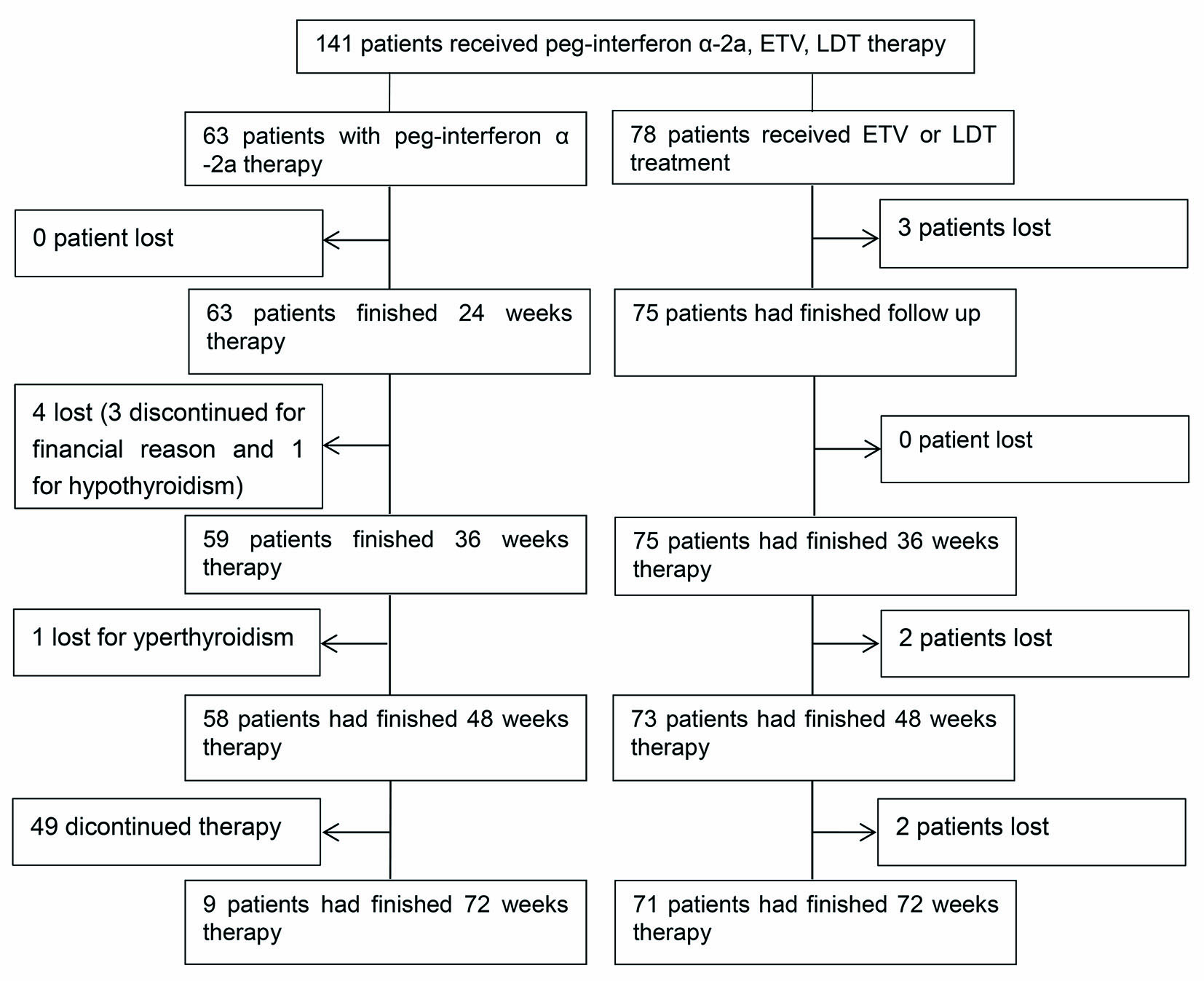
Figure 1. The flowchart of the study design.
