| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 12, Number 4, August 2019, pages 211-215
Kratom-Induced Cholestatic Liver Injury Mimicking Anti-Mitochondrial Antibody-Negative Primary Biliary Cholangitis: A Case Report and Review of Literature
Mahmoud Aldyaba, Peter F. Ellsb, Rosa Buib, Timothy D. Chapmanc, Hwajeong Leea, d
aDepartment of Pathology and Laboratory Medicine, Albany Medical College, Albany, NY 12208, USA
bGastroenterology, Albany Medical College, Albany, NY 12208, USA
cPathology, Bassett Healthcare Network, Cooperstown, NY 13326, USA
dCorresponding Author: Hwajeong Lee, Department of Pathology and Laboratory Medicine, Albany Medical Center, 47 New Scotland Ave., MC81, Albany, NY 12208, USA
Manuscript submitted July 20, 2019, accepted July 31, 2019
Short title: Kratom-Induced Granulomatous Liver Injury
doi: https://doi.org/10.14740/gr1204
| Abstract | ▴Top |
Kratom is an herbal supplement used to relieve chronic pain or opioid withdrawal symptoms. Recent news articles covering adverse effects associated with kratom use have brought attention to its organ toxicities. Reports of kratom-induced hepatic toxicity are limited and only three case reports of kratom-induced liver injury with histopathologic examination of the liver biopsies are available. A 40-year-old female presented with symptoms of mixed cholestatic and hepatocellular liver injury without clear etiology. The laboratory and imaging workup suggested possibilities of autoimmune hepatitis, autoimmune hepatitis-primary biliary cholangitis (PBC) overlap syndrome, or drug-induced liver injury. Autoantibodies including anti-mitochondrial antibody (AMA) were negative. Liver biopsy showed granulomatous hepatitis with prominent duct injury, suggestive of AMA-negative PBC. She subsequently was referred to a hepatologist and a history of recent kratom use was finally revealed. Kratom was discontinued and the symptoms improved. Kratom-induced hepatic toxicity may manifest with variable biochemical and clinical abnormalities. Histologically, it may mimic AMA-negative PBC. Our case highlights the importance of thorough history taking, interdisciplinary approach and communication for optimal patient care.
Keywords: Kratom; Cholestasis; Liver; Biopsy; Granuloma
| Introduction | ▴Top |
Kratom is the common name used for Mitragyna speciosa, which is an evergreen tree that is found mainly in Southeast Asia. Extracts from the leaves of the kratom tree have been historically used to relieve pain and improve energy. The extract of kratom consists of many constituents including psychoactive alkaloids, of which no less than 25 different alkaloids have been identified. It acts as a stimulant at low doses, opioid-like at moderate doses and causes sedation at high doses [1-4].
While several studies attempted to understand the addictive potential of kratom [5-7], reports describing kratom toxicity manifesting in specific organs are relatively few [8-11]. Also, although kratom-induced cholestatic and/or hepatocellular pattern liver injuries have been reported [12-14], reports with detailed histopathologic examination of the liver biopsy are rare [15-17].
We report an unusual case of kratom-induced liver injury that mimicked anti-mitochondrial antibody (AMA)-negative primary biliary cholangitis (PBC) histologically, due to the presence of granulomas and duct injury in the liver biopsy. This particular histologic pattern of drug-induced liver injury (DILI) has not been documented in association with kratom use, to the best of our knowledge.
| Case Report | ▴Top |
A 40-year-old female with history of prediabetes, gastroesophageal reflux disease (GERD), and cluster headaches presented for follow-up, after hospitalization for abdominal pain that had been attributed to “hepatitis”. About 8 weeks ago, she started a ketogenic diet in hopes to control her prediabetes. After 4 weeks, she suddenly developed acute abdominal pain associated with fevers. Lab work was notable for a total bilirubin of 5.1 mg/dL, aspartate aminotransferase (AST) 462 IU/L, alanine aminotransferase (ALT) 875 IU/L and alkaline phosphatase (ALP) 162 IU/L. A viral hepatitis panel and workup for Wilson’s disease and alpha-1 antitrypsin deficiency were negative. Autoantibodies including anti-nuclear antibody (ANA), anti-smooth muscle antibody (ASMA) and AMA were negative. Imaging studies including computed tomography (CT) of the abdomen and pelvis with contrast, and magnetic resonance cholangiopancreatography (MRCP) showed nonspecific mild periportal edema only. There were no stones in the biliary tree or gallbladder. There was no biliary dilatation or features to suggest bile duct obstruction. Liver, spleen, gallbladder and pancreas were unremarkable. A liver biopsy was performed with the working diagnosis of autoimmune hepatitis versus PBC. She was discharged on prednisone 40 mg, to be tapered, as well as ursodiol.
