| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 12, Number 2, April 2019, pages 53-59
The Flavone Luteolin, an Endocrine Disruptor, Relaxed Male Guinea Pig Gallbladder Strips
Loren Kline
School of Dentistry, University of Alberta, 5-470 ECHA, Edmonton, Alberta T6G 2E1, Canada
Manuscript submitted January 22, 2019, accepted February 19, 2019
Short title: Luteolin and the Gallbladder
doi: https://doi.org/10.14740/gr1142
| Abstract | ▴Top |
Background: Luteolin (3',4',5,7-tetrahydroxyflavone) is a flavone with a yellow crystalline appearance present in numerous plants such as broccoli, green chili, and carrot. Luteolin is considered to be an endocrine disruptor with potent estrogen agonist activity and potent progesterone antagonist activity. Luteolin has effects on smooth muscle. Luteolin relaxed guinea pig trachea smooth muscle as it inhibited both phosphodiesterase and reduced intracellular Ca2+. Luteolin also caused vasorelaxation in rat thoracic aorta smooth muscle by inhibiting intracellular Ca2+ release, inhibition of sarcolemmal Ca2+ channels, and activation of K+ channels. Luteolin or its glycosides from artichoke extracts may have an ameliorating effect on irritable bowel syndrome. The purpose of this study was to determine if luteolin had an effect on gallbladder motility.
Methods: An in vitro pharmacologic technique was utilized. Either cholecystokinin octapeptide (CCK) or KCl were used to induce tension in male guinea pig gallbladder strips maintained in Sawyer-Bartlestone chambers. Luteolin relaxed either the CCK- or KCl-induced tension in a concentration dependent manner. Various blockers were added to the chambers to determine which second messenger system(s) mediated the observed relaxation. Paired t-tests were used for statistical analysis. Differences between mean values of P < 0.05 were considered significant.
Results: Treatment of the gallbladder strips with luteolin prior to either KCl or CCK significantly (P < 0.001) decreased the amount of either KCl- or cholecystokinin-induced tension. The 2-aminoethoxydiphenylborane was used to ascertain if the release of intracellular Ca2+ mediated the luteolin-induced relaxation. It significantly (P < 0.001) decreased the amount of luteolin-induced relaxation. To ascertain if PKA mediated the luteolin-induced relaxation, PKA inhibitor 14-22 amide myristolated was used. It significantly (P < 0.01) reduced the amount of luteolin-induced relaxation. Neither KT5823, NG-methyl-L-arginine acetate salt, genistein, tetraethylammonium, nor fulvestrant had a significant effect. To ascertain if PKC mediated the luteolin-induced relaxation, the PKC inhibitors bisindolymaleimide IV and chelerythrine Cl- were used together. They had no significant effect.
Conclusions: Luteolin relaxed cholecystokinin- or KCl-induced tension by blocking extracellular Ca2+ entry as well as intracellular Ca2+ release. In addition, the actions of PKA are also involved in mediating the luteolin effect.
Keywords: Luteolin; Flavone; Gallbladder; Smooth muscle; Guinea pig; Calcium; PKA
| Introduction | ▴Top |
Flavonoids are common constituents of plants used as traditional remedies. Luteolin is a flavone, a subtype of flavonoid. Luteolin is present in numerous edible plants, many of which are used in traditional medicine [1]. For example, it is a constituent of broccoli, green chili, carrot, and clover blossoms Trifolium pretense L. [2]. Epidemiological evidence has suggested that flavonoids may have an important role in decreasing the possibility of developing chronic diseases associated with a diet deficient in plant-derived foods [3]. Luteolin has various physiological effects. Basu et al [4] demonstrated that luteolin had antiatherogenic properties due to its anti-inflammatory actions. The anti-inflammatory/anti-oxidant properties of luteolin also had anticancer effects [5, 6]. Smooth muscle also responds to luteolin. Luteolin relaxed guinea pig trachea smooth muscle as it inhibited both phosphodiesterase (PDE) and reduced intracellular Ca2+ [7]. Luteolin also caused vasorelaxation in rat thoracic aorta smooth muscle by inhibiting intracellular Ca2+ release, inhibition of sarcolemmal Ca2+ channels, and activation of K+ channels [8]. Luteolin or its glycosides from artichoke extracts may have an ameliorating effect on irritable bowel syndrome [9].
With these potential beneficial attributes, luteolin has been described as a “promiscuous endocrine disruptor” with potent estrogen agonist activity [10], but weak androgen activity [11].
The goal of the current study was to determine the mechanism of action of luteolin on cholecystokinin octapeptide- (CCK-) and KCl-induced tension in male guinea pig gallbladder strips in vitro.
| Materials and Methods | ▴Top |
Materials
The drugs and inhibitors which were purchased from Sigma Aldrich (St. Louis, MO, USA) were CCK, atropine, L-NMMA, and TEA. Those purchased from EMD Millipore (Etobicoke, Ontario, Canada) were PKA-IM, KT5823, genistein, and 2-aminoethoxydiphenylborane (2-APB). Luteolin, fulvestrant, chelerythrine Cl- , and bisindolymaleimide IV were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Charybdotoxin was purchased from Tocris Bioscience (Minneapolis, MN, USA). All agents were dissolved in either distilled water or dimethyl sulfoxide (DMSO). The amount of DMSO or distilled water (10 µL) injected to the chambers had no effect on the strips [12].