| Gastroenterology Research, ISSN 1918-2805 print, 1918-2813 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Gastroenterol Res and Elmer Press Inc |
| Journal website http://www.gastrores.org |
Original Article
Volume 10, Number 1, February 2017, pages 6-14
Antiviral Therapy in Chronic Hepatitis B With Mild Acute Exacerbation
Figures

Tables
| Annual transitional probabilities | Estimated (%) | Range | References |
|---|---|---|---|
| HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B envelope antigen; CHB: chronic hepatitis B; HCC: hepatocellular carcinoma. | |||
| From HBsAg clearance to | [19] | ||
| HCC | 0.039 | ||
| From HBeAg Positive CHB to | |||
| Spontaneous seroconversion | 7.0 | 2.0 - 23 | [18] |
| Compensated cirrhosis | 2.4 | 2.1 - 2.6 | [18] |
| HCC | 0.8 | 0.5 - 1.0 | [18] |
| Death | 0.6 | 0.2 - 0.9 | [18] |
| From HBeAg seroconversion to | |||
| HBsAg seroclearance | 0.7 | 0.4 - 1.2 | [18] |
| Compensated cirrhosis | 1.0 | 0.1 - 6.3 | [18] |
| HCC | 0.2 | 0.05 - 0.9 | [18] |
| From compensated cirrhosis to | |||
| Decompensated cirrhosis | 3.9 | 3.2 - 4.6 | [18] |
| HCC | 5.0 | 3.0 - 7.0 | [18] |
| Death | 5.6 | 3.1 - 8.0 | [18] |
| From decompensated cirrhosis to | |||
| HCC | 7.1 | 3.5 - 10.0 | [18] |
| Death | 15 | 9.9 - 20.0 | [18] |
| From HCC to | |||
| Death | 54.5 | 20.0 - 60.0 | [18] |
| Health state costs, RMB (Dollar) [18] | Health state utilities [20] | |
|---|---|---|
| HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B envelope antigen; CHB: chronic hepatitis B; HCC: hepatocellular carcinoma. | ||
| HBsAg clearance | 110 ($19) | 0.99 |
| HBeAg seroconversion | 110 ($19) | 0.95 |
| HBeAg-positive CHB | 1,162 ($170) | 0.85 |
| Compensated cirrhosis | 1,514 ($222) | 0.69 |
| Decompensated cirrhosis | 13,927 ($2,040) | 0.35 |
