| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 12, Number 3, June 2019, pages 176-180
Successful Hepatitis C Virus Eradication in a Hemodialysis Patient With 2k/1b Chimera Genotype: A Case Report and Literature Review
Mikhail Leonidovich Zubkina, c, Galina Sergeevna Shchepetkovaa, Olga Veniaminovna Balkarovab, Valeriy Ivanovich Chervinkoa, Evgeniy Vladimirovich Kryukova
aСlinical and Diagnostic Department, G.N. Gabrichevsky Research Institute for Epidemiology and Microbiology, 125212 Moscow, Russia
bNephrology Unit, City Hospital No. 24, 127015 Moscow, Russia
cCorresponding Author: Mikhail L. Zubkin, Clinical and Diagnostic Department, G.N. Gabrichevsky Research Institute for Epidemiology and Microbiology, Admiral Makarov Street 10, 125212 Moscow, Russia
Manuscript submitted March 5, 2019, accepted April 5, 2019
Short title: Antiviral Therapy of Hepatitis C in Dialysis
doi: https://doi.org/10.14740/gr1171
| Abstract | ▴Top |
Treatment of hemodialysis patients infected with two or three hepatitis C virus (HCV) genotypes (Gt) with interferon-free regimens has not been possible until the recent introduction of pan-genotypic next generation therapy. The main reason is that sofosbuvir (SOF)-containing regimens are contraindicated in patients with low glomerular filtration rate. We describe here a case of a chronic HCV infection in a patient with end-stage renal disease, successfully treated with gleсaprevir/pibrentasvir (GLE/PIB). Limited published data are available regarding the efficacy of antiviral therapy in patients with rare HCV recombinant Gt 2k/1b. We were not able to identify any reports describing treatment of hemodialysis patients with this recombinant type of HCV. We present a 57-year-old patient with autosomal-dominant polycystic kidney disease with liver involvement with end-stage of kidney disease. He was infected with HCV Gt 2k/1b variant after initiation of hemodialysis. This subtype appeared in Russia (Soviet Union that times) as a result of high frequency of virus mutations, and actually is widely spread in some states of the post-Soviet space, as well as in the countries with intensive migration from Russia and other former Soviet republics. In this particular case, we observed a tendency to a rapid progression of liver fibrosis despite mild clinical activity of chronic hepatitis C. A 12-week course of GLE/PIB allowed achieving sustained virologic response (SVR) and was well tolerated.
Keywords: Hepatitis C virus; Recombinant 2k/1b genotype; Hemodialysis; Direct-acting antiviral agents; Glecaprevir/pibrentasvir
| Introduction | ▴Top |
Last decades, a significant reduction of the number of patients infected with hepatitis C virus (HCV) has been observed in the hemodialysis units. However, hemodialysis patients have higher incidence of HCV infection compared to the general population. Despite the relatively mild clinical course of the disease, HCV infection is an independent and significant risk factor of the increased mortality in these patients [1-5]. Introduction of direct-acting antiviral agents (DAAs) into clinical practice allowed achieving high efficacy among various groups of HCV-infected patients, including those with liver cirrhosis. As all patients in dialysis units are regularly screened and evaluated for HCV infection, there is a realistic opportunity of HCV elimination in this group. However, there are a number of limitations which make this goal difficult to achieve. Until recent time, SOF-containing regimens were the only option to treat chronic HCV infection genotypes (Gt) 2 and 3, but they cannot be used in patients on hemodialysis. Moreover, so-called HCV recombinant Gt, in particular 2k/1b variant, proved to be resistant not only to interferon therapy, but also to some DAAs [6].
A new pan-genotypic DAA combination, gleсaprevir/pibrentasvir (GLE/PIB) with an extra-renal elimination, with proven efficacy in hemodialysis patients, is a promising option for this group [7]. However, it has not been studied in patients infected with 2k/1b HCV variant.