Tissue preparation
The experiments were performed under protocol #275 approved (February 3, 2017 and reapproved on January 16, 2018) by the University of Alberta Animal Care Committee-Health Sciences. The committee and I adhere to the Canadian Council on Animal Care (CCAC) guidelines. Male Hartley guinea pigs (225 - 375 g body weight) were killed by decapitation. The gallbladder was excised from the guinea pig, and liver, fat, and connective tissue were removed. The gallbladder was placed in a petri dish filled with Krebs-Henseleit solution (KHS) and gassed with 95% O2 and 5% CO2. The composition of the KHS was (in mM) NaCl, 115; KCl, 5; CaCl2, 2.1; MgSO4, 1.2; NaH2PO4, 1.2; NaHCO3, 25; and glucose, 11. Each gallbladder was dissected into strips roughly 1.5 × 0.5 cm, suspended in Sawyer-Bartlestone chambers using 6-0 surgical silk, and the chambers were filled with KHS, maintained at 37 °C, and had 95% O2 and 5% CO2 bubbled into the chambers. An optimal passive resting tension of 0.7 g was determined previously to work optimally, and used in the study [12-15]. Each gallbladder routinely produced four strips.
Data collection
The force developed by the gallbladder strips was quantified using calibrated Grass FT03 force displacement transducers (Grass Instruments Co., Quincy, MA, USA) and recorded on a Grass 7D polygraph (Grass Instruments Co., Quincy, MA, USA). After being suspended in the chambers, the strips were allowed to recover for 45 min before ascertaining if they were acceptable for further use. To block the effects of nervous action on the strips, each chamber had 2 µM (final concentration) atropine added, in every experiment, 3 min before 1.0 nM CCK or 40 mM KCl. Each strip was treated with 1.0 nM CCK, and the tension was recorded. Three changes of bathing solution were given, and a 20-min recovery period occurred between assessments. The assessment was repeated three times. A repeatable minimum active tension of 0.5 g was required if the strips were to be used in the experiments. All agents were injected directly to the chambers using Hamilton syringes (Reno, NV, USA). All concentrations are reported as the final concentration within the Sawyer-Bartlestone chambers.
Many male guinea pig gallbladder strips exhibited spontaneous activity (n = 6). Luteolin (10 µM) was added to the chambers with no other agent to ascertain if luteolin had an effect on this spontaneous activity.
A pharmacologic approach was used, namely, various inhibitors were used to determine which second messenger or system mediated the luteolin-induced relaxation. CCK (1.0 nM) produced a stable long lasting tension after 3 min. This stable tension lasted at least 10 min [10, 14]. To ascertain if luteolin relaxed CCK-induced tension, a noncumulative concentration response curve was generated. The CCK-induced tension attained a steady level (3 min). One concentration of luteolin was added into the chambers, the response was recorded until the relaxation reached a steady level (approximately 4 min), the KHS was changed three times, and the strips recovered for 30 min before assessing the effects of a different concentration of luteolin. The concentration of luteolin (10 µM) was utilized in ensuing experiments as it produced a reproducible relaxation.
When each inhibitor was used, the procedure followed was similar. CCK (1.0 nM) was added to the chambers. When a stable tension was attained, 10 µM luteolin was injected into the chambers, and the degree of relaxation was recorded. The chambers had the KHS changed three times, and the strips were recovered for 25 min. After the recovery period, the KHS was changed, a blocking agent was added, a period of time passed to allow for the blocking agent to take effect, and 1.0 nM CCK was injected into the chambers. When the tension reached a steady level, 10 µM luteolin was injected into the chambers, and the amount of relaxation was again recorded. The two relaxations, with and without a blocking agent, were compared.
To ascertain if Ca2+ released from the endoplasmic reticulum mediated the luteolin-induced relaxation 2-aminoethoxydiphenylborane (2-APB, 125 µM), an inhibitor of IP3-induced Ca2+ release, was added to the chambers 10 min prior to the CCK [16-19].
The protein kinase A (PKA) inhibitor PKA inhibitor 14-22 amide myristolated (PKA-IM; 180 nM) was added into the chambers 15 min prior to CCK to ensure adequate time for its entry into the smooth muscle. Each of the following agents was used separately in different experiments: KT5823 (585 nM), a protein kinase G (PKG) blocker, genistein (10 µM), a protein tyrosine kinase inhibitor, bisindolymaleimide IV (BIM, 0.5 µM), and chelerythrine Cl- (5 µM) were used together; L-NG-methyl-L-arginine acetate salt (L-NMMA; 20 µM), a nitric oxide synthase inhibitor, fulvestrant (10 µM), a blocker of estrogen receptors, tetraethylammonium chloride (TEA, 100 µM), a non-specific K+ channel blocker, and charybdotoxin (5 nM), a blocker of Ca2+ activated K+ channels. When used, each agent was injected in the chambers 5 min preceding the CCK. BIM and chelerythrine Cl- were used together as BIM blocks the translocation of PKC to its action site and chelerythrine Cl- blocks the catalytic domain of PKC. Using both blockers together produced consistent results [12, 14].
KCl was used to depolarize the smooth muscle cells directly by activating the opening of voltage-gated Ca2+ channels [20]. This would ascertain if luteolin blocked extracellular Ca2+ entry, 40 mM KCl was used to produce tension in the strips. The tension generated by 40 mM KCl was recorded, the KHS was changed three times, and the strips were allowed to equilibrate for 25 min. Luteolin (10 µM) was added to the chambers 3 min prior to the next addition of 40 mM KCl. The tension generated was monitored and compared to that observed when the KCl was added to the chambers with no luteolin. The same procedure was followed using 1.0 nM CCK instead of the KCl.
Statistical analysis
All experiments were performed on at least three separate occasions, and the results were pooled where appropriate. Statistical comparisons were performed using either the paired t-test or Mann-Whitney rank sum test. Results are expressed as mean ± SE. The number of animals used in each block of experiments is “n”. Each gallbladder usually produced four strips; therefore, an “n” of 4 means up to 16 strips were used.
| Results | ▴Top |
Concentration-dependent relaxation
Luteolin (10 µM) was added to the chambers with no other agent to determine its effect on the spontaneous activity observed in some of the gallbladder strips. It was observed that luteolin decreased the amount of spontaneous activity in the gallbladder strips by 81.2±12.9% (n = 6).