Additional lab works were resulted after the liver biopsy. Immunoglobulin M (IgM) was 202 (reference range: 48 - 312) mg/dL, and immunoglobulin G (IgG) was 1,090 (reference range: 681 - 1,648) mg/dL.
The biopsy consisted of two cores of benign liver. The portal tracts were mildly expanded by inflammatory cell infiltrate, consisting of histiocytes, lymphocytes, neutrophils, eosinophils and a lesser number of plasma cells. The interlobular bile ducts showed signs of injury with epithelial disarray and nuclear hyperchromasia, and infiltrating lymphocytes (Fig. 1a). A few damaged interlobular bile ducts were encased by vaguely formed granulomas (Fig. 1b). In addition, a few portal venous branches showed endotheliitis (Fig. 1c). A few small, poorly formed granulomas were identified in the lobules (Fig. 1d). Scattered areas of ballooned hepatocytes were also noted. Iron stain and copper stain were negative. There was no fibrosis. Given the prominent duct injury associated with granulomas in conjunction with cholestatic and hepatitis pattern biochemistry, autoimmune hepatitis-PBC overlap syndrome and AMA-negative PBC were considered. However, given the lack of features of autoimmune hepatitis such as prominence of plasma cells, interface activity and marked lobular injury, AMA-negative PBC was finally favored, histologically.
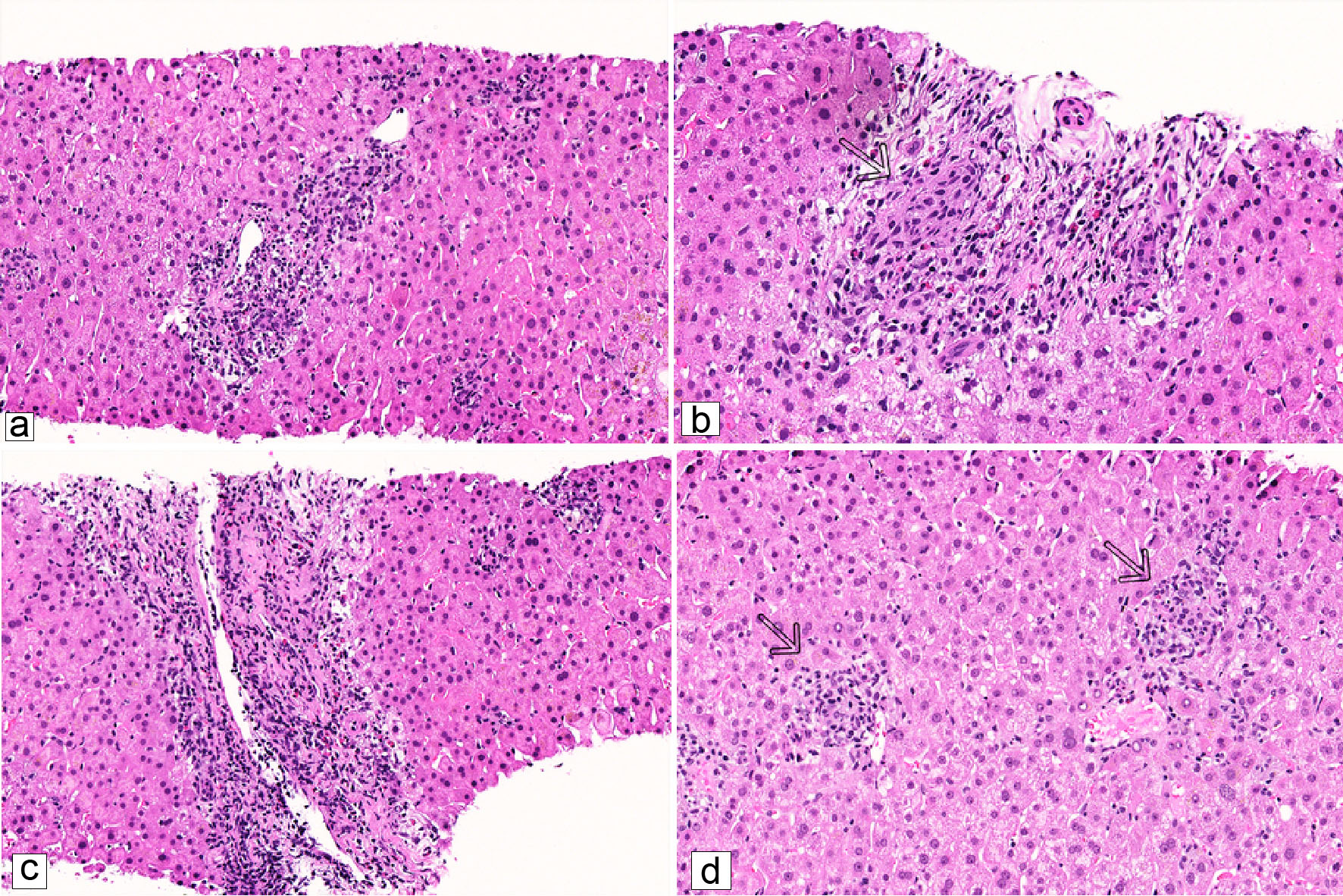 Click for large image | Figure 1. Representative microscopic images of the liver biopsy (H&E stain). (a) Marked interlobular bile duct injury with infiltrating lymphocytes within the ductal epithelium (× 200). (b) Vaguely formed granuloma encases a damaged bile duct (arrow) (× 309). (c) Endotheliitis with subendothelial lymphocytes (× 200). (d) Poorly formed lobular granulomas (arrows) (× 223). |
Approximately 5 weeks following the initial presentation, the patient was referred to a hepatologist at our institution for a second opinion. Recent blood work showed improved hepatic function panel. Also, her abdominal pain had improved. She denied fevers, chills, nausea, vomiting, or diarrhea. She reported some joint pain in her ankles and hands, changes in her vision and transient ankle swelling. On further questioning, she had taken oral contraceptive pills prior to her recent admission. She denied any alcohol use or intravenous drug use. However, she mentioned that she had been taking kratom once a week for the preceding month prior to the initial presentation. Also, she revealed the use of nettle leaf supplements for the past 4 - 5 years. The hepatologist recognized the association between kratom use and cholestatic hepatitis pattern injury. Taken together, the diagnosis of kratom-induced liver injury was rendered. The patient was advised to discontinue kratom, nettle leaf supplements, and oral contraceptive.