The purpose of our report is to provide information on the first experience of using GLE/PIB combination in a hemodialysis patient infected with the chimeric HCV Gt 2k/1b.
| Case Report | ▴Top |
A 57-year-old Caucasian man had an elevated blood pressure of 200/100 mm Hg and an increased serum creatinine (exact value is unknown) during routine outpatient check-up in 2006. After abdominal and kidney ultrasound, he was referred to nephrologist and diagnosed with autosomal-dominant polycystic kidney disease (ADPKD) with liver involvement. Family history was taken with no clear information about parents, but it was found that the patient’s elder son was diagnosed with ADPKD, while the younger son never underwent examination for ADPKD.
In 2011, serum creatinine was already 364 µmol/L, hemoglobin (Hb) was 124 g/L, bilirubin, aspartate transaminase (AST) and alanine transaminase (ALT) were all within normal range (Fig. 1). By April 2012, his creatinine increased to 916 µmol/L, and estimated glomerular filtration rate (eGFR) by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 8 mL/min/1.73 m2 [8]. In September 2012, patient was started on hemodialysis. Hepatitis B surface antigen (HBsAg) and anti-HCV were undetectable. In April 2013, bilateral nephrectomy was performed in preparation to kidney transplantation. In February 2014, his aminotransferases were found three times increasing upper normal limit, and antibodies to the HCV were detected. Gamma-glutamyltranspeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin were within normal ranges. By July 2014, AST and ALT decreased to 36 and 60 U/L, respectively. He underwent kidney transplantation, complicated by bleeding in the early postoperative period, and in August 2014, a transplantectomy was performed due to severe graft dysfunction. Hemodialysis treatment was resumed; patient received antihypertensive drugs (amlodipine and losartan) and erythropoietin on the regular basis.
