| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Case Report
Volume 11, Number 3, June 2018, pages 247-251
Cytoreductive Surgery and Normothermic Intraperitoneal Chemotherapy for Signet Ring Cell Appendiceal Adenocarcinoma With Peritoneal Metastases in the Setting of Cirrhosis
Bharat Panugantia, Ea-sle Changb, Cyril W. Helmc, Theresa Schwartzd, Eddy C. Hsuehd, Jinhua Piaoe, Jinping Laif, Jula Veerapongg, h
aDepartment of Surgery, University of California-San Diego, La Jolla, CA, USA
bDepartment of General Surgery, Saint Louis University, Saint Louis, MO, USA
cRoyal Cornwall Hospitals NHS Trust, Treliske, Truro, Cornwall, TR1 3LJ, United Kingdom
dDivision of Surgical Oncology, Department of General Surgery, Saint Louis University, Saint Louis, MO, USA
eDepartment of Pathology, Saint Louis University, Saint Louis, MO, USA
fDepartment of Pathology, Immunology, and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL, USA
gDivision of Surgical Oncology, Department of General Surgery, University of California-San Diego, La Jolla, CA, USA
hCorresponding Author: Jula Veerapong, Division of Surgical Oncology, Department of General Surgery, University of California - San Diego, UC San Diego Moores Cancer Center, 3855 Health Sciences Drive, #0987, La Jolla, CA 92093, USA
Manuscript submitted April 20, 2018, accepted May 15, 2018
Short title: CRS/IPC for Peritoneal Malignancy in Cirrhotics
doi: https://doi.org/10.14740/gr1029w
| Abstract | ▴Top |
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are combined to treat peritoneal surface malignancies (PSM). The objective of cytoreduction is to eradicate macroscopic disease, while HIPEC addresses residual microscopic disease. Currently, there are no protocols guiding treatment of cirrhotic patients with PSM. We report the case of a cirrhotic patient with signet ring cell (SRC) appendiceal adenocarcinoma who underwent normothermic, as opposed to hyperthermic intraperitoneal chemotherapy (IPC). A 50-year-old woman with compensated class A cirrhosis and chronic hepatitis B and C underwent a right hemicolectomy in 2007 and adjuvant chemotherapy in 2008 for appendiceal SRC adenocarcinoma. In 2011, she was found to have peritoneal disease after a laparotomy. She subsequently experienced intolerance to chemotherapy, with stable disease on serial restaging. In light of her cirrhosis, the decision was made to perform CRS and IPC without hyperthermia to treat her residual disease. In 2012, she underwent CRS (omentectomy, total abdominal hysterectomy, left salpingo-oophorectomy) and IPC with mitomycin C. Thirty-day postoperative morbidity included delayed abdominal closure (Clavien-Dindo Grade IIIb), prolonged ventilator support (IIIa), vasopressor requirements (II), and confusion (II). The patient’s liver function remained stable. Eight months later, she had evidence of recurrence on computed tomography. Twenty-two months later, she developed an extrinsic compression secondary to evolving disease, requiring a palliative endoscopic stent. The patient expired from her disease 29 months after her CRS and IPC. The criteria guiding selection of suitable candidates for CRS continues to evolve. Concomitant compensated cirrhosis in patients with PSM should not constitute a reason independently to exclude CRS with intraperitoneal chemotherapy, given the oncologic benefits of the procedure.
Keywords: Intraperitoneal chemotherapy; Peritoneal surface malignancy; Cirrhosis; Cytoreductive surgery
| Introduction | ▴Top |
Abdominal and pelvic peritoneal surface malignancies can be a challenging clinical entity to treat. A combined approach including cytoreductive surgery (CRS), meant to eliminate macroscopic disease, and hyperthermic intraperitoneal chemotherapy (HIPEC), has been employed with documented success. Signet ring cell (SRC) appendiceal carcinoma represents a rare, non-carcinoid tumor with a dismal 5-year survival when it is accompanied by distant peritoneal spread. Given the extensive nature of the CRS/HIPEC procedure, appropriate patient selection weighing oncologic prognosis against expected perioperative morbidity of the intervention can be a challenging clinical mandate. While the significance of liver disease as a predictor of perioperative complications in intra-abdominal surgery has been defined, there is a paucity of literature characterizing cirrhosis as a risk factor for adverse events in the prospective CRS/HIPEC population. Herein, we describe the case of a 50-year-old woman with SRC and class A compensated cirrhosis who underwent CRS with normothermic intraperitoneal chemotherapy, in whom hyperthermia was deferred given the uncertain potential effects on liver function.