About 10 weeks following her initial presentation, she has been in the process of tapering her medication, and was feeling completely well. Blood work at that time was normal. Seventeen weeks after the initial presentation, she has been off the prednisone for several weeks. She was advised to stop ursodiol. Twenty-one weeks after the initial presentation, she was off all medication for more than 3 weeks. Her lab work continues to be normal (Table 1).
 Click to view | Table 1. Liver Biochemistry |
| Discussion | ▴Top |
With dual effects as a stimulant as well as an analgesic [1, 2], kratom is increasingly used in an attempt to relieve chronic pain or to relieve opioid withdrawal symptoms [3, 18-20]. Although it is considered a safe herbal extract with positive mood effects by some, many reports linking kratom to variable harmful outcomes including altered mental status, agitation, seizure, kidney injury, liver injury and even death, have emerged [8, 11, 12, 21-23]. Thus, the US Food and Drug Administration (FDA) did not approve kratom for any medical indications, but identified it as an unapproved drug and warned against its use [6, 11, 24].
Of the over 25 alkaloids identified in kratom, mitragynine and 7-hydroxymitragynine (7-HMG) are considered major active compounds [2, 25, 26]. They stimulate µ- and δ-opioid receptors, and the effects of both compounds are dose-dependent. Mitragynine also stimulates postsynaptic α2 adrenergic receptors and suppresses cyclooxygenase 2 (COX2) mRNA and its protein expression [1, 2]. Metabolism of mitragynine occurs via phase I and phase II mechanisms: hydrolysis, o-demethylation, oxidative and reactive reaction that ends with conjugation of glucuronide and sulfate, yielding a final metabolite excreted in urine [1, 2, 27]. It also blocks neuronal calcium channels with an effect at the neuromuscular junction level [28]. Still, data on the pharmacokinetics of kratom are limited and additional study is needed to better understand different metabolites and their effects [3, 28, 29].
A few studies documented liver injury caused by kratom [8, 12, 13, 15-17, 30], three with histologic examination of the liver biopsies [15-17]. Seven of the reported cases are summarized in Table 2 [8, 12, 13, 15-17, 30]. In addition, hepatomegaly that is possibly associated with kratom use [31] has been reported.