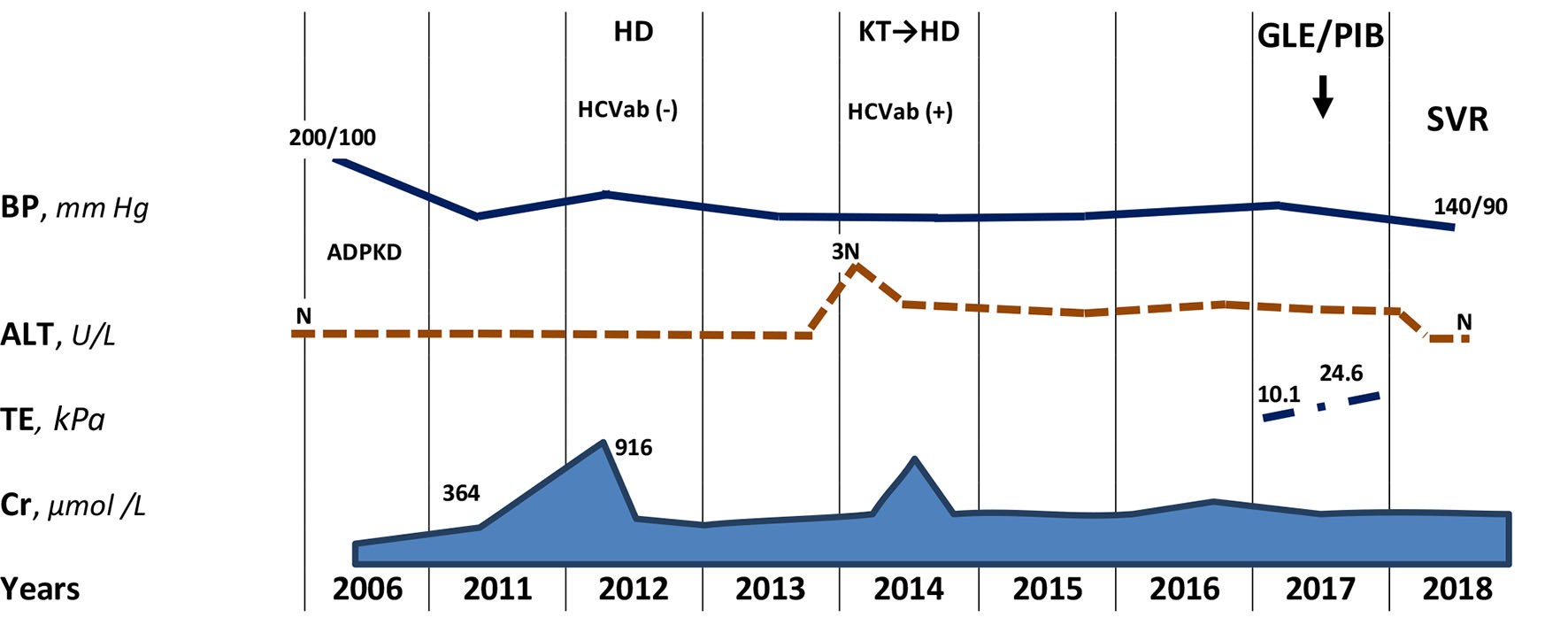 Click for large image | Figure 1. The first information about effective and safe treatment with glecaprevir/pibrentasvir combination of rare HCV recombinant 2k/1b genotype in hemodialysis patient. ADPKD: autosomal-dominant polycystic kidney disease; HD: hemodialysis; KT: kidney transplantation; SVR: sustained virologic response; BP: blood pressure; Сr: creatinine; TE: transient elastography; N: normal range. |
During next 3 years, only slight increase of ALT was observed. In 2017, Gt 2 of HCV (further 2k/1b variant) was identified. The viral load was 7.1 × 104 IU/mL. Other laboratory findings included Hb 121 g/L, white blood cell count (WBC) 3.7 × 109/L, platelets (PLT) 96 × 109/L, total protein 77 g/L, albumin 40 g/L, total bilirubin 10.1 µmol/L, AST 20 U/L, ALT 52 U/L, GGT 47 U/L, ALP 80 U/L, creatinine 838 µmol/L, alpha-fetoprotein 6.0 U/mL and international normalized ratio (INR) 1.04.
Abdomen ultrasound showed hepatosplenomegaly, increased liver echogenicity with well-preserved vascular pattern, heterogeneous echo-structure with multiple cysts up to 25 mm in diameter (Fig. 2). Diameter of the hepatic vein was 9.5 mm and the spleen vein was 6 mm. The results of transient elastography (TE) were not fully reliable due to cystic liver disease; however, the reason for such a rapid increase of the hepatic parenchyma density (from 10.1 to 24.6 kPa) within a few months remained unclear.
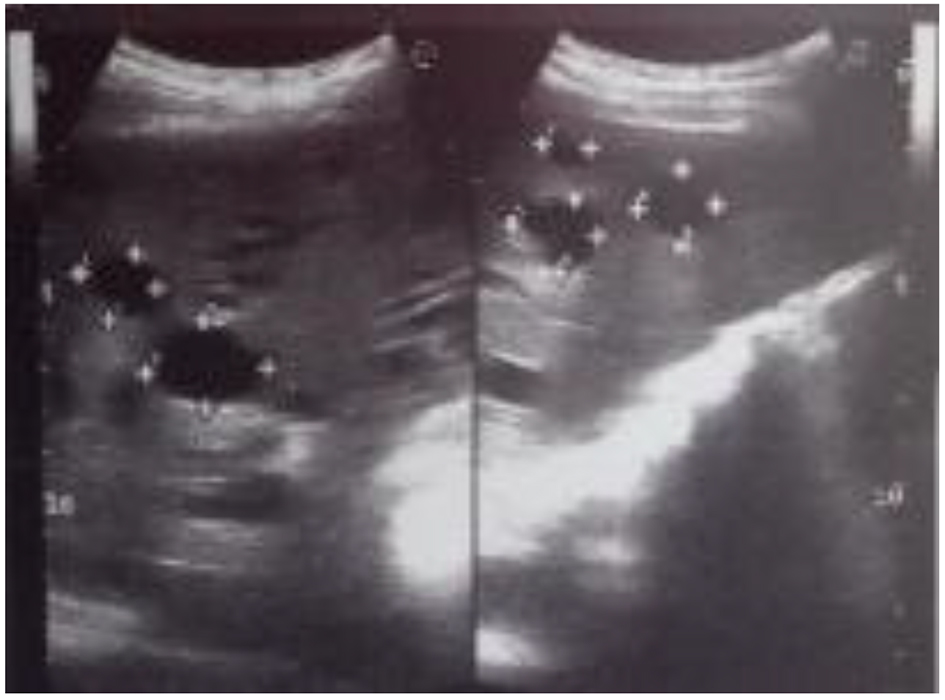 Click for large image | Figure 2. The ultrasound scan showed multiple cysts in the liver. |
Antiviral therapy was not initiated that time due to contraindications for SOF-containing regimens.