| Case Report | ▴Top |
We report a case of a 50-year-old Caucasian woman with Child-Turcotte-Pugh (CTP) class A compensated cirrhosis with the Model of End-Stage Liver Disease (MELD) Score of 9 who underwent CRS with normothermic intraperitoneal chemotherapy for recurrent SRC adenocarcinoma of the appendix, metastatic to the peritoneum. She initially presented to the emergency department in October 2007 with abdominal pain and obstructive symptoms. Comorbidities included chronic hepatitis B and C, chronic pancreatitis, and chronic obstructive pulmonary disease. After failure of initial conservative management, she was taken to the operating room for an exploratory laparotomy. Surgical exploration revealed a large cecal mass suspicious for malignancy. A right hemicolectomy was performed. Initial pathology demonstrated stage IIIc (T4N2Mx) SRC adenocarcinoma of the appendix with extension to the terminal ileum. She completed adjuvant chemotherapy of FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) in May 2008. Notably, at this time, she had undergone a liver biopsy for chronic hepatitis B and C, which demonstrated stage 2 periportal fibrosis.
Surveillance positron emission tomography (PET) scan in March 2011 demonstrated a 5 × 8 cm left cystic adnexal mass, mildly fluorodeoxyglucose (FDG) avid with no evidence of other FDG activity. In addition, her carcinoembryonic antigen (CEA) level had risen from a baseline of 3.5 ng/mL to 15.2 ng/mL. She was taken to the operating room for exploration and was found to have extensive carcinomatosis involving the peritoneum, omentum, and pelvis. Peritoneal and omental biopsies were consistent with mucinous adenocarcinoma with signet ring cells (Fig. 1). She then received systemic chemotherapy, consisting of FOLFOX and cetuximab for 12 cycles, followed by FOLFOX and bevacizumab for five cycles.
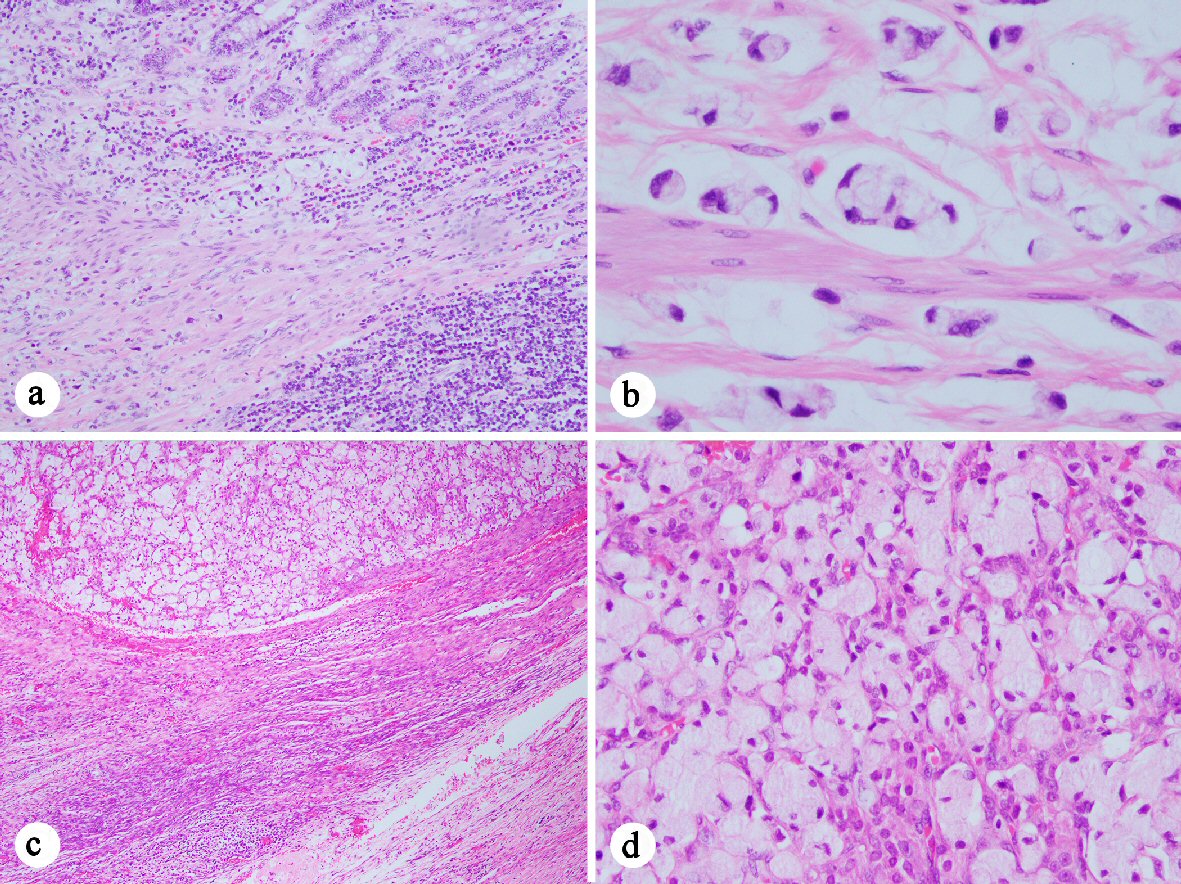 Click for large image | Figure 1. Histology of the signet ring cell adenocarcinoma ((a) H&E stain, 100 ×; (c) 40 ×; (b) and (d) 400 ×). (a-b) Signet ring cell carcinoma cells present at in the muscularis mucosae (a) and invading the muscularis propria (b) of the appendix. (c-d) Signet ring cell carcinoma present in the ovary. |