 Click to view | Table 2. Kratom-Induced Hepatotoxicity in the Literature |
Herein we report a case of granulomatous hepatitis pattern injury associated with kratom use. Liver function tests and overall clinical improvement after discontinuation of kratom support that it’s most likely the offending agent. Although the patient discontinued nettle leaf supplements and oral contraceptive at the same time, these are less likely to be the culprits as she has been taking the supplements for many years prior to the presentation, and granulomatous hepatitis pattern injury would be highly unusual in the setting of oral contraceptive use [32]. Given the lack of fibrosis and features of autoimmune hepatitis in the liver biopsy in conjunction with normal IgG, underlying chronic liver disease such as autoimmune hepatitis is also less likely.
There are limited data regarding liver injury associated with ketogenic diet. Elevation of transaminases and steatosis has been reported in animal and human studies, but cholestatic liver injury has not been described [33]. Cholestatic pattern injury, sometimes mixed with hepatocellular pattern injury, appears to be a common biochemical abnormality at presentation when kratom toxicity manifests in the liver. Histologically, all four biopsies including our case showed cholestatic pattern liver injury including cholestasis in three cases, and duct injury in three including our case, with relative sparing of the lobules [15-17]. Thus, it would be prudent to consider potential DILI when dealing with a liver biopsy in which the predominant injury pattern does not coincide with that of liver function tests. Alternatively, of course, the discrepancy between the histology and liver function tests in our case may be due to preprocedural steroid treatment that may have ameliorated the lobular hepatitis component more than the bile duct injury. Our case had another layer of challenge as the kratom use history was not available at the time of liver biopsy and histologic features mimicked PBC, which was not described previously in association with kratom use.
The finding of granulomas in a liver biopsy brings up a multitude of differential diagnosis including infection, foreign-body induced granulomas, PBC, drug-mediated injury and sarcoidosis. Also, vasculitis-related disorder such as Wegener granulomatosis and Sjogren syndrome may be a cause of liver granuloma. Patients with Hodgkin’s and non-Hodgkin’s lymphoma may develop granulomas as well [34]. Therefore, it would be crucial to obtain pertinent clinical histories including any drug/herbal supplement history and clinically correlate these with biopsy findings.
Kratom use and its side effects are drawing attention of the general public due to recent news article coverage [35-37]. With the increased awareness, additional kratom-induced hepatic injury cases are likely to be encountered and discovered. Our case and previous reports highlight the importance of thorough history taking, interdisciplinary approach and communication for optimal patient care.
Acknowledgments
Not applicable.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Anonymous case reports are exempt category reviews by the Institutional Review Board (IRB) at Albany Medical Center, Albany, NY, USA. The IRB does not require consent of the patient for an anonymous case review. Therefore, a formal IRB consent waiver was not required and was not obtained. Our study follows the principles of the Declaration of Helsinki.
Author Contributions
MA drafted and gave final approval of the version to be published. PEF, RB and TDC contributed to the editing and critical review of the manuscript, as well as final approval of the version to be published. They provided clinical histories and participated in the patient management. HL contributed to the drafting and editing, as well as gave final approval of the version to be published. All authors are agreeable to be accountable for all aspects of the work in ensuring that questions that arise are investigated and the information is accurate.
| References | ▴Top |
- Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, et al. From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013;37(2):138-151.
doi pubmed - Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130(1):127-138.
doi pubmed - National institute of health. Kratom. Liver Tox. 2018. Available from: https://livertox.nih.gov/Kratom.htm.
- Galbis-Reig D. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
- Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
doi pubmed - Eldridge WB, Foster C, Wyble L. Neonatal Abstinence Syndrome Due to Maternal Kratom Use. Pediatrics. 2018;142(6);e20181839.
doi pubmed - Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
doi pubmed - Castillo A, Payne JD, Nugent K. Posterior reversible leukoencephalopathy syndrome after kratom ingestion. Proc (Bayl Univ Med Cent). 2017;30(3):355-357.
doi pubmed - LaBryer L, Sharma R, Chaudhari KS, Talsania M, Scofield RH. Kratom, an emerging drug of abuse, raises prolactin and causes secondary hypogonadism: case report. J Investig Med High Impact Case Rep. 2018;6:2324709618765022.