On December 14, 2017, antiviral treatment using new pan-genotypic combination of glecaprevir and pibrentasvir (GLE/PIB, 300/120 mg/day) was initiated in frame of the pre-approval access program. Tolerability of therapy was satisfactory, and the patient noted only slight intensification of his skin itching. Four weeks after the start of therapy, aviremia was achieved, and persisted until the end of treatment. After 12 weeks of therapy, HCV elimination was confirmed, that is, SVR12 was achieved.
| Discussion | ▴Top |
Uses of so-called universal methods for the prevention of HCV infection lead to a significant reduction of the HCV-infection incidence in the dialysis units in developed countries last decades. In Russia, according to the National Registry, average proportion of HCV-antibodies carriers among hemodialysis patients is 11.6%, with a significant variability ranging from 1-4% to 25-50%, depending on the socio-economic development of the region [9].
The negative effect of chronic HCV infection on the prognosis of patients receiving renal replacement therapy (hemodialysis and kidney transplantation) has been convincingly proved. Therefore, the introduction DAAs with high efficacy and safety opened new opportunities for treatment of these patients. Excellent results of the combination of paritaprevir/ritonavir with ombitasvir and dasabuvir (“3D”) usage in HCV-infected hemodialysis patients with Gt 1 and 4 were shown. Abad et al and Ponziani et al reported 100% SVR12 in hemodialysis patients. It should be emphasized that the last study included only patients with liver cirrhosis and those who did not respond to previous interferon-containing therapy [10, 11]. No cases of early termination of treatment due to adverse events were reported.
Treatment of chronic HCV infection Gt 2 and 3 with SOF-containing regimens in hemodialysis patients was allowed only in selected cases, as SOF is contraindicated for patients with eGFR < 30 mL/min [12].
A new pan-genotypic combination of DAAs, GLE/PIB with the minimal level of renal excretion, showed its high efficiency in hemodialysis patients with chronic hepatitis C, including those with liver cirrhosis. In the EXPEDITION-4 study, 104 patients with impaired renal function (stage 4 and stage 5 of chronic kidney disease) were enrolled. Eighty-five of subjects received hemodialysis, and 28 of them were infected by HCV Gt 2 or 3 [7]. In 20 patients, compensated liver cirrhosis was diagnosed. SVR12 in this group was achieved by 98% of patients after the 12-week course of treatment. Serious adverse events associated with the use of this combination of drugs were not registered. In another study, all of 12 patients with severe renal dysfunction achieved SVR12 [13]. In this group, nine patients were infected with HCV Gt 2, four received hemodialysis and two had compensated liver cirrhosis. It is important to emphasize that patients without liver cirrhosis received antiviral therapy for 8 weeks, and the group of cirrhotic patients received antiviral therapy for 12 weeks.
We observed seven hemodialysis patients infected with HCV Gt 2 and 3, receiving GLE/PIB therapy. SVR12 was 100% (unpublished data). One of them was infected with 2k/1b variant of the HCV.