| HCC | 38,795 ($5,682) | 0.38 |
| Peginterferon α-2a (n = 63) | Nucleos(t)ide analogues (n = 78) | P value | |
|---|---|---|---|
| HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B envelope antigen; ALT: alanine aminotransferase. *Expressed as median (interquartile range). | |||
| Baseline | |||
| Age (years)* | 25 (21 - 32) | 32 (26 - 35) | < 0.001 |
| Male sex, n (%) | 49/63 (77.78%) | 67/78 (85.9%) | 0.209 |
| Genotype (B/C) | 22:10 | 27:12 | 0.965 |
| Biopsy | |||
| G* | 3 (2 - 3) | 3 (2 - 3) | 0.446 |
| S* | 3 (2 - 3) | 3 (2 - 3) | 0.887 |
| Duration of pretreatment (days)* | 13 (7 - 17) | 4 (2 - 8) | < 0.001 |
| ALT (IU/L)* | |||
| Highest | 703 (506 - 1,028) | 757 (542 - 1,185) | 0.348 |
| Begin pegIFN | 311 (280 - 357) | ||
| TBIL(μmol/L) | |||
| Highest | 17.62 ± 5.44 | 19.10 ± 5.92 | 0.154 |
| Begin pegIFN | 13.82 ± 4.56 | 15.35 ± 5.68 | 0.110 |
| PT(s)* | 11.80 (11.40 - 12.50) | 11.70 (11.40 - 12.43) | 0.685 |
| HBV DNA (log10 IU/mL) | 6.87 ± 1.11 | 6.70 ± 0.99 | 0.368 |
| HBsAg (log IU/mL) | 4.20 ± 0.59 | 4.18 ± 0.70 | 0.896 |
| HBeAg (s/co)* | 740.50 (122.13 - 1,232.29) | 712.14 (126.79 - 1,071.35) | 0.404 |
| Results | |||
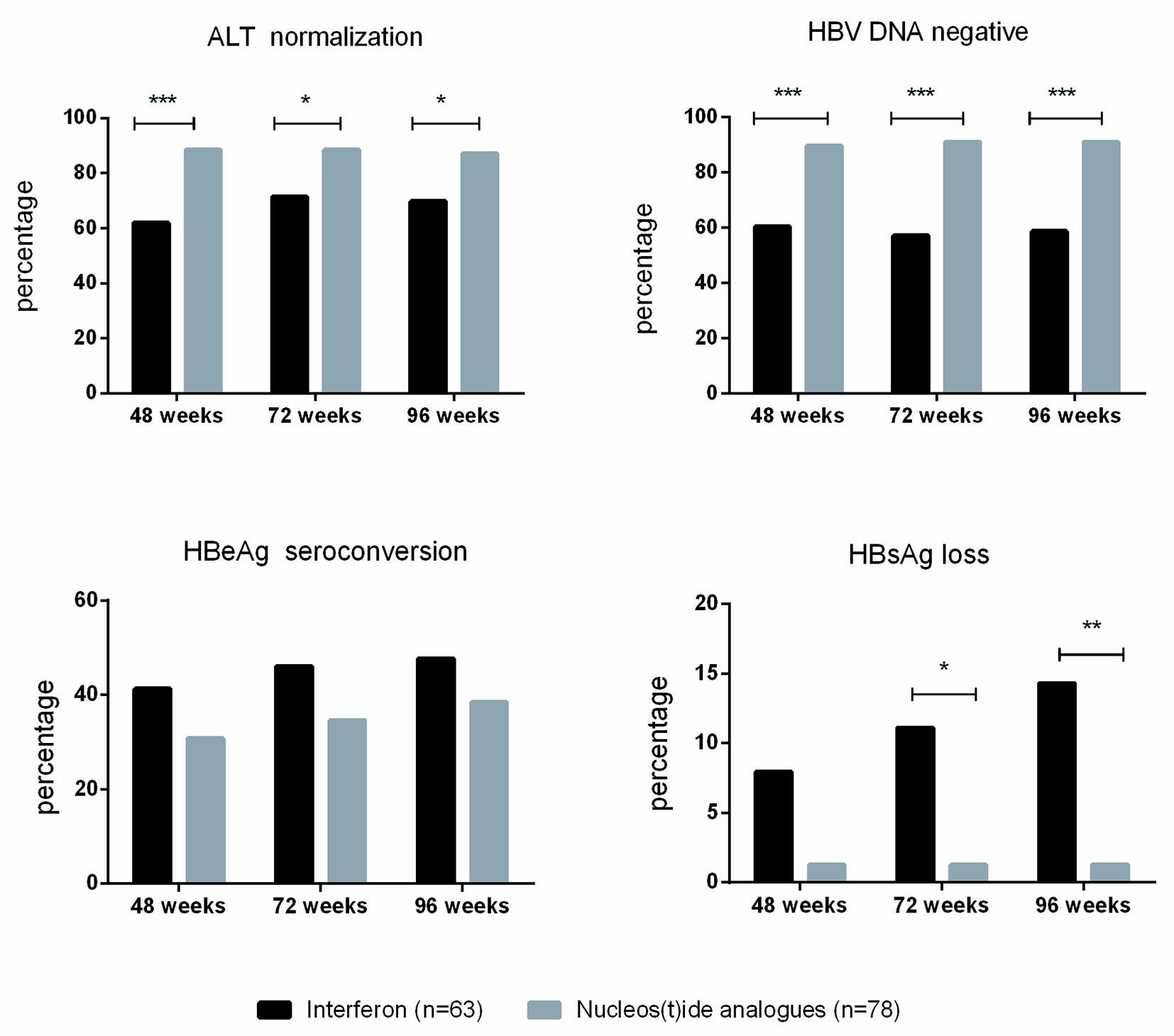
| ALT normalization, n/total (%) | |||
| Week 12 | 19/63 (30.16%) | 44/78 (56.41%) | 0.002 |
| Week 24 | 25/63 (39.68%) | 60/78 (76.92%) | 0.000 |
| Week 36 | 34/63 (53.97%) | 69/78 (88.46%) | 0.004 |
| Week 48 | 39/63 (61.90%) | 69/78 (88.46%) | < 0.001 |
| Week 72 | 45/63 (71.43%) | 69/78 (88.46%) | 0.011 |
| Week 96 | 44/63 (69.84%) | 68/78 (87.18%) | 0.011 |
| HBV DNA negative, n/total (%) | |||
| Week 12 | 14/63 (22.22%) | 26/78 (33.33%) | 0.146 |
| Week 24 | 27/63 (42.86%) | 47/78 (60.26%) | 0.040 |
| Week 36 | 36/63 (57.14%) | 55/78 (70.51%) | 0.099 |
| Week 48 | 38/63 (60.32%) | 70/78 (89.74%) | < 0.001 |