doi pubmed - Post S, Spiller HA, Chounthirath T, Smith GA. Kratom exposures reported to United States poison control centers: 2011-2017. Clin Toxicol (Phila). 2019;57(10):847-854.
doi pubmed - Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
doi pubmed - Osborne CS, Overstreet AN, Rockey DC, Schreiner AD. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167.
doi pubmed - Pantano F, Tittarelli R, Mannocchi G, Zaami S, Ricci S, Giorgetti R, Terranova D, et al. Hepatotoxicity Induced by "the 3Ks": Kava, Kratom and Khat. Int J Mol Sci. 2016;17(4):580.
doi pubmed - Riverso M, Chang M, Soldevila-Pico C, Lai J, Liu X. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
doi pubmed - Kapp FG, Maurer HH, Auwarter V, Winkelmann M, Hermanns-Clausen M. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
doi pubmed - Fernandes CT, Iqbal U, Tighe SP, Ahmed A. kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138.
doi pubmed - Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction. 2008;103(6):1048-1050.
doi pubmed - Bestha D. Kratom and the Opioid Crisis. Innov Clin Neurosci. 2018;15(5-6):11.
- Ward J, Rosenbaum C, Hernon C, McCurdy CR, Boyer EW. Herbal medicines for the management of opioid addiction: safe and effective alternatives to conventional pharmacotherapy? CNS Drugs. 2011;25(12):999-1007.
doi pubmed - Nelsen JL, Lapoint J, Hodgman MJ, Aldous KM. Seizure and coma following Kratom (Mitragynina speciosa Korth) exposure. J Med Toxicol. 2010;6(4):424-426.
doi pubmed - Gershman K, Timm K, Frank M, Lampi L, Melamed J, Gerona R, Monte AA. Deaths in Colorado Attributed to Kratom. N Engl J Med. 2019;380(1):97-98.
doi pubmed - Grundmann O, Brown PN, Henningfield J, Swogger M, Walsh Z. The therapeutic potential of kratom. Addiction. 2018;113(10):1951-1953.
doi pubmed - U.S. Food and Drug Administration. FDA and Kratom. 2019. Available from: https://www.fda.gov/newsevents/publichealthfocus/ucm584952.htm.
- Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
doi pubmed - Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K. Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2004;74(17):2143-2155.
doi pubmed - European Monitoring Centre for Drugs and Drug Addiction. Kratom (Mitragyna speciosa) drug profile. 2015. Available from: http://www.emcdda.europa.eu/publications/drug-profiles/kratom.
- Suhaimi FW, Yusoff NH, Hassan R, Mansor SM, Navaratnam V, Muller CP, Hassan Z. Neurobiology of Kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(Pt 1):29-40.
doi pubmed - White CM. Pharmacologic and clinical assessment of kratom. Am J Health Syst Pharm. 2018;75(5):261-267.
doi pubmed - Mousa MS, Sephien A, Gutierrez J, O'Leary C. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
doi pubmed - Griffiths CL, Gandhi N, Olin JL. Possible kratom-induced hepatomegaly: A case report. J Am Pharm Assoc (2003). 2018;58(5):561-563.
doi pubmed - Molleken K. [Liver biopsy findings after intake of oral contraceptives (author's transl)]. Zentralbl Allg Pathol. 1979;123(3):195-201.
- Arslan N, Guzel O, Kose E, Yilmaz U, Kuyum P, Aksoy B, Calik T. Is ketogenic diet treatment hepatotoxic for children with intractable epilepsy? Seizure. 2016;43:32-38.
doi pubmed - Lagana SM, Moreira RK, Lefkowitch JH. Hepatic granulomas: pathogenesis and differential diagnosis. Clin Liver Dis. 2010;14(4):605-617.
doi pubmed - Miller R, Garrison J. What is kratom and what's it made from? Increasingly popular herbal drug tied to over 90 fatal overdoses. USA Today. 2019. Available from: https://www.usatoday.com/story/news/health/2019/04/13/kratom-what-popular-herbal-supplement-tied-fatal-overdoses/3450421002/.
- Oppel Jr R, Kovaleski S. Opioid users call kratom a godsend. The F.D.A. says it's a menace. The New York Times. 2019. Available from: https://www.nytimes.com/2019/04/17/us/kratom-overdose-deaths.html.
- Schwartz A. Kratom, an addict's alternative, is found to be addictive itself. The New York Times. 2016. Available from: https://www.nytimes.com/2016/01/03/us/kratom-an-addicts-alternative-is-found-to-be-addictive- itself.html.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