The 2k/1b chimeras were first detected in blood samples from patients with chronic HCV infection in St. Petersburg (Russia) by Kalinina et al in 2002 [14]. Presumably this recombinant genotype emerged in Russia (Soviet Union those times) between 1923 and 1956 [15]. Recombination resulted from the high frequency of mutation of the virus and led to the formation of genetic diversity of HCV. HCV 2k/1b variant appeared to be more viable than others detected HCV chimeras (2i/6p, 2b/1b, 2/5, 2b/6w, 2b/1a and 2a/1b). The frequency of its detection in St. Petersburg region reaches 7.5% of the total number of infected patients, and 6.3% of infected hemodialysis patients in this city [16]. In general, the prevalence of recombinant 2k/1b virus in Russia is 4% among all HCV-infected patients, and 40% among those infected with Gt 2 [17]. There is a significant variability in infection rate with this recombinant variant in the former Soviet republics. For example, in Georgia, prevalence of 2k/1b variant among all patients with chronic HCV infection reaches 19.7-22.2%, and 72-76% among patients with Gt 2, while in Uzbekistan, prevalence of 2k/1b variant does not exceed 1% of the total infected population [18-20].
An interesting study, conducted by German group, showed high incidence of recombinant HCV 2k/1b strains in patients infected with HCV Gt 2 in Germany (13%), and especially in Israel (25%), countries with the high proportion of emigrants from the former Soviet Union, and vice versa, the absence of recombinant variants in Italy, where the number of such emigrants is much lower [6]. More international studies are needed to address the prevalence of 2k/1b variant in other countries with a significant number of emigrants from the Soviet Union and countries of the post-Soviet space.
HCV Gt 1b is most prevalent type of virus in Russia, in particular in Moscow hemodialysis units. At the same time, HCV Gt 2 is a rare. However, the high prevalence of the 2k/1b chimera among Gt 2 HCV is a reason for clinical concerns about infection with this recombinant variant of the HCV. Taking into consideration this possibility, the deep sequencing analysis of HCV geno-/subtype was performed in our patient and the 2k/1b was identified.
The unique feature of our patient was the combination of chronic HCV infection with liver cysts in frame of autosomal-dominant polycystic kidney disease. This disease is typically complicated by severe kidney damage, which, like in our case, often leads to the end stage of kidney disease, requiring renal replacement therapy. Clinical activity of chronic hepatitis C was low, which is typical for hemodialysis patients; according to different authors, ALT in these patients usually does not exceed 18 or 27 U/L [21, 22].
Absence of correlation between aminotransferases levels and disease activity in hemodialysis patients complicates assessment of disease severity. It is well known that liver biopsy is the optimal method for diagnostics of inflammatory activity and fibrosis stage [23]. However, the high risk of complications, especially bleeding, in dialysis patients, increases the value of non-invasive methods. Liver TE usage in patients receiving hemodialysis was evaluated by Liu et al, 2011 [24]. The authors showed that it is more informative than the AST to platelet ratio index (APRI). However, the lack of information on the correlation of data obtained with TE and liver biopsy in hemodialysis patients, as well as the possible effect of liquid overload in the interdialysis period on the results of the examination, requires more evidence to prove the adequacy of replacing liver biopsy with TE [25]. Presence of multiple cysts additionally influenced the reliability of TE results in our patient.
Evaluation of DAAs efficacy in patients with HCV Gt 2k/1b is currently under investigation. It was shown that the combination of SOF/ribavirin demonstrated good results in patients infected with HCV Gt 2, but had poor efficacy in subjects infected with recombinant variant [26]. In contrast, the usage of pan-genotypic DAAs’ combinations (SOF/daclatasvir and SOF/velpatasvir), as well as combinations recommended for treatment of HCV Gt 1 (SOF/ledipasvir, “3D” therapy) showed good results as initial treatment in patients with HCV variant 2k/1b (n - 9), as well as in cases of unsuccessful previous treatment with SOF/ribavirin (n - 13). SVR12 was achieved in 21 of 22 patients (95.5%) [6]. It should be noted that 15 out of these 22 patients received treatment with SOF-containing regimens and seven received “3D” therapy.