| Week 72 | 36/63 (57.14%) | 71/78 (91.03%) | < 0.001 |
| Week 96 | 37/63 (58.73%) | 71/78 (91.03%) | < 0.001 |
| HBeAg seroconversion, n/total (%) | |||
| Week 12 | 6/63 (9.52%) | 6/78 (7.69%) | 0.698 |
| Week 24 | 8/63 (12.70%) | 14/78 (17.95%) | 0.393 |
| Week 36 | 10/63 (15.87%) | 16/78 (20.51%) | 0.480 |
| Week 48 | 26/63 (41.27%) | 24/78 (30.77%) | 0.195 |
| Week 72 | 29/63 (46.03%) | 27/78 (34.62%) | 0.168 |
| Week 96 | 30/63 (47.62%) | 30/78 (38.46%) | 0.274 |
| HBsAg loss, n/total (%) | |||
| Week 12 | 0/63 (0.00%) | 0/78 (0.00%) | |
| Week 24 | 0/63 (0.00%) | 1/78 (1.28%) | 1.000 |
| Week 36 | 1/63 (1.59%) | 1/78 (1.28%) | 1.000 |
| Week 48 | 5/63 (7.94%) | 1/78 (1.28%) | 0.089 |
| Week 72 | 7/63 (11.11%) | 1/78 (1.28%) | 0.022 |
| Week 96 | 9/63 (14.29%) | 1/78 (1.28%) | 0.005 |
| HBsAg seroconversion, n/total (%) | |||
| Week 12 | 0/63 (0.00%) | None | |
| Week 24 | 0/63 (0.00%) | ||
| Week 36 | 1/63 (1.59%) | 0.447 | |
| Week 48 | 2/63 (3.17%) | 0.198 | |
| Week 72 | 3/63 (4.76%) | 0.087 | |
| Week 96 | 3/63 (4.76%) | 0.087 | |
| ETV (n = 38) | LDT (n = 40) | P value | |
|---|---|---|---|
| HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B envelope antigen; ALT: alanine aminotransferase. *Expressed as median (interquartile range). | |||
| Baseline | |||
| Age (years)* | 35 (20 - 39) | 27 (24 - 32) | < 0.001 |
| Male sex, n (%) | 32/38 (84.21%) | 35/40 (87.50%) | 0.677 |
| Genotype (B/C) | 15:07 | 12:05 | 0.872 |
| Biopsy | |||
| G* | 3 (2 - 3) | 3 (2 - 3) | 0.719 |
| S* | 3 (2 - 3) | 3 (2 - 3) | 0.509 |
| Duration of pretreatment (days)* | 5 (2 - 8) | 4 (2 - 7) | 0.360 |
| ALT (IU/L)* | |||
| Highest | 756 (555 - 1,248) | 757 (531 - 1,021) | 0.803 |
| TBIL (μmol/L) | |||
| Highest | 19.07 ± 6.13 | 19.12 ± 5.80 | 0.972 |
