| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 12, Number 4, August 2019, pages 208-210
Chronic Pancreatitis Leading to Pancreatogenic Diabetes Presenting in Diabetic Ketoacidosis: A Rare Entity
Gabriel Melkia, c, Linda Lahama, Gres Karima, Fnu Komala, Vinod Kumara, Shaker Barhamb, Matthew Grossmanb, Sugabramya Kurua, Hadir Mohameda, Rana Garrisa, Walid Baddourab
aDepartment of Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
bDepartment of Gastroenterology, St. Joseph’s University Medical Center, Paterson, NJ, USA
cCorresponding Author: Gabriel Melki, Department of Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
Manuscript submitted July 8, 2019, accepted July 31, 2019
Short title: DM3c in DKA
doi: https://doi.org/10.14740/gr1203
| Abstract | ▴Top |
Diabetes mellitus type 3c (DM3c) is an uncommon cause of diabetes due to pancreatic pathology. Its prevalence reaches about 5-10% among all diabetics in the Western world, largely due to chronic pancreatitis. DM3c occurs due to the destruction of the endocrine islet cells. Glucagon and insulin levels are both decreased due to the destruction of alpha and beta cells, respectively. This makes the development of diabetic ketoacidosis (DKA) a rare process in patients with DM3c because of the destruction of glucagon, which facilitates ketone production. We report a case of DM3c presenting with DKA. The patient presented with a history of chronic pancreatitis and was on pancreatic enzyme replacement therapy. Prior records revealed that HbA1c levels were normal. Prior computed tomography evidence revealed diffuse pancreatic calcifications. The patient was admitted for DKA, presenting with hyperglycemia, blood glucose of 703 mg/dL, bicarbonate of 16 mmol/L, ketones in the urine and acetone in the blood. The patient’s anion gap corrected for albumin was 27. The patient was admitted to the medical intensive care unit where he was treated with intravenous (IV) insulin and IV hydration. Once the anion gap closed, the patient was transitioned to long-acting insulin. HbA1c level on admission was elevated, autoimmune causes of diabetes were sent and were negative, ruling out late onset type 1 diabetes. This shows that although it is a rare phenomenon, diabetics with DM3c can present in DKA.
Keywords: Chronic pancreatitis; Diabetic ketoacidosis; Pancreatogenic diabetes; Diabetes mellitus type 3c
| Introduction | ▴Top |
Diabetes mellitus type 3c (DM3c) is a cause of diabetes largely due to chronic pancreatitis [1-3]. Its prevalence reaches about 5-10% among all diabetic patients in the Western world [2, 4]. About 75% of patients presenting with DM3c have a history of chronic pancreatitis, while others have hemochromatosis, cystic fibrosis or pancreatic cancer [2, 4]. Presence of pancreatic calcifications, longer duration of disease and smoking increase the likelihood of developing DM3c [5]. DM3c is mainly due to cytokine production and endocrine islet cell destruction [2]. It can also occur because of pancreatic manipulation, such as pancreatic surgery for pancreatic cancer [3]. Due to its association with pancreatic disease, patients are more likely to be undernourished with various vitamin and mineral deficiencies, in addition to metabolic derangement [5]. The management of DM3c tends to be more challenging than that of DM type 1 and 2 due to a variety of metabolic features, such as low glycogen stores and normal glycated hemoglobin [1, 2, 5]. Glucagon and insulin levels are both low due to destruction of alpha and beta cells, respectively. Glucose levels can vary as the destruction of glucagon producing alpha cells can lead to hypoglycemia, while the destruction of insulin producing beta cells can lead to hyperglycemia [1, 2]. This in turn makes the development of diabetic ketoacidosis (DKA) a rare process in patients with DM3c because of the deficiency of glucagon, which normally would facilitate ketone production [1, 2]. We report a case of DM3c presenting with DKA, a very rare finding, as the cells responsible have been compromised.