CCK was used to stimulate tension and luteolin was injected into the chambers to ascertain if luteolin induced a relaxation of the CCK-induced tension (Fig. 1a). Luteolin produced a concentration-dependent relaxation of CCK-induced tension (Fig. 1b).
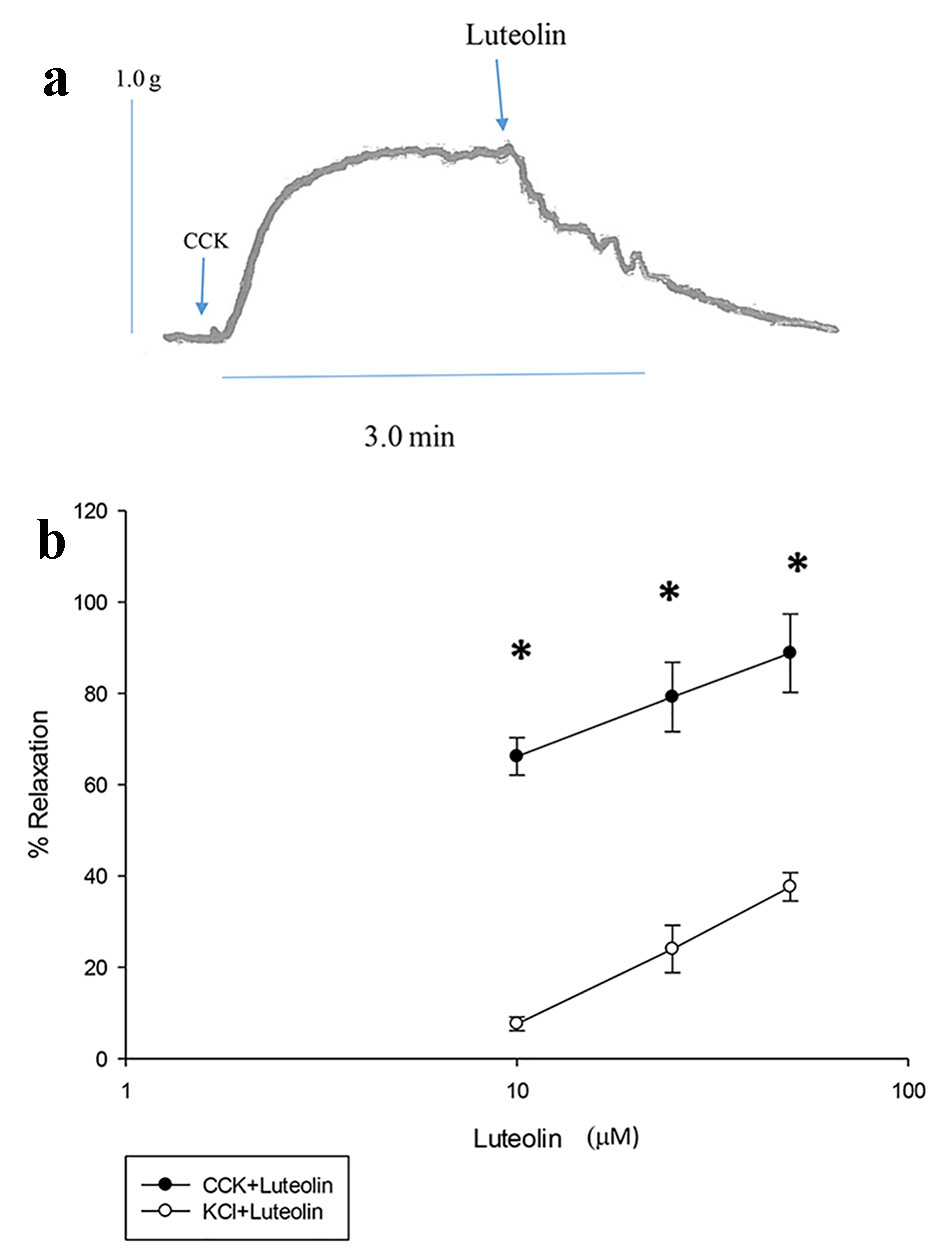 Click for large image | Figure 1. Effect of luteolin on CCK-induced tension (a). A data trace showing the relaxation caused by luteolin on CCK-induced tension in a male guinea pig gallbladder strip. Arrow indicates when CCK was added to the chamber (b). Luteolin was used to relax the CCK- or KCl-induced tension. The responses are concentration-dependent. Values are means ± SE. |
Luteolin was injected into the chambers 3 min before KCl (40 mM). A significant decrease (P < 0.001) in the tension generated (1.1 ± 0.09 g vs. 0.97 ± 0.08 g, n = 5; Fig. 2) was recorded. When 10 µM luteolin was injected into the chambers 3 min before CCK (1.0 nM), a significant (P < 0.001) diminution in the amount of tension generated (1.02 ± 0.08 g vs. 0.79 ± 0.1 g, n = 4; Fig. 2) was recorded. Luteolin had a significantly (P < 0.01) greater effect on the CCK-induced tension (28.7±5.5%), then on the KCl-induced tension (12.5±1.1%).