| PT(s)* | 11.90 (11.50 - 12.50) | 11.60 (11.30 - 12.38) | 0.278 |
| HBV DNA (log10 IU/mL) | 6.54 ± 0.98 | 6.85 ± 0.99 | 0.168 |
| HBsAg (log IU/mL) | 4.11 ± 0.66 | 4.25 ± 0.75 | 0.454 |
| HBeAg (s/co) | 537.49 (116.81 - 1,071.12) | 741.59 (184.91 - 1,102.27) | 0.470 |
| Results | |||
| ALT normalization, n/total (%) | |||
| Week 12 | 19/38 (50.00%) | 25/40 (62.50%) | 0.266 |
| Week 24 | 24/38 (63.16%) | 36/40 (90.00%) | 0.005 |
| Week 36 | 25/38 (65.79%) | 35/40 (87.50%) | 0.023 |
| Week 48 | 33/38 (86.84%) | 36/40 (90.00%) | 0.734 |
| Week 72 | 33/38 (86.84(%) | 36/40 (90.00%) | 0.734 |
| Week 96 | 32/38 (84.21%) | 36/40 (90.00%) | 0.670 |
| HBV DNA negative, n/total (%) | |||
| Week 12 | 16/38 (42.11%) | 10/40 (25.00%) | 0.109 |
| Week 24 | 24/38 (63.16%) | 23/40 (57.50%) | 0.610 |
| Week 36 | 27/38 (71.05%) | 28/40 (70.00%) | 0.919 |
| Week 48 | 37/38 (97.37%) | 33/40 (82.50%) | 0.057 |
| Week 72 | 36/38 (94.74%) | 35/40 (87.50%) | 0.432 |
| Week 96 | 36/38 (94.74%) | 35/40 (87.50%) | |
| HBeAg seroconversion, n/total (%) | |||
| Week 12 | 3/38 (7.89%) | 3/40 (7.50%) | 1.000 |
| Week 24 | 7/38 (1.84%) | 7/40 (1.75%) | 0.916 |
| Week 36 | 7/38 (1.84%) | 9/40 (22.50%) | 0.656 |
| Week 48 | 10/38 (26.32%) | 14/40 (35.00%) | 0.406 |
| Week 72 | 13/38 (34.21%) | 14/40 (35.00%) | 0.942 |
| Week 96 | 14/38 (36.84%) | 16/40 (40.00%) | 0.774 |
| HBsAg loss, n/total (%) | |||
| Week 12 | 0/38 (0.00%) | 0/40 (0.00%) | |
| Week 24 | 1/38 (2.63%) | 0/40 (0.00%) | 0.487 |
| Week 36 | 1/38 (2.63%) | 0/40 (0.00%) | 0.487 |
| Week 48 | 1/38 (2.63%) | 0/40 (0.00%) | 0.487 |
| Week 72 | 1/38 (2.63%) | 0/40 (0.00%) | 0.487 |
| Week 96 | 1/38 (2.63%) | 0/40 (0.00%) | 0.487 |
| HBsAg seroconversion, n/total (%) | |||
| Week 12 | None | None | |
| Week 24 | |||
| Week 36 | |||
| Week 48 | |||
| Week 72 | |||
| Week 96 | |||