| Case Report | ▴Top |
A 50-year-old male with a past medical history of chronic pancreatitis (secondary to chronic alcohol use) presented to the emergency department (ED) with complaints of epigastric abdominal pain and generalized weakness for 2 months in duration. He reported chronic abdominal pain. He denied nausea, vomiting, diarrhea, constipation, and change in urinary habits, recent travel, or sick contacts. The patient was on acetaminophen/oxycodone for pain control and pancreatic enzyme replacement therapy for his chronic pancreatitis. Vital signs on presentation were blood pressure 100/92 mm Hg, heart rate of 94 bpm, respiratory rate of 16 breaths/min and O2 saturation of 97% on room air. Physical exam was positive for epigastric tenderness. A computed tomography (CT) of abdomen from prior records revealed a pancreas with diffuse calcifications, suggestive of chronic pancreatitis (Fig. 1). In the ED, he was found to be hyperglycemic with a blood glucose of 703 mg/dL, serum bicarbonate of 16 mmol/L with ketones in the urine and acetone in the blood. The patient’s anion gap corrected for albumin was 27. The patient met the criteria for DKA. The patient was subsequently admitted to the medical intensive care unit for management of DKA. He was made nil per os (NPO) and started on intravenous (IV) lactated Ringer’s solution with potassium and an IV insulin drip. The patient’s pancreatic enzyme replacement was held, as patient was NPO. Cardiac enzymes and lipase were sent to rule out other causes of epigastric pain. Cardiac enzymes were subsequently reported to be within normal limits and lipase was elevated to 156. A septic workup was also initially sent, which returned negative. The patient’s blood glucose was monitored every hour while electrolytes and albumin were monitored every 4 h. IV hydration continued throughout his treatment with IV insulin. Adjustments of potassium and dextrose were made for IV hydration. Once the patient’s anion gap closed, the patient was transitioned to long-acting insulin detemir 10 Units SQ QHS, and was placed on a diabetic diet 2 h after detemir administration. The patient was also placed on an insulin sliding scale and sent to the medical floor. Diabetic teaching was provided to the patient during this hospital admission. While the patient was admitted, the insulin regimen was optimized. The patient was subsequently discharged with insulin aspart 3 Units TID with meals and insulin detemir 10 Units QHS and 6 Units QAM. An autoimmune workup for diabetes was sent and returned negative. The patient was given ketorolac for abdominal pain and was told to follow up with his primary care physician. The patient’s prior HbA1c values were normal; however, during this admission, it was 16%. This case is an unusual case of DM3c presenting in DKA. The destruction of beta cells in the endocrine pancreas is one of the main reasons why DM occurs in patients with chronic pancreatitis [1, 2]. These patients rarely present in DKA because they typically have a destruction of glucagon secreting alpha cells which are required to create ketones [1, 2]. This patient was diagnosed with DM3c and presented in DKA.