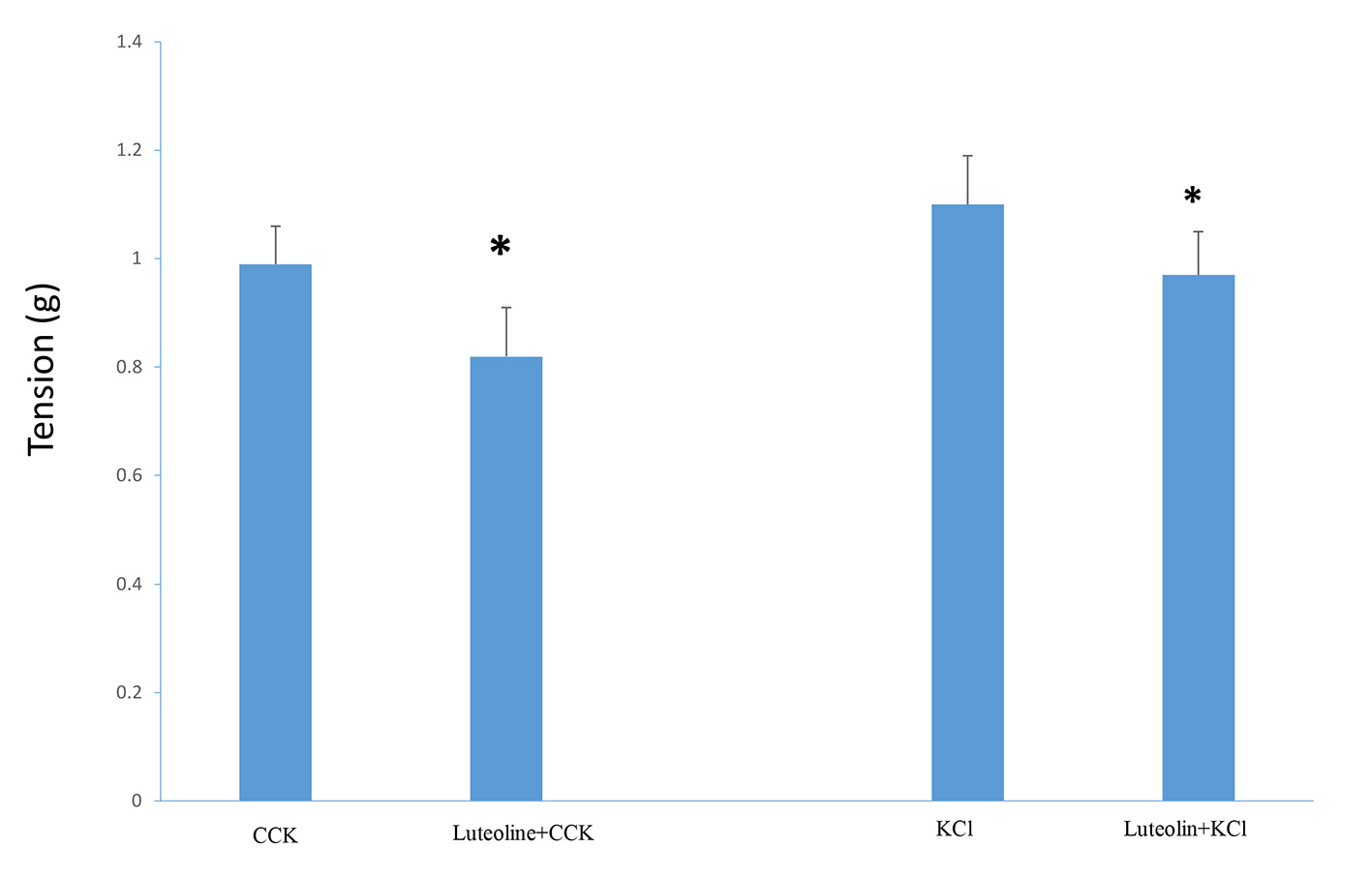 Click for large image | Figure 2. The effect of adding luteolin (10 µM) prior to CCK or KCl. The luteolin significantly (P < 0.001) decreased the amount of CCK-induced tension. A similar result (P < 0.001) was observed when luteolin was added to the chambers prior to KCl. Values are means ± SE. |
Effect of blocking agents
When TEA (100 µM) was injected into the chambers before CCK, no significant change in the tension (1.08 ± 0.1 g vs. 1.0 ± 0.09 g; n = 7) was observed. TEA had no significant effect on the percentage of luteolin-induced relaxation (37.4±3.4% vs. 38.8±3.9%; n = 7; Fig. 3).