We could not use SOF-containing regimens in our patient due to very low GFR. Taking into account German experience, we preferred to use the pan-genotypic combination GLE/PIB in frame of the pre-approval access program in Russia. We could not reliably estimate the severity of liver fibrosis, because the TE results could be greatly distorted by the presence of numerous cysts. Aminotransferases were just moderately elevated, especially taking into account normal values for hemodialysis patients. However, splenomegaly, leukopenia and thrombocytopenia indicated high probability of progressive liver fibrosis, despite a relatively short time from the hit of HCV infection. On this basis, it was decided to initiate a 12-week course of antiviral therapy. Aviremia was reached by the fourth week of treatment, and SVR12 was achieved, despite severe comorbidity in our patient.
Conclusions
In our experience, the combination of GLE/PIB used for 12 weeks for treatment of HCV GT 2k/1b variant in a patient on hemodialysis proved to be effective, well tolerated and safe. The number of patients in European and American hemodialysis units infected with this variant of the virus is still unknown, however, may be substantial due to migration. Therefore, studies for evaluation of hepatitis C chimeric genotypes prevalence will help to identify patients population which may benefit from pan-genomic DAAs administration.
Acknowledgments
We thank Dr. Helena Zakharova for help in preparing of our manuscript.
Financial Disclosure
None to declare.
Conflict of Interest
Zubkin M.L. is a lecturer for AbbVie, Bristol-Myers Squibb, MSD, Gilead Sciences. Other authors do not have any conflict of interest.
Informed Consent
Patient’s informed consent was obtained.
Author Contributions
Zubkin ML and Kryukov EV designed the report; Shchepetkova GS and Balkarova OV collected the patients clinical data; Zubkin ML, Chervinko VI and Kryukov EV analyzed the data and wrote the paper.
| References | ▴Top |
- Furusyo N, Hayashi J, Ariyama I, Sawayama Y, Etoh Y, Shigematsu M, Kashiwagi S. Maintenance hemodialysis decreases serum hepatitis C virus (HCV) RNA levels in hemodialysis patients with chronic HCV infection. Am J Gastroenterol. 2000;95(2):490-496.
doi pubmed - Fabrizi F, Lunghi G, Finazzi S, Colucci P, Pagano A, Ponticelli C, Locatelli F. Decreased serum aminotransferase activity in patients with chronic renal failure: impact on the detection of viral hepatitis. Am J Kidney Dis. 2001;38(5):1009-1015.
doi pubmed - Ishida H, Agishi T, Koyama I, Sawada T, Murakami T, Utsumi K, Tsuji K, et al. Hemodialysis paradox: survey on the incidence rate of hepatocellular carcinoma in antihepatitis virus C-antibody-positive chronic hemodialysis patients. Artif Organs. 2001;25(1):58-60.
doi pubmed - Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: Effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther. 2004;20(11-12):1271-1277.
doi - Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, Han DS, Jha V, et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24(5):1598-1603.
doi pubmed - Susser S, Dietz J, Schlevogt B, Zuckerman E, Barak M, Piazzolla V, Howe A, et al. Origin, prevalence and response to therapy of hepatitis C virus genotype 2k/1b chimeras. J Hepatol. 2017;67(4):680-686.
doi pubmed - Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Brau N, Brown A, Pol S, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377(15):1448-1455.
doi pubmed - Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
doi pubmed - Bikbov BT, Tomilina NA. The contingent and treatment quality indicators in patients on replacement therapy of end stage renal disease in the Russian Federation in 1998-2013 years. Report of the register of renal replacement therapy of the Russian Dialysis Society, part II. Nephrologiya I Dialys. 2016;18:98-164.