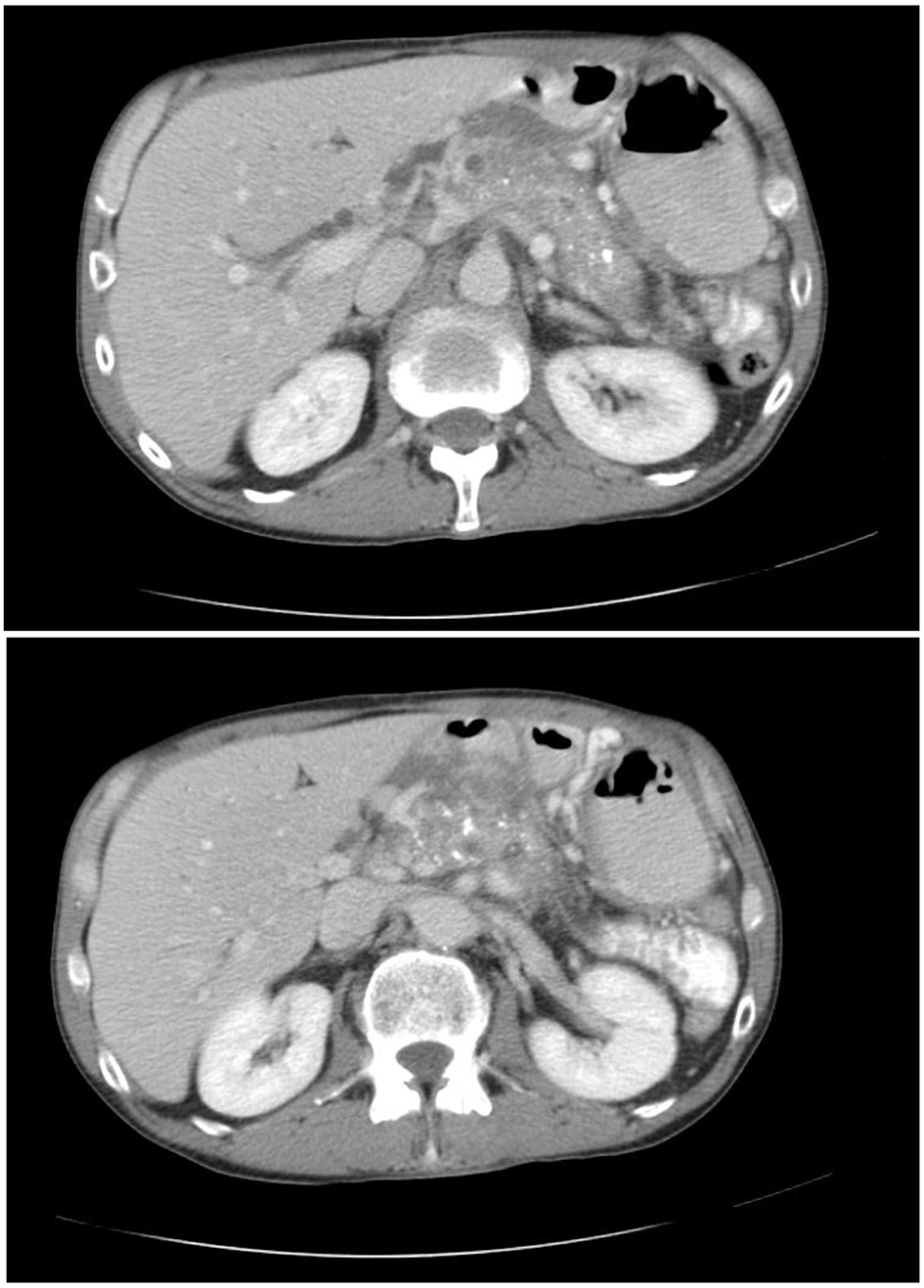 Click for large image | Figure 1. Computed tomography of abdomen pelvis showing diffuse calcifications and multiple pseudocyst formation consistent with chronic pancreatitis. |
| Discussion | ▴Top |
DM3c is most commonly associated with recurrent or chronic pancreatitis [1, 2, 4]. DM3c remains underdiagnosed as an underlying etiology of diabetes, being mistaken for type 2 diabetes mellitus most frequently [4]. Typically, exocrine insufficiency precedes endocrine insufficiency, resulting in fat malabsorption with the later development of diabetes [1, 2, 5]. The pathophysiology of DM3c commonly involves inflammation of the pancreatic islet cells with irreversible fibrotic changes [1, 2]. Early on, the beta cells and the pancreatic polypeptide (PP) cells are affected, but as the disease progresses, glucagon secreting alpha cells are destroyed [1, 2]. Due to defective digestion, incretin secretory function is lost. This disease process can be accelerated by the presence of multiple risk factors for type 2 diabetes. The diagnostic criteria of DM3c require imaging evidence of pancreatic pathology, the presence of exocrine insufficiency and the absence of type 1 DM autoimmune markers [1, 2, 5]. Further evidence of incretin, PP, or insulin secretory defects would also support the diagnosis. Confirmation can be made by absent PP response to mixed-nutrient ingestion, which best discriminates the pathological process of type 3c versus type 2 DM [1, 2, 5]. In our case, chronic pancreatitis was diagnosed based on prior CT scans, which revealed diffuse calcifications. No pathological diagnosis was made, making this a limitation to the case report. Recognition of DM3c is crucial, as the underlying pancreatic disease would require special treatment. Involvement of oral pancreatic enzyme replacement to correct fat malabsorption is crucial in order to optimize incretin section and enable the absorption of vitamin D and other fat-soluble vitamins. Pancreatic enzyme replacement can improve the insulin secretion, incretin levels, as well as glucose intolerance [5]. DKA is typically seen in DM1 due to high glucagon and low insulin levels [1, 2]. Hyperglycemia is due to increased gluconeogenesis, increased glycogenolysis and decreased glucose uptake into cells. Ketosis is due to insulin deficiency, causing a mobilization and oxidation of fatty acids, increased substrate for ketogenesis, increased ketogenic state of the liver and decreased ketone clearance [1, 2].