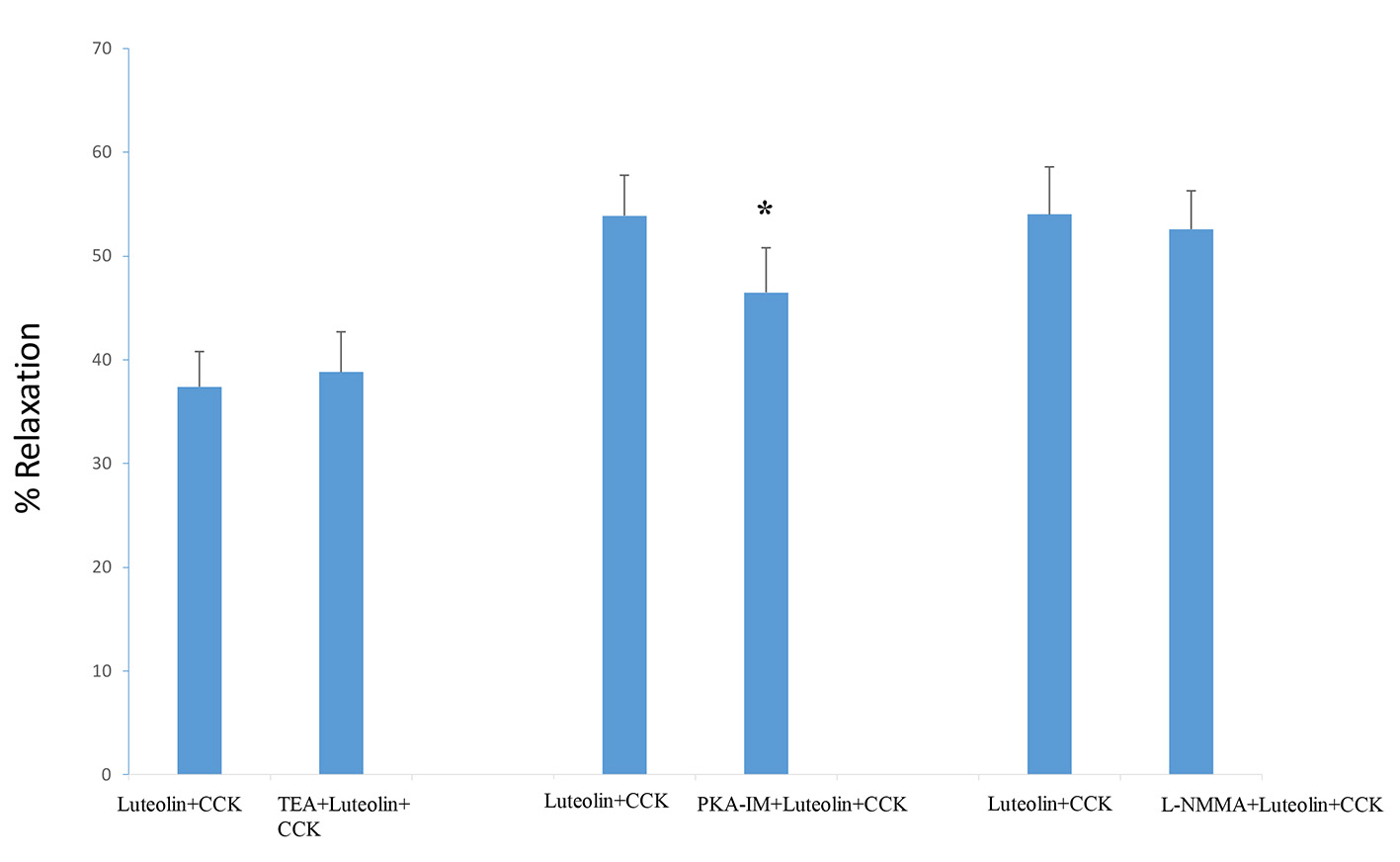 Click for large image | Figure 3. TEA (100 µM), a non-specific blocker of K+ channels, had no significant effect on luteolin-induced relaxation. The PKA blocker, PKA-IM (180 nM), significantly (P < 0.01) decreased the amount of luteolin-induced relaxation. The nitric oxide synthase blocker L-NAME (20 µM) had no significant effect on the amount of luteolin-induced relaxation. Values are means ± SE. |
PKA-IM produced a significant (P < 0.01) increase in the CCK-induced tension (0.83 ± 0.08 g vs. 0.94 ± 0.08 g, n = 5). A significant (P < 0.01) diminution in the luteolin-induced relaxation (53.9±3.9% vs. 46.5±4.3%, n = 5; Fig. 3) was also recorded.
L-NMMA, a nitric oxide (NO) synthase blocker, had no significant effect on the CCK-induced tension (0.78 ± 0.04 g vs. 0.79 ± 0.04 g, n = 5) nor on the luteolin-induced relaxation (54.0±4.6% vs. 52.6±3.7%, n = 5; Fig. 3) in the CCK-treated gallbladder strips.
KT5823, a PKG blocker, had no significant effect on the amount of CCK-induced tension (0.78 ± 0.05 g vs. 0.80 ± 0.6 g, n = 4), nor on the luteolin-induced relaxation of CCK-induced relaxation (51.5 ± 4.5 vs. 50.1±3.2%, n = 4; Fig. 4). Genistein had no significant effect on the amount of luteolin-induced relaxation of CCK-induced tension (47.7±3.7% vs. 59.6±4.4%, n = 4; Fig. 4). No significant change in the CCK-induced tension (0.73 ± 0.04 g vs. 0.71 ± 0.05 g) was recorded in the presence or absence of genistein.