The patient’s progressive intolerance of her chemotherapy, including weight loss, failure to thrive, and thrombocytopenia along with stable disease on numerous restaging images prompted the consideration of CRS and HIPEC as a treatment modality. Preoperative liver biopsy in February 2012 confirmed stage 4 cirrhosis (MELD = 9, CTP = 6, platelets = 70,000 /µL, ALT = 40 IU/L, AST = 39 IU/L, total bilirubin = 0.3 mg/dL, albumin = 3.1 g/dL, INR = 1.4). Careful consideration was taken in regards to the possibility of precipitating liver failure with the inflow temperature of 42 °C for the hyperthermia required by the HIPEC procedure. The decision was made to proceed with CRS and normothermic intraperitoneal chemotherapy (CRS/IPC). Findings in the operating room were consistent with macronodular cirrhosis (Fig. 2). The cytoreduction included extensive adhesiolysis, omentectomy, excision of retroperitoneal lymph nodes, total abdominal hysterectomy, left salpingo-oophorectomy (Fig. 3) with subsequent administration of 30 mg of intraperitoneal mitomycin C at an inflow temperature of 38 °C for 90 min. Her peritoneal carcinomatosis index (PCI) score was 4 out of 39 and her completeness of cytoreduction score was CC-0 (i.e. no gross residual disease). Her abdominal wall was unable to be closed at the time of initial cytoreduction due to elevated peak ventilatory airway pressures, marked edema of the abdominal viscera, and loss of domain from a pre-existing ventral hernia defect. A negative pressure wound therapy device was placed and she was recovered in the intensive care unit. She returned to the operating room two additional times for placement of a feeding jejunostomy tube, partial abdominal wall closure with bioprosthetic mesh, and negative pressure wound therapy, followed by definitive closure 1 week after her initial surgery. She was subsequently discharged to a skilled nursing facility after a prolonged hospital stay of 30 days, mainly for deconditioning and mental status issues. Of note, the patient did not demonstrate significant changes in her liver function tests (ALT = 50 IU/L, AST = 54 IU/L, total bilirubin = 0.3 mg/dL, albumin = 2.9 g/dL). Although the patient’s INR was not available from the day of her discharge, INR measured 3 weeks after her discharge from the hospital was also stable (1.5).