- Abad S, Vega A, Hernandez E, Merida E, de Sequera P, Albalate M, Macias N, et al. Universal Sustained viral response to the combination of ombitasvir/paritaprevir/ritonavir and dasabuvir with/without ribavirin in patients on hemodialysis infected with hepatitis C virus genotypes 1 and 4. Am J Nephrol. 2017;45(3):267-272.
doi pubmed - Ponziani FR, Siciliano M, Lionetti R, Pasquazzi C, Gianserra L, D'Offizi G, Gasbarrini A, et al. Effectiveness of Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir in Hemodialysis Patients With Hepatitis C Virus Infection and Advanced Liver Fibrosis: Case Reports. Am J Kidney Dis. 2017;70(2):297-300.
doi pubmed - European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66(1):153-194.
doi - Kumada H, Watanabe T, Suzuki F, Ikeda K, Sato K, Toyoda H, Atsukawa M, et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol. 2018;53(4):566-575.
doi pubmed - Kalinina O, Norder H, Mukomolov S, Magnius LO. A natural intergenotypic recombinant of hepatitis C virus identified in St. Petersburg. J Virol. 2002;76(8):4034-4043.
doi pubmed - Raghwani J, Thomas XV, Koekkoek SM, Schinkel J, Molenkamp R, van de Laar TJ, Takebe Y, et al. Origin and evolution of the unique hepatitis C virus circulating recombinant form 2k/1b. J Virol. 2012;86(4):2212-2220.
doi pubmed - Kalinina O. Genome organization and geographical distribution of the natural intergenotypic recombinant of hepatitis С RF1_2k/1b. Infekciya I Immunitet. 2012;2:677-686.
doi - Karandashova IV, Bulatova KV, Chulanov VP. The prevalence of the recombinant form of RF1_2k/1b of the hepatitis C virus in Russia. In: Pokrovskiy V.I. editors. Molecular diagnostics. 2017: Proceedings of the 9th Russia scientific conference with international participation; 2017. p.18-20; Moscow, Russia. Tambov: Yulis. 2017;1:56-57.
- Zakalashvili M, Zarkua J, Weizenegger M, Bartel J, Raabe M, Zangurashvili L, Kankia N, et al. Identification of hepatitis C virus 2k/1b intergenotypic recombinants in Georgia. Liver Int. 2018;38(3):451-457.
doi pubmed - Karchava M, Waldenstrom J, Parker M, Hallack R, Sharvadze L, Gatserelia L, Chkhartishvili N, et al. High incidence of the hepatitis C virus recombinant 2k/1b in Georgia: Recommendations for testing and treatment. Hepatol Res. 2015;45(13):1292-1298.
doi pubmed - Kurbanov F, Tanaka Y, Chub E, Maruyama I, Azlarova A, Kamitsukasa H, Ohno T, et al. Molecular epidemiology and interferon susceptibility of the natural recombinant hepatitis C virus strain RF1_2k/1b. J Infect Dis. 2008;198(10):1448-1456.
doi pubmed - Guh JY, Lai YH, Yang CY, Chen SC, Chuang WL, Hsu TC, Chen HC, et al. Impact of decreased serum transaminase levels on the evaluation of viral hepatitis in hemodialysis patients. Nephron. 1995;69(4):459-465.
doi pubmed - Espinosa M, Martin-Malo A, Alvarez de Lara MA, Soriano S, Aljama P. High ALT levels predict viremia in anti-HCV-positive HD patients if a modified normal range of ALT is applied. Clin Nephrol. 2000;54(2):151-156.
- Marinaki S, Boletis JN, Sakellariou S, Delladetsima IK. Hepatitis C in hemodialysis patients. World J Hepatol. 2015;7(3):548-558.
doi pubmed - Liu CH, Liang CC, Huang KW, Liu CJ, Chen SI, Lin JW, Hung PH, et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6(5):1057-1065.
doi pubmed - Taneja S, Borkakoty A, Rathi S, Kumar V, Duseja A, Dhiman RK, Gupta KL, et al. Assessment of liver fibrosis by transient elastography should be done after hemodialysis in end stage renal disease patients with liver disease. Dig Dis Sci. 2017;62(11):3186-3192.
doi pubmed - Hedskog C, Doehle B, Chodavarapu K, Gontcharova V, Crespo Garcia J, De Knegt R, Drenth JP, et al. Characterization of hepatitis C virus intergenotypic recombinant strains and associated virological response to sofosbuvir/ribavirin. Hepatology. 2015;61(2):471-480.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