The stress hormones that upregulate the ketone production are glucagon, cortisol, catecholamines and growth hormone [6, 7]. In the state of relative insulin deficiency, glucagon enhances lipolysis, which in turn increases the supply of fatty acids to the liver [6, 7]. Glucagon’s main effect is on the liver, where it inhibits malonyl-CoA synthesis, which activates the carnitine acyltransferase system [6, 7]. This increases fatty acid oxidation and ketogenesis [6, 7]. In DM3c, the low glucagon levels lead to an enhancement of other stress hormones, cortisol, catecholamines and growth hormone [6, 7]. The most prominent one in an acute stressed state such as DKA is catecholamines, which may be the enhanced hormone that upregulates ketone production [6, 7]. Catecholamines upregulate ketone production by stimulating lipolysis in adipocytes and providing free fatty acids to the liver to produce ketones [6, 7].
Conclusion
Diagnostic studies that support a state of DKA are an increased anion gap metabolic acidosis, positive ketones in the serum and urine, increased serum glucose, and elevated blood urea nitrogen and creatinine. In patients who suffer from chronic pancreatitis and present in DKA, it is of crucial importance to think about DM3c, as this would affect long-term management [5]. DM3 is an underdiagnosed and overlooked disease, often mistaken for DM1 or DM2 [4]. Patients with chronic pancreatitis should be screened for diabetes with HbA1c. Once diabetes is confirmed, an autoimmune workup for DM1 should be sent to rule out late onset DM1.
Learning points
1) DM3c is a new entity for pancreatic diabetes. 2) DM3c is due to pancreatic pathology. 3) Pathogenesis includes local islet cell destruction, islet cell fibrosis, local cytocine release or surgical removal. 4) The leading cause of DM3c is chronic pancreatitis. 5) Patients presenting in DKA should not assume to have DM1, especially if they have a history of chronic pancreatitis. 6) Patients with chronic pancreatitis should undergo regular monitoring for the development of diabetes with HbA1c levels. 7) When patients with chronic pancreatits reveal laboratory evidence of glucose intolerance (e.g. elevated HbA1c), patients should have an autoimmune workup sent to rule out DM1. 8) The diagnosis of DM3c requires evidence of glucose intolerance, imaging evidence of pancreatitc compromise and negative autoimmune causes of diabetes.
Acknowledgments
We thank Dr. Patrick Michael (Program Director of the Internal Medicine Residency), Dr. Michael Agnelli (Associate Program Director of the Internal Medicine Residency), Dr. Monisha Singhal (Associate Program Director of the Internal Medicine Residency) and Dr. Robert Lahita (Chairman of the Department of Medicine) for their continuous support and guidance.
Financial Disclosure
No funding was provided to any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient for this case report.
Author Contributions
All authors contributed to the revision and approval of the manuscript.
| References | ▴Top |
- Andersen DK, Korc M, Petersen GM, Eibl G, Li D, Rickels MR, Chari ST, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66(5):1103-1110.
doi pubmed - Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11(3):279-294.
doi pubmed - Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology. 2011;11(2):268-276.
doi pubmed - Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)—are we neglecting an important disease? Eur J Intern Med. 2013;24(3):203-206.
doi pubmed - Duggan SN, Ewald N, Kelleher L, Griffin O, Gibney J, Conlon KC. The nutritional management of type 3c (pancreatogenic) diabetes in chronic pancreatitis. Eur J Clin Nutr. 2017;71(1):3-8.
doi pubmed - Gosmanov AR, Gosmanova EO, Kitabchi AE. Hyperglycemic crises: Diabetic Ketoacidosis (DKA), and Hyperglycemic Hyperosmolar State (HHS) [Updated 2018 May 17]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279052/.
- Alberti KG, Johnston DG, Gill A, Barnes AJ, Orskov H. Hormonal regulation of ketone-body metabolism in man. Biochem Soc Symp. 1978;43:163-182.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