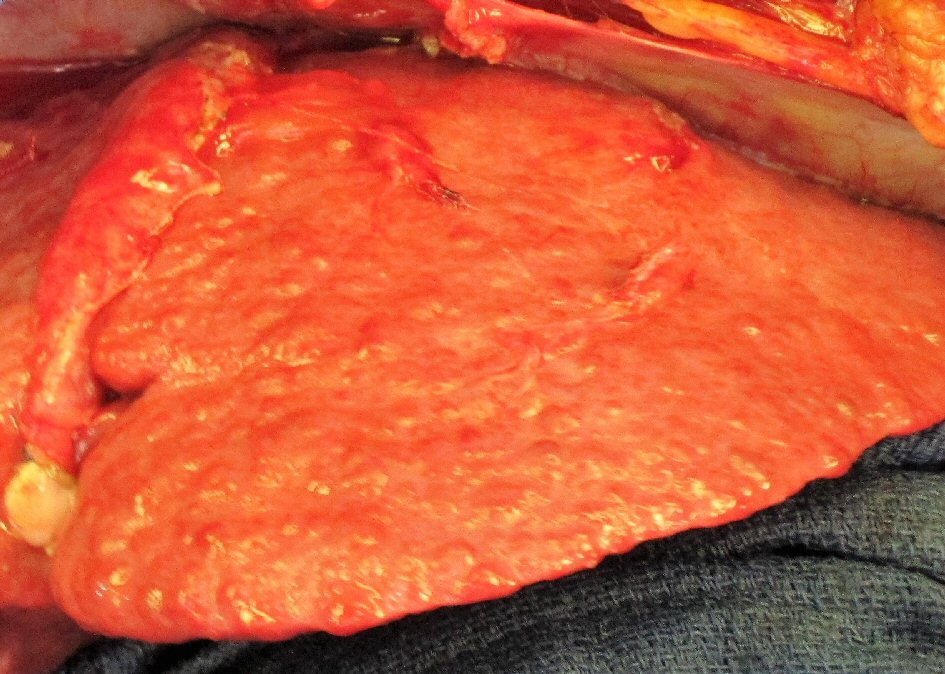 Click for large image | Figure 2. Characteristic surface nodularity of cirrhotic liver observed during cytoreductive surgery. |
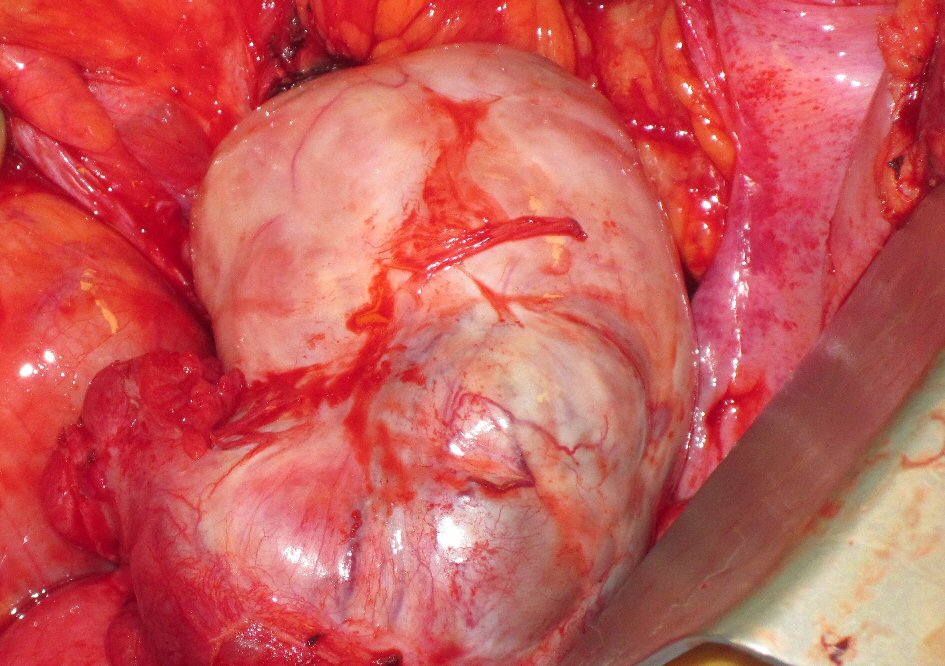 Click for large image | Figure 3. Adenocarcinoma involving pelvic viscera. Intraoperative picture of enlarged left ovary involved by primary appendiceal signet ring cell adenocarcinoma, revealed during exploration of pelvis for cytoreductive surgery. |
Restaging imaging demonstrated evidence of peritoneal recurrence 8 months post-operatively. She underwent treatment with capecitabine, oxaliplatin, and cetuximab for six cycles and was subsequently transitioned to single agent cetuximab. Twenty-two months after her CRS/IPC procedure, she developed a malignant bowel obstruction at her rectosigmoid junction, which required the placement of a palliative endoscopic stent. She stopped systemic therapy and opted for expectanct management. She expired 29 months after CRS/IPC.
| Discussion | ▴Top |
CRS and HIPEC comprise a treatment modality for wide variety of peritoneal surface malignancies. HIPEC is used to treat gastrointestinal malignancies including appendiceal neoplasms and colon cancers, gynecologic malignancies such as epithelial ovarian cancer, primary peritoneal carcinomas, and diffuse malignant peritoneal mesotheliomas. The objective of CRS is to address all gross macroscopic disease, while the goal of intraoperative HIPEC is to eradicate residual microscopic disease, invisible to the naked eye and serving as microscopic “floaters” that can potentially re-implant in the abdominal cavity. Peritoneal surface malignancies are commonly the result of exfoliation, widespread dissemination, and subsequent invasion by malignant cells from the abdominal or pelvic viscera (e.g. stomach, colon, appendix, and ovaries).
