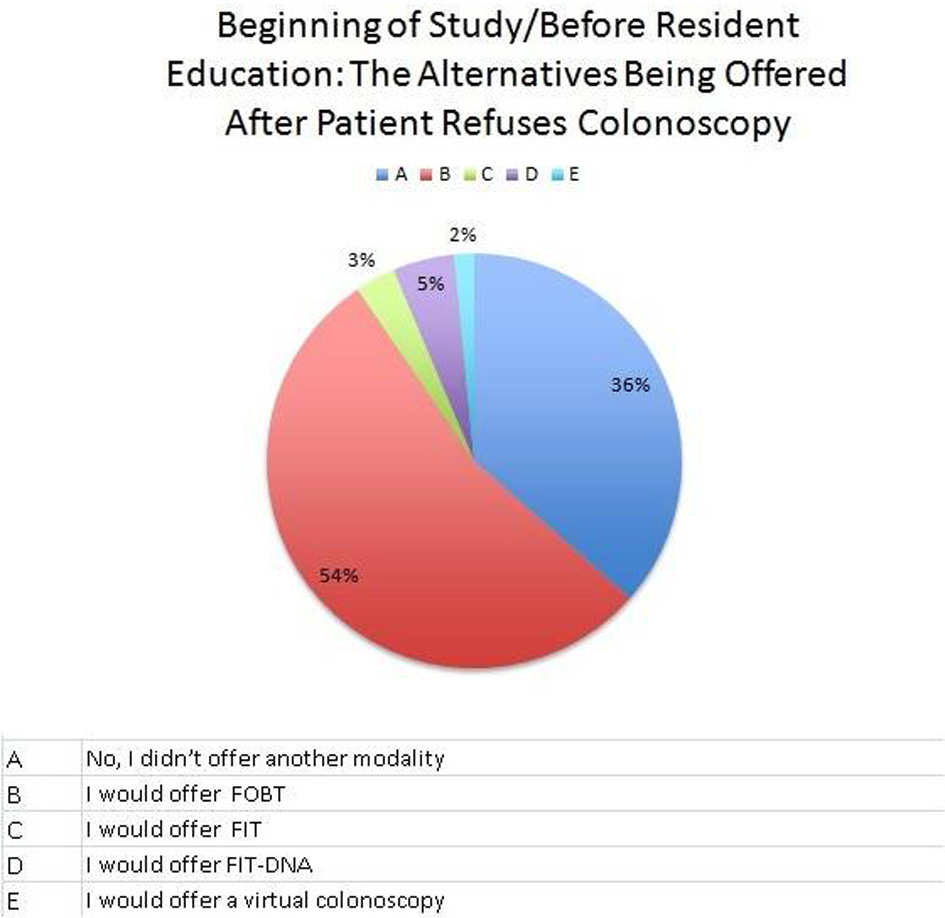 Click for large image | Figure 2. A pie graph at the beginning of the study focusing on what residents offer when a patient refuses colonoscopy (alternative less invasive procedures). |
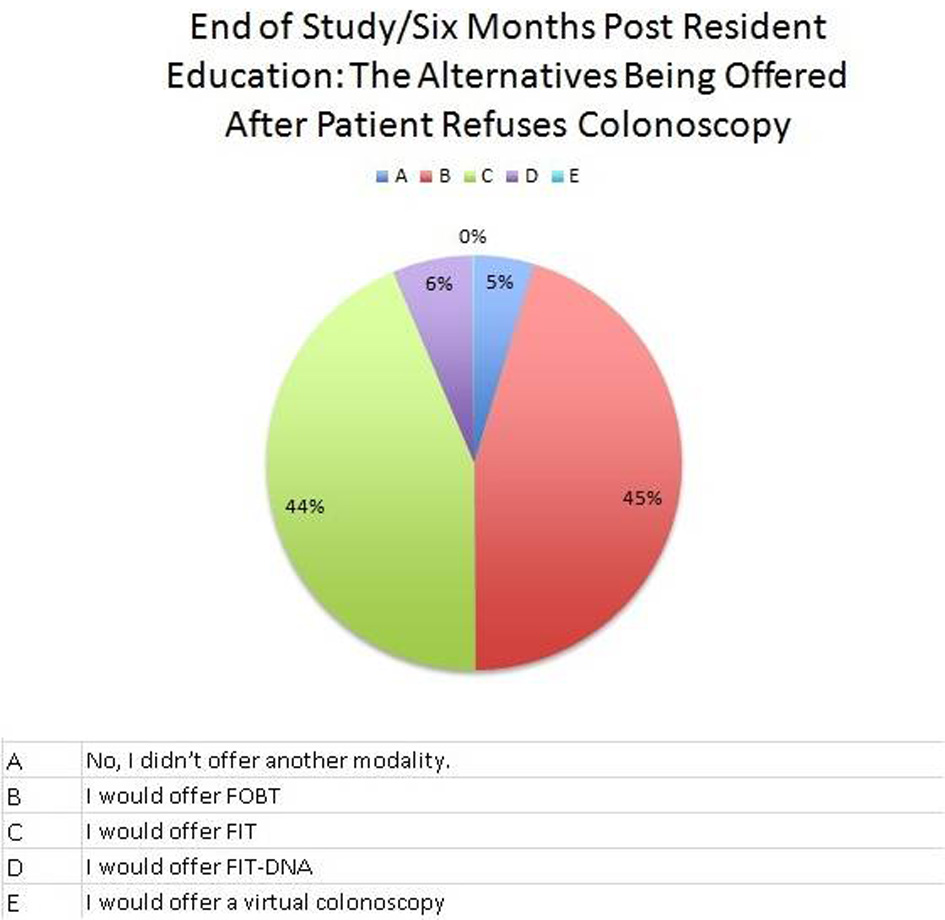 Click for large image | Figure 3. Pie graph at the end of the study focusing on what residents offer when a patient refuses colonoscopy (alternative less invasive procedures). |
| Discussion | ▴Top |
Most cases of CRC develop from polyps; therefore early detection and removal by colonoscopy reduce the risk of CRC development [1-3]. Colonoscopy is the gold standard for CRC screening; however other modalities are also approved such as FOBT test yearly, and it is important to offer these modalities when colonoscopies are refused [1-3]. It was noticed that residents were not offering these test when patients refused colonoscopies and this is why this quality improvement (QI) project was created. After 6 months of re-education, residents went from offering less invasive testing once refusal of colonoscopy from 64% to 95%, revealing a percent change of 48.4375% in terms of residents offering less invasive testing when patients refused colonoscopy. The data of the study were gathered from an anonymous survey issued before and 6 months after resident re-education. At the time of the study, the facility only had paper charts. Relying on resident’s response on a survey is less ideal than analyzing medical chart work in order to determine what was offered to the patients for CRC screening and what was offered to the patient when they refused colonoscopy. This is a limitation of the study. Future studies could focus on documented refusal and alternative tests offered by looking through an electronic medical record. However, after re-education, residents reported that they were offering less invasive testing (such as FOBT or FIT) when patients refused colonoscopy. The attending preceptors at the institution realized this and are now offering the test automatically when patients refuse colonoscopy. This would increase the number of patients screened. Patients could have many reasons to refuse a screening colonoscopy including thinking they do not need it since they have no symptoms or CRC risk factors, discomfort with bowel prep for colonoscopy and discomfort with the invasive procedure itself [4]. By offering FOBT or FIT for screening when patients refuse colonoscopy, the patients that refuse colonoscopy due to invasive testing, discomfort with procedure and bowel prep regimen will be screened using noninvasive methods. If fecal tests are