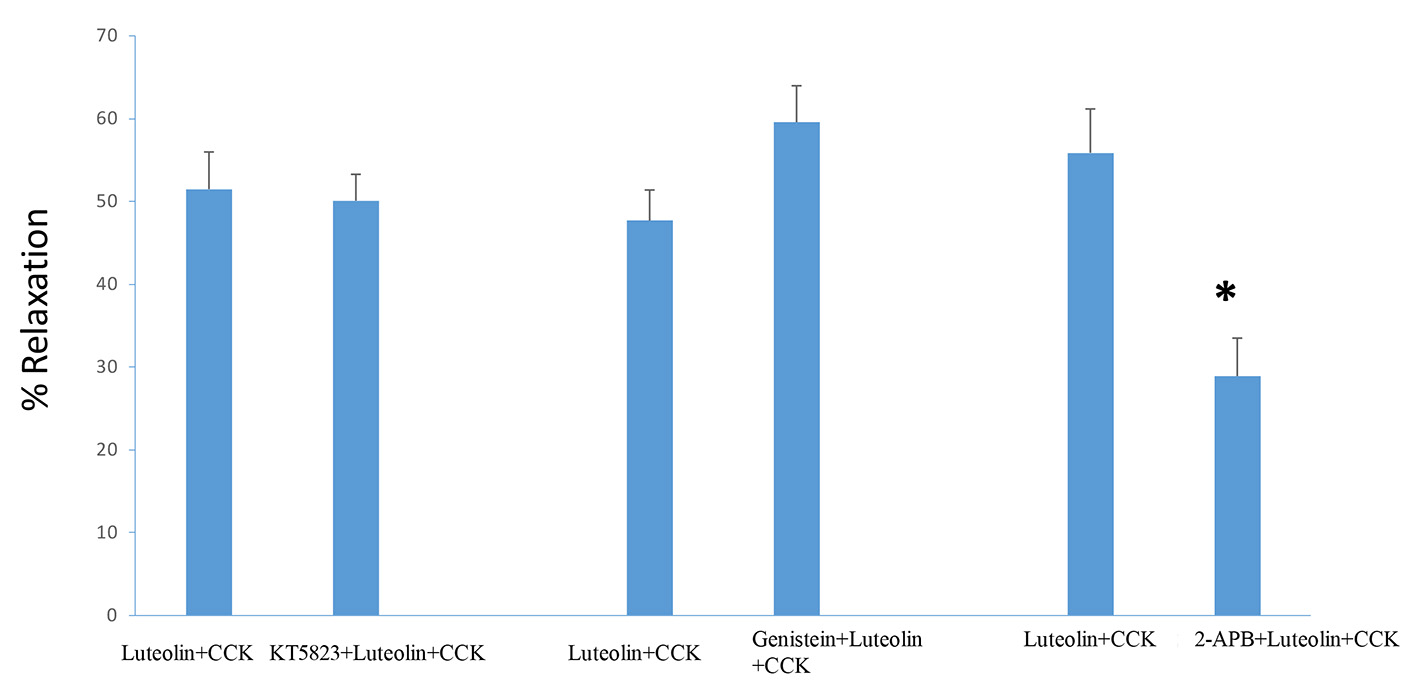 Click for large image | Figure 4. The effect of the PKG blocker KT5823 (585 nM) had no significant effect on the amount of luteolin-induced relaxation. Genistein (10 µM), a tyrosine kinase inhibitor, had no significant effect on the amount of luteolin-induced relaxation. The use of 2-APB (125 µM) significantly (P < 0.001) decreased the amount of luteolin-induced relaxation. Values are means ± SE. |
The 2-APB significantly decreased the amount of CCK-induced tension (0.96 ± 0.14 g vs. 0.46 ± 0.08 g, P < 0.001, n = 4), as well as on the luteolin-induced relaxation (55.9 ± 5.3% vs. 28.9 ± 4.6%, n = 4; P < 0.001; Fig. 4).
PKC inhibitors BIM and chelerythrine Cl- had no significant effect on the CCK-induced tension when the tensions generated with or without the PKC blockers were compared (0.78 ± 0.06 g vs. 0.84 ± 0.04 g; n = 4). The combination of PKC blockers had no effect on the luteolin-induced relaxation when compared to those not treated with the PKC inhibitors (45.0 ± 4.7% vs. 42.7 ± 4.5%; n = 4; Fig. 5).
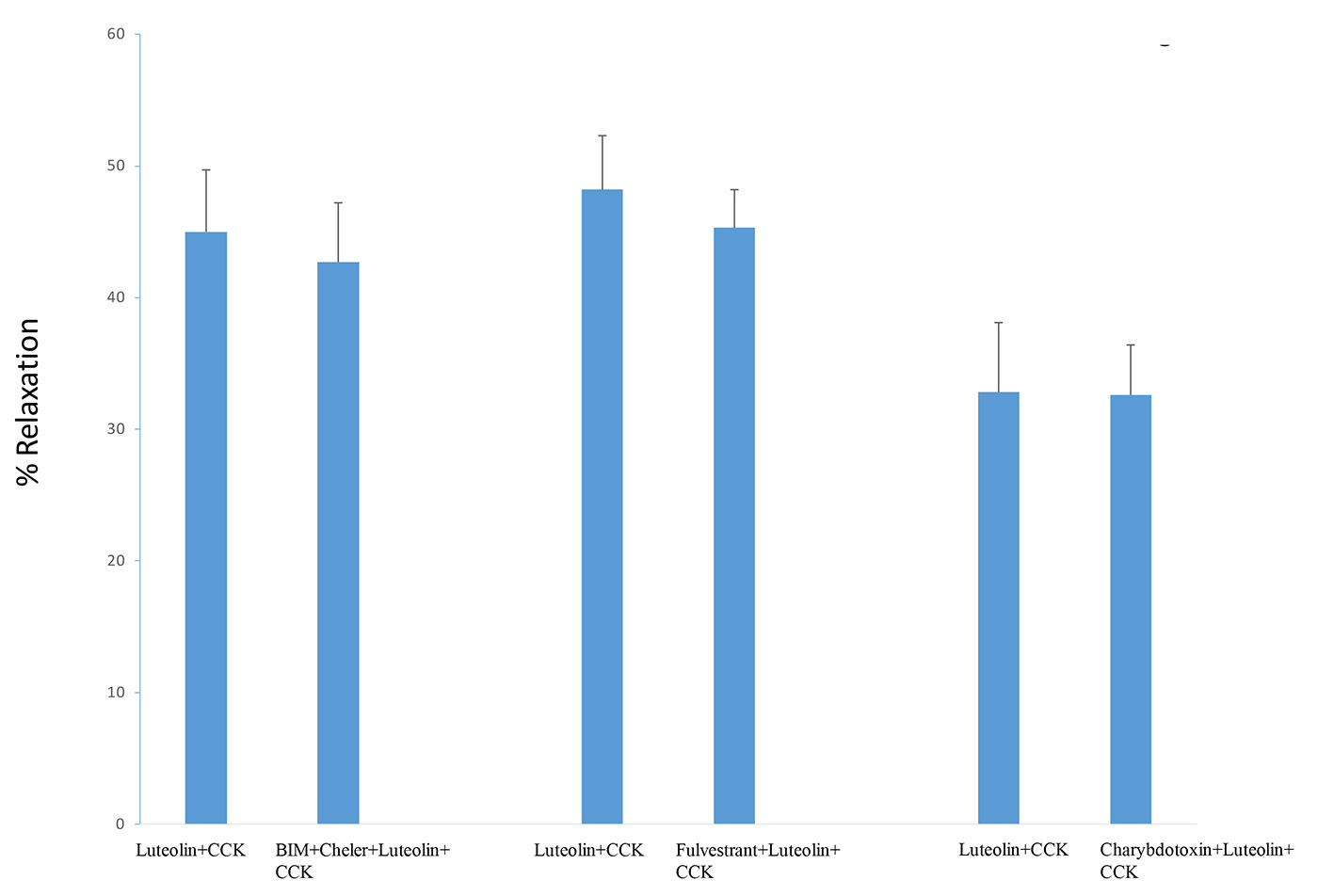 Click for large image | Figure 5. The use of the PKC blockers BIM (0.5 µM) and chelerythrine Cl- (5.0 µM), fulvestrant (10 µM), or charybdotoxin (5 nM) had no significant effect on the amount of luteolin-induced relaxation. Values are means ± SE. |
Charybdotoxin (5 nM, n = 3) had no significant effect on the luteolin-induced relaxation (Fig. 5) nor on the CCK-induced tension. Fulvestrant had no significant effect on the CCK-induced tension (0.88 ± 0.08 g vs. 0.92 ± 0.1 g; n = 4), nor a significant effect on the luteolin-induced relaxation (48.2 ± 4.1% vs. 45.3 ± 2.9%; n = 4; Fig. 5).