Peritoneal carcinomatosis generally carries a poor prognosis. SRC appendiceal adenocarcinoma is a rare, non-carcinoid tumor that represents only 4% of all appendiceal neoplasms [1]. It is associated with only a 7% 5-year survival when it is accompanied by distant peritoneal spread [2], which has proven to be a common complication. In fact, McGory et al [2] reported that in their cohort of 113 cases, 60% of patients with appendiceal SRC carcinoma were discovered associated with distant disease, while McCusker et al similarly posited that primary appendiceal SRC carcinoma present with distant spread in 76% of cases [3].
CRS and HIPEC are considered by many to be the treatment of choice for peritoneal carcinomatosis [4]. Reporting on a cohort of patients with appendiceal SRC carcinoma, Chua et al describe a median overall survival (OS) of 27 months in patients treated with CRS/HIPEC compared to a median OS of 15 months in patients treated with systemic chemotherapy [5]. Peritoneal carcinomatosis is associated with a considerable likelihood of chemoresistance as the peritoneum is hypovascular with limited systemic perfusion. The intraperitoneal route allows the delivery of high concentrations of chemotherapy (e.g. mitomycin C or a platinum-based drug) directly to the peritoneal surface while the plasma-peritoneum barrier prevents the significant side effects commonly associated with systemic chemotherapy [6]. Although survival benefits of CRS/HIPEC have become apparent since Dr. Paul Sugarbaker has developed the procedure, definitive guidelines to advise optimal patient selection for CRS/HIPEC have yet to be established. A consensus statement from 2006 does exist, addressing patient variables such as performance status in cases of peritoneal carcinomatosis of colorectal origin [7]. No literature currently exists to advise the management of peritoneal carcinomatosis in patients with concomitant cirrhosis.
Based on our experience, CRS and intraperitoneal chemotherapy (CRS/IPC) may be a reasonable treatment modality with manageable risk in patients with peritoneal surface malignancies and compensated cirrhosis. Clinician reticence before performing the procedure in patients with varying degrees of liver disease may stem from a series of risk factors directly related to impaired liver function, including coagulopathy (e.g. thrombocytopenia, clotting factor deficiencies), hypoalbuminemia (associated with poorer postoperative outcomes), and hyponatremia. Cirrhotic livers are affected by baseline hypoperfusion secondary to portal hypertension and impaired arterial blood flow due to depressed autoregulation [8]. A hemorrhagic or hypotensive perioperative event may have a more pronounced effect in these patients, particularly considering the depressive effect that general inhaled anesthetics have on hepatic blood flow, though agents such as isoflurane and propofol may have a less prominent such effect [8]. In spite of the aforementioned risks, Befeler et al posited that a MELD score of less than 14 effectively predicts a low probability (9%) of adverse postoperative outcomes, including death, the need for a liver transplant within 90 days of the procedures, and hospital stay of greater than 21 days, following intra-abdominal surgeries [9]. As such, concomitant cirrhosis in patients with peritoneal surface malignancies should not elicit reflexive exclusion from CRS/IPC. This point is particularly applicable to patients whose overall survival is more likely to be limited by their tumor biology than their liver disease, as is the case with SRC appendiceal adenocarcinoma. Although the MELD score was originally developed to predict survival after transjugular intrahepatic portosystemic shunt placement, it was eventually shown to be a viable predictor of 3-month mortality in cirrhotic patients. For example, a MELD of 9 is associated with a 3 month mortality of less than 1.9% and generally reflects a low severity of liver disease [10]. Said et al indicated that MELD serves as an effective prognosticator of 1-year mortality, although the score’s predictive ability decreases significantly for long-term mortality (5 years) [11]. Clinicians are advised to consider factors such as hepatic encephalopathy and continued alcohol use in cases of alcoholic cirrhosis when attempting to approximate the long-term prognosis of their cirrhotic patients.