positive, at a follow-up visit, a discussion is made about having a colonoscopy. Patients generally agree to colonoscopy when noninvasive screening tests are abnormal. Unfortunately, some patients refuse to undergo a colonoscopy despite a positive FIT for various reasons but mostly related to procedural discomfort. For these patients, we recommend re-educating them at every visit about the benefits of CRC screening, the possibility of curing colon cancer when detected early or preventing it by resecting polyps with malignant potential [2].
This QI project aims to improve compliance with CRC screening using FIT or FOBT once patients refuse colonoscopy. Our intervention with re-educating primary care clinic medical staff resulted in improvement in screening compliance. This ultimately means providing better medical care.
| Supplementary Material | ▴Top |
Supplementary Material 1. Colorectal Cancer Screening Survey.
Acknowledgments
We thank Dr. Patrick Michael (Program Director of the Internal Medicine Residency), Dr. Michael Agnelli (Associate Program Director of the Internal Medicine Residency), Dr. Monisha Singhal (Associate Program Director of the Internal Medicine Residency) and Dr. Robert Lahita (Chairman of the Department of Medicine) for their continuous support and guidance.
Financial Disclosure
No funding was provided to any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained for this research article.
Author Contributions
GM was the principle investigator, who contributed to project conception/design, manuscript preparation and writing, survey creation, methods, survey administration, data collection and analysis. MG, HM, AK, FA, AM and AW contributed to survey creation, and data collection. SB was involved in manuscript writing and editing, and mentorship. SK, AF and RG contributed to technical methods and editing. CC and MG were the supervising attending physicians, playing a role in mentorship and guidance. WB was the supervising attending physicians, playing a role in mentorship guidance and manuscript editing. All authors reviewed the final manuscript.
| References | ▴Top |
- Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23(28):5086-5096.
doi pubmed - Freeman HJ. Early stage colon cancer. World J Gastroenterol. 2013;19(46):8468-8473.
doi pubmed - US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr., Garcia FAR, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564-2575.
doi pubmed - Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, Marcus AC, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20(11):989-995.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