| Discussion | ▴Top |
Flavonoids such as luteolin are antioxidants and may therefore aid in preventing cancer [3, 5]. Luteolin is found in many edible tropical plants as well as vegetables such as celery and carrots [1, 2]. Flavonoids are also anti-inflammatory agents. Luteolin has been shown to have antiatherogenic properties which could reduce the incidence of cardiovascular diseases [4]. The principal mechanism of the anti-inflammatory properties of plant flavonoids is the inhibition of enzymes which generate eicosanoids such as phospholipase A2, cyclooxygenases, and lipoxygenases [6, 21].
Luteolin has been described as a promiscuous endocrine disruptor. It displays potent progesterone antagonist and estrogen agonist activities [10]. These activities of luteolin are significant at low micromolar levels, levels achievable by supplementation in vivo [22, 23]. Both 17β-estradiol (E2) and progesterone (P) had an inhibitory effect upon gallbladder contractility in men and premenopausal and postmenopausal women [24, 25]. Both E2 and P relaxed CCK-induced tension in male and female guinea pig gallbladder strips. The inhibition of extracellular Ca2+ entry mediated both P-induced and E2-induced relaxation of CCK- and KCl-induced tension in male and female guinea pig gallbladder strips [15, 26-28].
Bolton [20] used KCl to directly depolarize vascular smooth muscle cells by opening voltage-gated Ca2+ channels. Nifedipine could not be used to block extracellular Ca2+ entry as it abolishes contractile activity and resting gallbladder tone in both guinea pig and human gallbladders [29]. Extracellular Ca2+ entry promotes intracellular Ca2+ release. This sustains the long lasting contraction of gallbladder smooth muscle [30, 31]. Thus, the significant decrease in tension generated by KCl when luteolin was added to the chambers prior to the KCl demonstrates that luteolin, in part, blocks extracellular Ca2+ entry.
Luteolin was shown to produce vasorelaxation of the smooth muscle of the thoracic aorta of the rat via Ca2+ and K+ channels [8]. In the guinea pig gallbladder when luteolin was injected into the chambers before either CCK or KCl, luteolin significantly decreased the amount of tension. Thus, the luteolin-induced relaxation is mediated, in part, by blocking extracellular Ca2+ entry. Luteolin blocks extracellular Ca2+ entry as has been shown using quercetin or curcumin [23, 24]. In addition, the use of 2-APB demonstrated that luteolin inhibited intracellular Ca2+ release. Neither charybdotoxin nor TEA had an effect on the luteolin-induced relaxation; therefore, K+ channels were not involved in mediating the effect of luteolin in the guinea pig gallbladder which differs from the findings of Jiang et al [8] in the rat thoracic artery smooth muscle. In addition, the use of 2-APB demonstrated that luteolin inhibited intracellular Ca2+ release. Ko et al [7] also showed that luteolin relaxed tracheal smooth muscle in the guinea pig via PDE activity and reducing intracellular Ca2+ release. PKA-IM significantly decreased the luteolin-induced relaxation suggesting that cAMP-PDE also mediated the luteolin-induced relaxation. Luteolin was shown to relax both noradrenaline- and KCl-induced tension in rat aortic rings. The relaxation induced by luteolin was shown to be mediated by NO [32]. In the guinea pig gallbladder L-NMMA had no significant effect on the luteolin-induced relaxation; therefore, NO had no role in mediating the luteolin-induced relaxation. However, luteolin relaxed KCl-induced tension in the gallbladder strips much as described by Uydes-Dogan et al [32] in rat aortic rings.
Luteolin relaxes CCK- or KCl-induced tension by blocking extracellular Ca2+ entry and intracellular Ca2+ release. In addition, the actions of PKA are also involved in mediating the luteolin effect. Luteolin exerted its effects utilizing similar systems as E2 and P [15, 26]. The concentrations at which luteolin may exert its effects are achievable by supplementation [22, 23]. Keane et al [25] demonstrated that P and E2 had an inhibitory effect on gallbladder motility which could lead to increased incidence of gallstones. Since luteolin has potent estrogen agonist activity, luteolin could affect gallbladder contractility which could lead to gallstones.
Financial Disclosure
The author has no financial disclosure to be made.