The rationale underlying hyperthermia in intraperitoneal chemotherapy is to augment cytotoxicity and curtail the effects of residual disease. However, the effects of hyperthermia in patients with comorbid liver disease have not been fully investigated. The treatment team in this case opted for normothermic IPC to avoid hepatic failure in a patient with limited functional reserve. It is possible that the patient would have avoided hepatic decompensation even if hyperthermia was employed. However, Bouchereau et al described two cases of hepatic necrosis following HIPEC but hypothesize the mechanism to be related to the entrapment of oxaliplatin in liver recesses created by electrofulguration of hepatic nodules [12]. Reports of fulminant hepatic failure following heat stroke (body temperature > 40.6 °C) in patients with normal hepatic reserve were documented [13]. One proposed mechanism of hepatic failure in these patients includes direct thermal injury conducted from the surface to the enclosed parenchyma. Liu et al [14] proposed that fibrotic hepatic parenchyma demonstrates less thermal conductivity than the normal liver. However, in patients undergoing HIPEC, the patient’s liver, and mesenteric-to-portal circulation penetrating to the deep hepatic parenchyma are heated. Accordingly, the risks of hepatic decompensation following a prolonged surgical procedure with general anesthesia warrant particular caution before using hyperthermia with intraperitoneal chemotherapy in patients with impaired hepatic reserves.
In the interval since this patient’s surgery, our group has performed CRS with HIPEC in a small cohort (n = 4) of select patients with well-compensated cirrhosis, including a less severe thrombocytopenia than the patient described in this report. Although longitudinal follow-up is so far lacking, they demonstrated acceptable 30-day perioperative morbidity, commensurate with the published literature. Ultimately, future studies need to be conducted to investigate the specific effects of HIPEC on hepatic function, with the goal of correlating acceptable temperatures and duration of perfusion with the extent of hepatic impairment.
Conclusions
The criteria guiding selection of suitable candidates for CRS/IPC continues to evolve. Currently, there are no protocols guiding treatment of cirrhotic patients with peritoneal surface malignancies. A comorbidity as severe as cirrhosis merits unique consideration when selecting patients for cytoreduction and intraperitoneal chemotherapy. Concomitant compensated cirrhosis in patients with peritoneal surface malignancies should not solely constitute a reason to exclude CRS/IPC, particularly when the approximate risk of mortality from the patient’s peritoneal malignancy exceeds that of his or her liver disease.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Conflict of Interest
The authors of this manuscript have no financial disclosure or competing interest to declare.
Author Contributions
All authors were involved in the conception of the paper; analysis and interpretation of associated data and relevant literature; and in drafting the manuscript and revising it critically for accuracy.
| References | ▴Top |
- Fusari M, Sorrentino N, Bottazzi EC, Del Vecchio W, Cozzolino I, Maurea S, Salvatore M, et al. Primary signet ring cell carcinoma of the appendix mimicking acute appendicitis. Acta Radiol Short Rep. 2012;1(9).
doi pubmed - McGory ML, Maggard MA, Kang H, O'Connell JB, Ko CY. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48(12):2264-2271.
doi pubmed - McCusker ME, Cote TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94(12):3307-3312.
doi pubmed - Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69-76.
doi - Chua TC, Pelz JO, Kerscher A, Morris DL, Esquivel J. Critical analysis of 33 patients with peritoneal carcinomatosis secondary to colorectal and appendiceal signet ring cell carcinoma. Ann Surg Oncol. 2009;16(10):2765-2770.
doi pubmed - Van der Speeten K, Stuart OA, Sugarbaker PH. Using pharmacologic data to plan clinical treatments for patients with peritoneal surface malignancy. Curr Drug Discov Technol. 2009;6(1):72-81.
doi pubmed - Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14(1):128-133.
doi pubmed - Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192-204; discussion 205.
pubmed - Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140(7):650-654; discussion 655.
doi pubmed - Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91-96.
doi pubmed - Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey MR. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40(6):897-903.
doi pubmed - Bouchereau M, Gervais MK, Sideris L, Loriot MH, Ahern SP, Dube P. Hepatic necrosis and hemorrhage following hyperthermic intraperitoneal chemotherapy with oxaliplatin: A review of two cases. J Gastrointest Oncol. 2011;2(2):113-116.
pubmed - Grogan H, Hopkins PM. Heat stroke: implications for critical care and anaesthesia. Br J Anaesth. 2002;88(5):700-707.
doi pubmed - Liu Z, Ahmed M, Weinstein Y, Yi M, Mahajan RL, Goldberg SN. Characterization of the RF ablation-induced 'oven effect': the importance of background tissue thermal conductivity on tissue heating. Int J Hyperthermia. 2006;22(4):327-342.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gastroenterology Research is published by Elmer Press Inc.