Grant Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This research is dedicated to the memory of my longtime friend and collaborator Dr. Edward Karpinski.
| References | ▴Top |
- Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009;9(1):31-59.
doi pubmed - Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49(6):3106-3112.
doi - Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke. 2004;35(8):1999-2010.
doi pubmed - Basu A, Das AS, Majumder M, Mukhopadhyay R. Antiatherogenic roles of dietary flavonoids chrysin, quercetin, and luteolin. J Cardiovasc Pharmacol. 2016;68(1):89-96.
doi pubmed - Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8(7):797-807.
doi pubmed - Ziyan L, Yongmei Z, Nan Z, Ning T, Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007;73(3):221-226.
doi pubmed - Ko WC, Shih CM, Leu IJ, Chen TT, Chang JP. Mechanisms of relaxant action of luteolin in isolated guinea pig trachea. Planta Med. 2005;71(5):406-411.
doi pubmed - Jiang H, Xia Q, Wang X, Song J, Bruce IC. Luteolin induces vasorelaxation in rat thoracic aorta via calcium and potassium channels. Pharmazie. 2005;60(6):444-447.
doi - Verspohl EJ, Ploch M, Windeck T, Klaes M, Schmidt T, Bauer K. Effect of two artichoke extracts (36_U and 36_EB) on rat ileum (with respect to bowel syndrome) and the peristaltic threshold. Phytomedicine. 2008;15(11):1002-1009.
doi pubmed - Nordeen SK, Bona BJ, Jones DN, Lambert JR, Jackson TA. Endocrine disrupting activities of the flavonoid nutraceuticals luteolin and quercetin. Horm Cancer. 2013;4(5):293-300.
doi pubmed - Lin FM, Chen LR, Lin EH, Ke FC, Chen HY, Tsai MJ, Hsiao PW. Compounds from Wedelia chinensis synergistically suppress androgen activity and growth in prostate cancer cells. Carcinogenesis. 2007;28(12):2521-2529.
doi pubmed - Kline LW, Kaneko T, Benishin CG, Pang PK. Calcitonin gene-related peptide: an inhibitor of guinea pig gallbladder contraction. Can J Physiol Pharmacol. 1991;69(8):1149-1154.
doi pubmed - Kline LW, Pang PK. Calcitonin gene related peptide relaxes cholecystokinin-induced contraction in guinea pig gallbladder strips in vitro. Can J Physiol Pharmacol. 1992;70(12):1571-1575.
doi - Kline LW, Karpinski E. Testosterone and dihydrotestosterone inhibit gallbladder motility through multiple signalling pathways. Steroids. 2008;73(11):1174-1180.
doi pubmed - Kline L, Karpinski E. A comparison of the effects of various sex steroids on cholecystokinin- and KCl-induced tension in female guinea pig gallbladder strips. Gen Comp Endocrinol. 2013;185:37-43.
doi pubmed - Kline LW, Karpinski E. Progesterone inhibits gallbladder motility through multiple signaling pathways. Steroids. 2005;70(9):673-679.
doi pubmed - Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16(10):1145-1150.
doi pubmed - Selli C, Tosun M. Effects of cyclopiazonic acid and dexamethasone on serotonin-induced calcium responses in vascular smooth muscle cells. J Physiol Biochem. 2016;72(2):245-253.
doi pubmed - Zhou H, Iwasaki H, Nakamura T, Nakamura K, Maruyama T, Hamano S, Ozaki S, et al. 2-Aminoethyl diphenylborinate analogues: selective inhibition for store-operated Ca2+ entry. Biochem Biophys Res Commun. 2007;352(2):277-282.
doi pubmed - Bolton TB. Calcium metabolism in vascular smooth muscle. Br Med Bull. 1986;42(4):421-429.
doi pubmed - Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96(3):229-245.
doi pubmed - Zhou P, Li LP, Luo SQ, Jiang HD, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem. 2008;56(1):296-300.
doi pubmed - Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, et al. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438(3):220-224.
doi - Kern F, Jr., Everson GT, DeMark B, McKinley C, Showalter R, Erfling W, Braverman DZ, et al. Biliary lipids, bile acids, and gallbladder function in the human female. Effects of pregnancy and the ovulatory cycle. J Clin Invest. 1981;68(5):1229-1242.
doi pubmed - Keane P, Colwell D, Baer HP, Clanachan AS, Scott GW. Effects of age, gender and female sex hormones upon contractility of the human gallbladder in vitro. Surg Gynecol Obstet. 1986;163(6):555-560.
pubmed - Kline L, Karpinski E. Gallbladder motility and the sex of the guinea pig. Physiol Rep. 2016;4(12):e12843.
doi pubmed - Kline LW, Karpinski E. Curcumin Relaxes Precontracted Guinea Pig Gallbladder Strips via Multiple Signaling Pathways. Gastroenterology Res. 2015;8(5):253-259.
doi pubmed - Kline L, Karpinski E. Quercetin relaxes guinea pig gallbladder strips. Nutr Res. 2016;36(10):1098-1104.
doi pubmed - Clas D, Hould FS, Rosenthall L, Arzoumanian A, Fried GM. Nifedipine inhibits cholecystokinin-induced gallbladder contraction. J Surg Res. 1989;46(5):479-483.
doi - Morales S, Camello PJ, Alcon S, Salido GM, Mawe G, Pozo MJ. Coactivation of capacitative calcium entry and L-type calcium channels in guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G1090-1100.
doi pubmed - Yu P, Chen Q, Xiao Z, Harnett K, Biancani P, Behar J. Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am J Physiol. 1998;275(2 Pt 1):G203-211.
pubmed - Uydes-Dogan BS, Takir S, Ozdemir O, Kolak U, Topcu G, Ulubelen A. The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. Vascul Pharmacol. 2005;43(4):220-226.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


